Academic Editors: Hiroki Teragawa and Hiroki Ikenaga
Background: Rotational atherectomy (RA) is an important technique for
the management of severe coronary calcification. However, optimal rotational
speed is yet to be defined. Methods: A total of 372 coronary heart
disease (CHD) patients were retrospectively analyzed between February 2017 and
January 2022. The patients were divided into four groups based on the maximum RA
speed: group 1 (
With the expansion of the social economy and the aging population, the incidence of coronary heart disease (CHD) is increasing year by year, and the proportion of coronary calcification lesions has dramatically increased. Since early 1990s, rotational atherectomy (RA) has been the mainstay tool used to effectively debulk and modify calcified plaques, increasing the lumen area, facilitating the delivery of intraoperative balloons and stents, as well as improving stent expansion and apposition, thereby increasing the success rate of percutaneous coronary intervention (PCI) [1, 2, 3]. The rotation speed varies considerably among different interventional cardiologists (140,000 to 220,000 rpm) in clinical practice [4, 5, 6]. Moreover, there is limited data on the relationship between RA speed and long-term prognosis in patients with CHD, and determination of the optimal rotation speed remain controversial.
This study investigated interventional outcomes of RA at different rotational speeds and analyzed clinical outcomes in patients with CHD.
This observational, retrospective study was conducted between February 2017 and
December 2021. The study enrolled a total of 372 CHD patients with severe
coronary calcification who were treated with RA. The patients were divided into
four groups according to the maximum RA speed: group 1 (
Demographic and clinical characteristics of the patients, including age, sex, and comorbidities such as a history of cardiovascular diseases (CVD) or stroke were collected. Some auxiliary examination data such as serum creatinine or fasting plasma glucose (FPG) were also collected. Lesion characteristics and procedural details, as well as any complications during RA operation and six-month outcomes were analyzed.
This study protocol was approved by the Medical Research Ethics Committee of the First Affiliated Hospital of USTC (Anhui Provincial Hospital) (No. 2022-RE-063).
Our center used the Siemens DTC (or PHILIPS FD1010) angiography system for coronary angiography and interventional therapy. The Rotablator System (Boston Scientific Corporation, Natick, MA, USA) was used to perform all the RA procedures. Prior to the procedure, the patients received aspirin (100 mg/daily) and a thienopyridine (clopidogrel 75 mg/daily or ticagrelor 100 mg bid). In addition, intravenous unfractionated heparin (70 to 100 units/kg) was used to achieve an appropriate activated coagulation time (250 seconds) during the PCI. PCI was performed at the operator’s discretion based on the characteristics of the patient’s specific lesions and peripheral vascular access conditions. Guiding catheters up to 7F in diameter were used. With the help of a Finecross or Crossair microcatheter, a 0.009 in (1 in = 2.54 cm) rotawire floppy was exchanged to the distal of the target lesion. Each RA session lasted less than 30 seconds, and the interval between each RA was 60 seconds. During the RA procedure, unfractionated heparin (UFH) nitroglycerine flushing solution was continuously instilled under pressure. Non-compliant balloon dilation and stent placement were performed after RA based on the characteristics of the lesions. Because our experienced surgeons were less likely to experience bradyarrhythmias when using small burrs and lower rotational speeds, temporary pacemakers are rarely used. Patients with bradyarrhythmia after RA were either instructed to cough forcefully or administered with intravenous atropine. Atropine and temporary pacemakers were prepared in advance for patients with a dominant right coronary artery or circumflex. The decision to insert an intra aortic balloon Pump (IABP) was left to the discretion and guidance of the supervising cardiologists.
Perioperative endpoints in our study included occurrence of hypotension, vasospasm, dissection, slow flow, perforation, bradyarrhythmia, burr entrapment, rotawire fracture during RA, as well as the incidence of heart failure, stent thrombosis, and cardiac death during hospitalization. Long-term primary endpoint was six-month occurrence of major cardiovascular and cerebrovascular events (MACCE), which include a composite of myocardial infarction (MI), stent thrombosis, target vessel revascularization (TVR), cardiogenic death, all-cause death, and stroke. On the other hand, long-term secondary endpoint was six-month chronic heart failure.
Severely calcified lesions can be assessed visually by coronary angiography
(defined as radiometric turbidity without cardiac movement before contrast medium
injection) or (intravascular ultrasound) IVUS showing superficial calcification
involving more than 3 quadrants. Planned RA was defined as RA performed directly
before balloon predilation, while bail RA was performed after balloon predilation
failure or stent delivery to the target lesion. Procedural success was defined as
final stenosis of less than 30%, with a Thrombolysis in Myocardial Infarction (TIMI) flow grade of 3. The procedure was
considered a failure if patients received emergent coronary artery bypass
grafting (CABG) and/or PCI, or other severe RA-related complications (coronary
perforation, death) developed before discharge. Hypotension was defined by
transient drop in blood pressure to 90/60 mmHg or a 20% drop from the original
blood pressure level. Vasospasm was characterized by transient total or sub-total
occlusion of epicardial arteries or severe diffuse vasoconstriction. Dissection
referred to separation of true and false lumens that appear on angiography. Slow
flow/no re-flow was defined as less than TIMI III flow grade in the absence of dissection or thrombus immediately after
RA [7]. Coronary perforation referred to marked extravasation of contrast media
or blood from the coronary artery visible on angiography during or following the
interventional procedure [8]. Bradyarrhythmia was defined as transient sinus
arrest, overt sinus bradycardia (heart rate
Means (standard deviation) and number (proportions) were calculated according to RA speed categories. To compare characteristics of the study subjects between the RA speed categories, we employed analysis of variance for continuous variables and chi-squared or Kruskal-Wallis rank-sum test for categorical variables. Multiple unconditional logistic regression analyses were performed to estimate the relationship between the RA speed and primary endpoints with adjustments for potential confounders. In addition, odds ratios (OR) and 95% confidence intervals (95% CI) as well as regression models for different clinical subgroups were conducted. All p values were 2-tailed, with a significance threshold of 0.05. Data management and analyses were performed using R software (4.0.2, Vienna, Austria).
