1 Department of Cardiology, University Medical Centre Ljubljana, 1000 Ljubljana, Slovenia
2 Faculty of Medicine, University of Ljubljana, 1000 Ljubljana, Slovenia
Abstract
Background: “Ablate and pace” strategy is a reasonable treatment option
in refractory atrial fibrillation (AF) when sinus rhythm (SR) cannot be achieved
with catheter ablation or pharmacological therapy. Atrioventricular node ablation
(AVNA) combined with conduction system pacing (CSP), with left bundle branch
pacing (LBBP) or His bundle pacing (HBP), is gaining recognition since it offers
the most physiological activation of the left ventricle. However, the incidence
of conversion to SR after AVNA with CSP is not known. The purpose of the
investigation was to determine the incidence of spontaneous conversion to SR and
its predicting factors in patients undergoing CSP and AVNA. Methods:
Consecutive refractory symptomatic AF patients undergoing AVNA with CSP at our
institution between June 2018 and December 2022 were retrospectively analyzed.
Twelve lead electrocardiogram (ECG) recordings were analyzed at each outpatient follow-up visit.
Echocardiographic and clinical parameters were assessed at baseline and six
months after the implantation. Results: Sixty-eight patients (male
42.6%, age 71
Keywords
- atrial fibrillation
- conduction system pacing
- left bundle branch pacing
- His bundle pacing
- atrioventricular node ablation
- sinus rhythm
Atrial fibrillation (AF), the most prevalent supraventricular tachyarrhythmia (SVT), results in disorganized atrial activity, reduced cardiac output, and hemodynamic deterioration. The progressive nature of AF has been attributed to alterations in the electrical, contractile, and structural properties of the atria. Some of these changes appear to be reversible upon the improvement of hemodynamics [1, 2, 3].
There are two basic approaches for the treatment of AF: rate and rhythm control. Antiarrhythmic drugs and/or pulmonary vein isolation (PVI) are used to maintain sinus rhythm (SR) as part of rhythm control management. The guidelines for the management of SVT recommend atrioventricular node ablation (AVNA) with permanent ventricular pacing (‘pace and ablate’ strategy) when SR is no longer pursued or attainable (Class I, level of evidence C) [4]. AVNA with subsequent right ventricular (RV) or biventricular (BiV) pacing in these patients results in symptomatic improvement, reduced heart failure (HF) hospitalizations, and improved survival [5, 6]. In addition, analysis of retrospective data raises the possibility that a rate control strategy with BiV pacing may even contribute to spontaneous SR restoration [7, 8, 9]. As BiV pacing still causes non-physiologic cardiac activation in patients with narrow QRS [8, 9, 10, 11], left bundle branch pacing (LBBP) and His bundle pacing (HBP) have recently evolved as conduction system pacing (CSP) options allowing more physiological activation of the myocardium which preserves left ventricular (LV) function [12, 13, 14, 15, 16, 17, 18].
The incidence of SR restoration after the “ablate and pace” strategy with CSP has been unexplored. The purpose of the investigation was to determine the incidence of spontaneous conversion to SR and its predicting factors in patients with refractory AF undergoing AVNA and CSP.
This study retrospectively investigated the incidence of spontaneous conversion to SR and its predicting factors in patients who underwent attempted AVNA and CSP for symptomatic AF between June 2018 and December 2022 at University Medical Centre Ljubljana. Consecutive patients with symptomatic AF refractory to rhythm and pharmacological rate control therapy were included. Patients with severe valvular disease were excluded. SR restoration was defined as the spontaneous conversion to SR documented on 12-lead electrocardiogram (ECG) in the device clinic during follow-up after AVNA and CSP.
All patients were asked for written informed consent before data collection. The study design was approved by the Republic of Slovenia National Medical Ethics Committee.
Pacing device implantations were always performed first, followed by AVNA, preferably during the same hospitalization. All device implantations were performed by two experienced operators.
The HBP procedures were conducted as previously reported [13, 19, 20]. His
bundle (HB) potential mapping was performed using a continuous recording of
intracardiac electrograms with the electrophysiological recording system
(EP-TRACER 2 Portable CardioTek B.V., Sittard, The Netherlands or LAB system Pro,
BARD Boston Scientific, Lowell, MA, USA). The tricuspid valve annulus ring was
imaged by contrast angiography to facilitate HB localization. The sheath and the
pacing lead were advanced to the HB area, where larger ventricular and smaller
atrial signals were detected (ventricular to atrial electrogram ratio at least
3:1). The pacing lead was screwed into position, and threshold measurement was
performed at the pulse width of 1 ms. HBP threshold
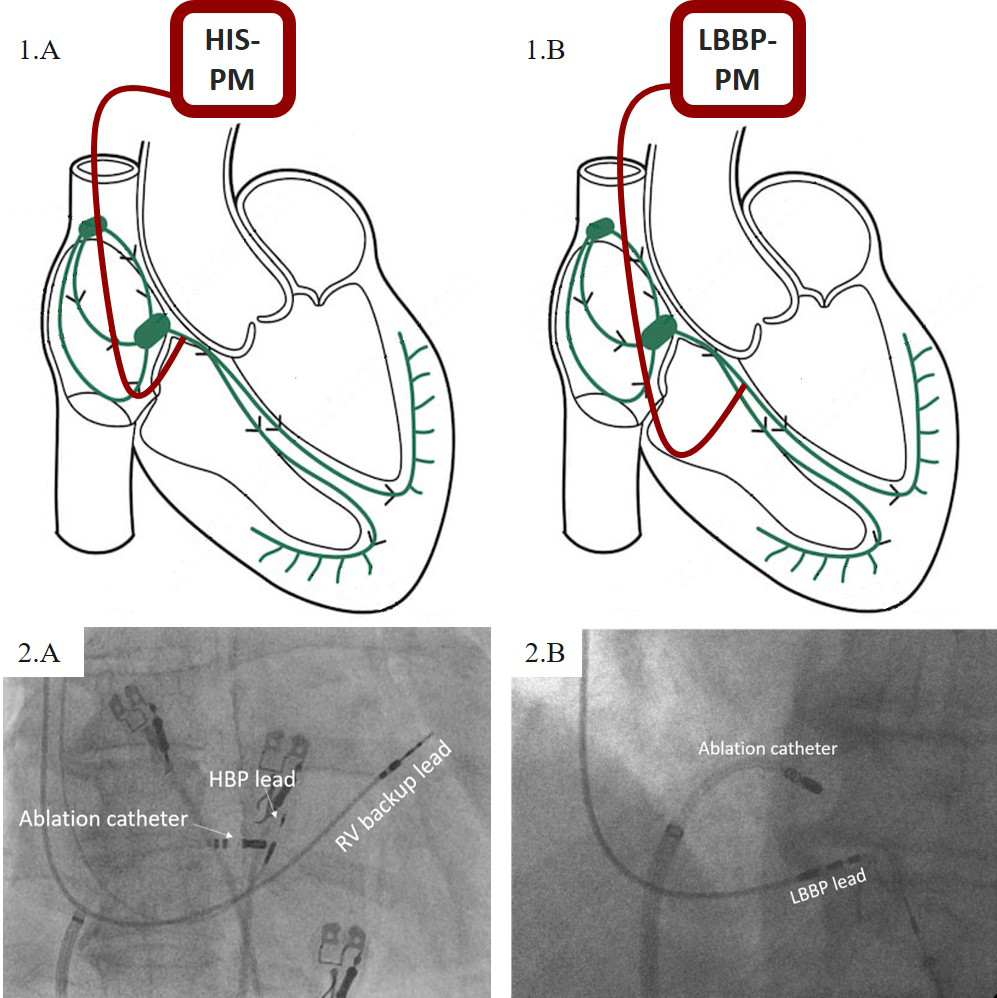
 Fig. 1.
Fig. 1.Schematic representation of pacemaker (PM) lead positions (1) and their relation to the ablation catheter under fluoroscopy (2). (A) His bundle pacing (HBP). (B) Left bundle branch pacing (LBBP). For clarity, the backup pacing lead is not illustrated.
The procedure was performed as previously described [21]. After the localization of the HB area, either fluoroscopically or determined with the use of intracardiac signals, the LBBP lead was positioned approximately 1–1.5 cm below the distal HB location along the line towards the RV apex. Following the optimal lead positioning with the use of fluoroscopy and paced QRS morphology (“w” pattern in lead V1), the lead was screwed into the interventricular septum with constant monitoring of pacing impedance, current of injury, and QRS morphology. Trans-septal lead advancement was stopped when typical left bundle branch capture morphology was reached. Both lumen-less and stylet-driven leads were used for LBBP. While backup pacing leads were never implanted due to low and stable pacing parameters, the ICD lead, if needed, was connected in the same fashion as in HBP procedures [22]. Schematic illustration and fluoroscopic view of the pacemaker lead position are presented in Fig. 1B.
Prior to AVNA, the previously implanted pacemaker was temporarily set to 40 bpm
for the duration of the procedure. After achieving femoral vein access, the
ablation catheter with a 3.5- or 4-mm tip (FlexAbility
Twelve-lead ECGs were assessed at baseline and each outpatient follow-up visit: at 1 month, 6 months, and every 6 months thereafter. Assessment of clinical outcomes (echocardiographic parameters, laboratory parameters, and symptomatic evaluation) was performed at baseline and at 6 months or immediately after SR on the ECG strip was detected.
Categorical variables are reported as frequencies and percentages and were
compared using Chi-square and Fisher exact test as appropriate. Continuous
variables are expressed as mean
Baseline patient characteristics according to the occurrence of spontaneous
conversion to SR are presented in Table 1. Sixty-eight consecutive patients
undergoing CSP combined with AVNA were included. The mean age of the patients was
71
| SR (n = 6) | NSR (n = 62) | p-value | ||
| Baseline characteristics | ||||
| Age [years] | 70 ( |
71 ( |
0.673 | |
| Male sex | 2 (33.3%) | 27 (43.5%) | 1 | |
| QRS [ms] | 108 (94–119) | 109 (95–120) | 0.792 | |
| LBBB | 0 | 6 (9.7%) | 1 | |
| RBBB | 0 | 2 (3.2%) | 1 | |
| Atypical atrial flutter | 1 (16.7%) | 11 (17.7%) | 1 | |
| LVEF [%] | 40 ( |
40 ( |
1 | |
| LAVI [mL/m |
45 (41–51) | 60 (52–75) | 0.002 | |
| Initial NYHA class | 3.5 (3–4) | 3 (3–3) | 0.082 | |
| Arterial hypertension | 4 (66.6%) | 45 (72.6%) | 1 | |
| Diabetes | 1 (16.7%) | 17 (27.4%) | 1 | |
| Coronary artery disease | 1 (16.7%) | 13 (21%) | 1 | |
| Medications | ||||
| Loop diuretic | 3 (50%) | 39 (62.9%) | 0.668 | |
| ACEi/ARB/ARNI | 2 (33.3%) | 40 (64.5%) | 0.193 | |
| MRA | 0 | 24 (38.7%) | 0.083 | |
| Beta-blocker | 5 (83.3%) | 59 (95.1%) | 0.315 | |
| Digoxin | 1 (16.7%) | 16 (25.8%) | 1 | |
| Amiodarone | 2 (33.3%) | 15 (24.2%) | 0.635 | |
Legend: SR, sinus rhythm group; NSR, non-sinus rhythm group; LBBB, left bundle
branch block; RBBB, right bundle branch block; LVEF, left ventricular ejection
fraction; LAVI, left atrial volume index; ACEi, angiotensin-converting enzyme
inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor
neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association. Bold value
denotes statistical significance at the p
The median follow-up time was 16 months (6–27). Spontaneous conversion to SR
during follow-up was registered in 6 patients (8.8%); 3 in the HBP group and 3
in the LBBP group. In patients who converted to SR, baseline LAVI was smaller (45
mL/m
To further clarify the predictors of spontaneous conversion to SR after AVNA, we performed a multiple regression analysis (Table 2). Covariates that were considered clinically relevant were age, gender, baseline LVEF, LAVI, and indexed left ventricular end-diastolic volume index (LVEDVi). Even after consideration of these covariates, LAVI remained a significant predictor for conversion to SR (odds ratio 1.273, 95% confidence interval [1.027, 1.578], p = 0.028).
| OR (95% CI) | p-value | |
| Sex | 0.315 (0.028, 3.585) | 0.352 |
| Age | 1.022 (0.899, 1.162) | 0.738 |
| Initial LVEF [%] | 1.117 (0.960, 1.300) | 0.151 |
| Initial LVEDVi [mL/m |
1.112 (0.976, 1.267) | 0.111 |
| Initial LAVI [mL/m |
1.273 (1.027, 1.578) | 0.028 |
Legend: OR, odds ratio; CI, confidence interval; LVEF, left ventricular ejection
fraction; LVEDVi, left ventricular end-diastolic volume index; LAVI, left atrial volume index. Bold value denotes
statistical significance at the p
All device implantations and subsequent AVNAs were successful without any acute adverse events. Apart from two patients in whom an existing ICD device was upgraded with a CSP lead, all other procedures were de-novo implantations. The median pacemaker implantation fluoroscopy time was 6 minutes (4.2–8.1). An ICD device for primary prevention was used in 3 (8%) patients who received HBP and 7 (22%) patients who underwent LBBP. Additional atrial lead was implanted in only one patient. Periprocedural increase in HBP threshold after AVNA was documented in 1 patient. However, the HB capture was maintained at higher outputs, and the lead revision was not required.
At baseline, NYHA class of patients who converted to SR (SR group) did not
differ from patients who remained in atrial arrhythmia (non-sinus rhythm (NSR) group) (p =
0.082). As reported in Table 3, NYHA class improvement was registered
regardless of SR restoration (p = 0.026 for SR; p
| SR (n = 6) | NSR (n = 62) | p-value — comparing groups | ||
| NYHA class | ||||
| Baseline NYHA class | 3.5 (3–4) | 3 (3–3) | 0.082 | |
| Nb. in NYHA class 2 | 0 | 14 (22.6%) | ||
| Nb. in NYHA class 3 | 3 (50%) | 37 (59.7%) | ||
| Nb. in NYHA class 4 | 3 (50%) | 11 (17.7%) | ||
| Follow-up NYHA class | 1.5 (1–2) | 2 (1–2) | 0.419 | |
| Nb. in NYHA class 1 | 3 (50%) | 16 (25.8%) | ||
| Nb. in NYHA class 2 | 2 (33.3%) | 39 (62.9%) | ||
| Nb. in NYHA class 3 | 1 (16.7%) | 7 (11.3%) | ||
| Nb. in NYHA class 4 | 0 | 0 | ||
| p-value: baseline vs. follow-up | 0.026 | |||
| Loop diuretics | ||||
| Baseline | 3 (50%) | 39 (62.9%) | 0.668 | |
| Follow-up | 2 (33.3%) | 27 (43.5%) | 1 | |
| p-value: baseline vs. follow-up | 1 | 0.047 | ||
| NT-proBNP [pg/mL] | ||||
| Baseline (n = 61) | 5122 (2800–12,059) | 2894 (1552–7285) | 0.339 | |
| Follow-up (n = 55)* | 1437 (1042–2229) | 2034 (976–3001) | 0.599 | |
| p-value: baseline vs. follow-up | 0.625 | |||
| eGFR [mL/min/1.73 m | ||||
| Baseline (n = 66) | 54 (32–67) | 52 (43-63) | 0.240 | |
| Follow-up (n = 60)* | 54 (49–90) | 67 (46-75) | 0.214 | |
| p-value: baseline vs. follow-up | 0.688 | |||
Legend: SR, sinus rhythm group; NSR, non-sinus rhythm group; NYHA, New York
Heart Association; NT-proBNP, N-terminal pro-b-type natriuretic peptide;
eGFR, estimated glomerular filtration rate. * 7 patients in the NSR
group and 1 in the SR group did not have follow-up GFR values, 11 patients in NSR
and 1 patient in the SR group did not have NT-proBNP values at follow-up. Bold
values denote statistical significance at the p
At baseline, median N-terminal pro-b-type natriuretic peptide (NT-proBNP) was 2969 (1569–3635) pg/mL and did not differ between both groups (p = 0.339). At follow-up, NT-proBNP decreased in both groups, although the decrease in the SR group, despite being relatively higher, did not reach statistical significance due to the smaller sample size.
Four patients in the NSR group (3 with HBP and one with LBBP) died during follow-up. While the death in the LBBP group was associated with progressive HF, the other 3 deaths were determined as non-cardiac.
At baseline, 6 patients had left bundle branch block (LBBB), and 2 patients had right bundle branch block (RBBB). While none of these 8 patients converted to sinus rhythm, there was no difference in baseline QRS width between SR (108 ms (94–119)) and NSR group (109 ms (95–120)), p = 0.792. Post-procedural QRS width was similar to baseline QRS (p = 0.109 for SR; p = 0.08 for NSR).
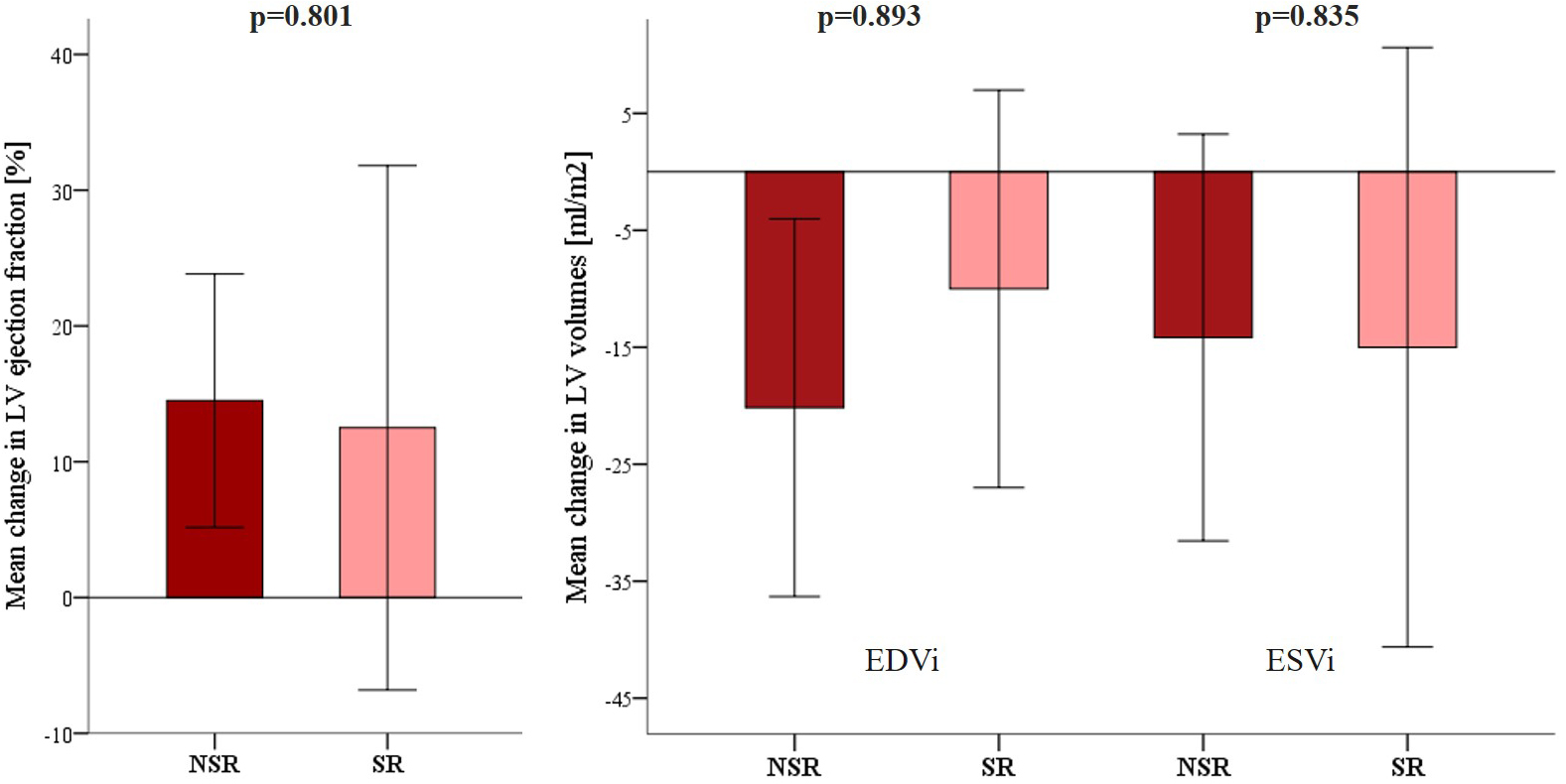
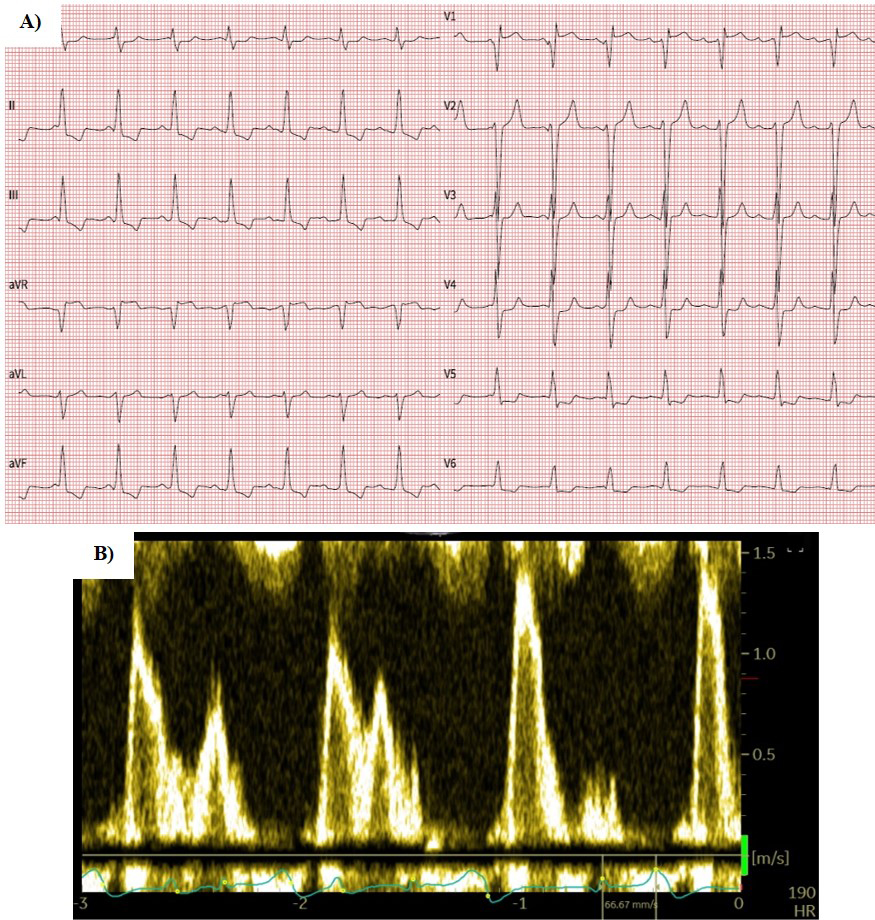
While there were no significant differences in baseline LVEF (p = 1), LVEDVi (p = 0.214), and indexed LV systolic volume (LVESVi) (p = 0.311), baseline LAVI was, as previously described, significantly smaller in patients who converted to SR (p = 0.002) (Table 4). At follow-up, LAVI did not change in any group. The increase in LVEF was numerically comparable in both groups, although, in the SR group, it did not reach statistical significance due to the smaller sample size. Similarly, LVESVi decreased in both groups; however, in the SR group, the decrease did not reach statistical significance due to both smaller sample size and smaller initial volumes. As for LVESVi, we observed consistent changes in LVEDVi. A comparison of mean changes in echocardiographic parameters is presented in Fig. 2. The follow-up electrocardiogram and echocardiographic mitral inflow pattern of the patient who converted to SR after LBBP with subsequent AVNA are presented in Fig. 3.
| SR (n = 6) | NSR (n = 62)* | p-value — comparing groups | |
| Baseline LVEF [%] | 40 ( |
40 ( |
1 |
| Follow-up LVEF [%] | 52 ( |
50 ( |
0.671 |
| p-value — baseline vs. follow-up | 0.174 | ||
| Baseline LVEDVi [mL/m |
56 (51–69) | 76 (53–94) | 0.214 |
| Follow-up LVEDVi [mL/m |
53 (49–57) | 63 (47–82) | 0.228 |
| p-value — baseline vs. follow-up | 0.144 | 0.001 | |
| Baseline LVESVi [mL/m |
32 (27–41) | 47 (27–63) | 0.311 |
| Follow-up LVESVi [mL/m |
25 (25–26) | 29 (21–46) | 0.184 |
| p-value — baseline vs. follow-up | 0.176 | ||
| Baseline LAVI [mL/m |
45 (41–51) | 60 (52–75) | 0.002 |
| Follow-up LAVI [mL/m |
48 (44–54) | 66 (52–77) | 0.018 |
| p-value — baseline vs. follow-up | 0.345 | 0.508 |
Legend: SR, sinus rhythm group; NSR, non-sinus rhythm group; LVEF, left
ventricle ejection fraction; LVEDVi, left ventricular end-diastolic volume index; LVESVi, left ventricular end-systolic volume index;
LAVI, left atrial volume index. * In the NSR group
follow-up, LVEF value was available in 56 patients and left ventricle volumes
were available in 59 patients at baseline and 56 patients at follow-up. Bold
values denote statistical significance at the p
 Fig. 2.
Fig. 2.Comparison of the mean (
 Fig. 3.
Fig. 3.12-lead ECG (A) and mitral inflow pattern (B) of the patient who converted to sinus rhythm after left bundle branch pacing and atrioventricular node ablation. Note the VVI pacing mode and atrioventricular dissociation due to the lack of atrial lead. ECG, electrocardiogram.
To the best of our knowledge, this is the first study reporting the incidence of spontaneous conversion to SR after CSP and AVNA in patients with refractory symptomatic AF. The main finding of the present study was that spontaneous conversion to SR after AVNA combined with CSP is not uncommon, as it occurred in 8.8% of the patients. Smaller LAVI was identified as the only independent predicting factor for SR restoration in patients undergoing this treatment option.
According to guidelines, antiarrhythmic drugs or PVI are considered to restore
and maintain SR as part of rhythm control management [24]. Success rates of
rhythm control strategy in patients with paroxysmal AF seem to be better than in
patients with persistent AF, where approximately 50% SR maintenance is
achievable, according to the literature [25, 26]. As the ‘pace and ablate’
strategy is considered only as a rate control strategy, SR restoration is not
anticipated [4]. Nonetheless, AVNA and BiV pacing has been associated with
spontaneous reversions to SR in patients with persistent AF, ranging from 7% in
one report [7] and 10.3% in the other [9]. In our study, spontaneous SR
restoration during follow-up was registered in 6 patients (8.8%), predominantly
in patients with smaller initial LAVI. This is in line with the previous BiV
study, where LA diameter
Several beneficial clinical outcomes of CSP modalities combined with AVNA have already been published [16, 17]. In our study, symptomatic improvement was achieved in both groups: 75.8% of patients in the NSR group and all patients in the SR group improved for at least one functional class. Similarly, the number of patients receiving loop diuretics decreased in both groups, however not significantly in the SR group. This difference could be explained by a smaller sample size of the SR group. Echocardiographic outcomes of the ‘pace and ablate’ strategy in this study resemble those mentioned in the previously published literature [14, 15, 17, 27]. LV volumes and LVEF improved in the NSR group. Similar, although not statistically significant, improvement of LVEF and reduction of LV volumes was observed in the SR group. The mean change of LVEF and LV volumes between both groups did not differ. There are several reasons that could be attributed to these findings. First, as atrial leads were not implanted in patients with SR, the patients did not gain any additional benefit from restored atrioventricular (AV) synchrony (Fig. 3). Furthermore, the SR group was numerically smaller with smaller, albeit not statistically, initial LV volumes which might have influenced the power of statistical analysis.
Larger studies are warranted to clarify the predicting variables of SR restoration in patients scheduled for an “ablate and pace” strategy with CSP. The incidence of SR restoration is clinically important as these patients may require an upgrade to a dual-chamber device to ensure AV synchrony that could further maintain SR and prevent potential pacemaker syndrome. On the contrary, initial atrial lead implantation in all patients may imply an unnecessary increase in lead burden. Therefore, the ability to identify which patients are expected to experience SR during follow-up is certainly important to optimally manage these patients.
The retrospective design of this single-center study with a relatively small number of included patients limits the strength of our findings. Furthermore, a smaller sample size in the SR group might have affected the outcomes compared to NSR. The potential exclusion of patients with severe valvular disease might have had an impact on the results of the study, while the probability of SR restoration in these patients is very low. However, none of the patients were, in fact, excluded due to this exclusion criterion. The study only recorded SR restoration in the device clinic during regular follow-ups that were documented on 12-lead ECG, while potential intermittent conversions to sinus rhythm were not detected. However, persistent sinus rhythm was not achieved in the NSR group. The data on the duration of AF before the ‘pace and ablate’ intervention could not be obtained in several patients. As some of the patients who converted to SR were urgently admitted to the hospital due to acute decompensated HF, a possible shorter duration of AF in these patients could have resulted in less structural and electrical left atrial changes and increased the likelihood of the SR restoration [1, 2, 3]. Therefore, our findings should be interpreted with caution and need to be confirmed in larger studies with longer follow-ups.
Spontaneous conversion to SR after AVNA combined with CSP is not uncommon, especially in AF patients with smaller left atria. Further studies are warranted to clarify which patients should be considered for an initial dual-chamber device to provide AV synchrony in case of SR restoration.
The data presented in this study are available upon request from the corresponding author and are not publicly available due to ethical issues.
DŽ and AZM designed the research study, MI and MM performed the research and analyzed the data. MI and DŽ wrote the manuscript, MM and AZM contributed to editorial changes in the manuscript. All authors have made a substantial contribution to the work and approved the submitted manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Republic of Slovenia National Medical Ethics Committee (number: 0120-193/2022/3). Written informed consent was obtained from all patients.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.