A total of 372 CHD patients with severe coronary calcification were treated with
RA, and included 76 (20%) patients in group 1 (
| Variables | Total (n = 372) | Group 1 (n = 76) | Group 2 (n = 156) | Group 3 (n = 90) | Group 4 (n = 50) | p value |
|---|---|---|---|---|---|---|
| Clinical presentation | ||||||
| SCAD, n (%) | 89 (23.9) | 13 (17.1) | 37 (23.7) | 22 (24.4) | 17 (34) | 0.191 |
| UA, n (%) | 241 (64.8) | 61 (80.3) | 102 (65.4) | 50 (55.6) | 28 (56) | 0.005 |
| NSTEMI, n (%) | 41 (11.0) | 2 (2.6) | 16 (10.3) | 18 (20) | 5 (10) | 0.005 |
| STEMI, n (%) | 1 (0.3) | 0 (0) | 1 (0.6) | 0 (0) | 0 (0) | 1.000 |
| Patients characteristics | ||||||
| Male, n (%) | 217 (58.3) | 48 (63.2) | 97 (62.2) | 44 (48.9) | 28 (56) | 0.165 |
| Age, Median (IQR) | 72.0 (66.0, 78.0) | 73.0 (67.0, 78.0) | 72.0 (65.8, 78.0) | 71.5 (67.2, 78.0) | 70.0 (66.0, 76.0) | 0.559 |
| CVD history, n (%) | 334 (89.8) | 68 (89.5) | 145 (92.9) | 76 (84.4) | 45 (90) | 0.211 |
| Stroke history, n (%) | 98 (26.3) | 21 (27.6) | 46 (29.5) | 18 (20) | 13 (26) | 0.435 |
| DM, n (%) | 142 (38.2) | 31 (40.8) | 66 (42.3) | 30 (33.3) | 15 (30) | 0.301 |
| Smoking, n (%) | 115 (30.9) | 21 (27.6) | 54 (34.6) | 27 (30) | 13 (26) | 0.576 |
| Clinical characteristics | ||||||
| Creatinine (umol/L), Median (IQR) | 73.0 (61.0, 90.0) | 72.5 (61.8, 95.0) | 74.5 (61.8, 91.2) | 73.5 (63.0, 89.8) | 70.0 (60.0, 84.2) | 0.593 |
| GPT (IU/L), Median (IQR) | 23.0 (16.0, 36.2) | 23.0 (16.8, 35.0) | 23.0 (15.8, 40.2) | 22.0 (15.2, 32.0) | 25.0 (18.0, 40.0) | 0.359 |
| FPG (mmol/L), Median (IQR) | 6.2 (5.0, 8.3) | 6.6 (5.2, 8.5) | 6.2 (5.0, 8.6) | 6.1 (4.9, 7.8) | 5.6 (4.8, 7.2) | 0.193 |
| HGB (g/L), Median (IQR) | 124.0 (112.0, 133.2) | 124.5 (117.5, 133.0) | 124.5 (112.0, 135.2) | 122.5 (112.2, 131.0) | 126.0 (111.5, 136.0) | 0.453 |
| TC (mmol/L), Median (IQR) | 3.7 (3.1, 4.4) | 3.6 (3.1, 3.9) | 3.8 (3.1, 4.4) | 3.8 (3.3, 4.7) | 3.7 (3.2, 4.6) | 0.141 |
| TG (mmol/L), Median (IQR) | 1.2 (1.0, 1.7) | 1.2 (0.9, 1.6) | 1.3 (1.0, 1.7) | 1.2 (1.0, 1.6) | 1.1 (1.0, 1.6) | 0.536 |
| LDL-C (mmol/L), Median (IQR) | 1.8 (1.4, 2.4) | 1.7 (1.3, 2.1) | 1.8 (1.5, 2.4) | 1.9 (1.5, 2.7) | 1.8 (1.4, 2.3) | 0.060 |
| LVEF (%), Median (IQR) | 61.0 (50.0, 67.2) | 62.0 (51.8, 68.0) | 60.0 (48.0, 68.0) | 59.5 (48.2, 67.0) | 62.0 (56.2, 68.0) | 0.178 |
| LVEF |
39 (10.5) | 6 (7.9) | 20 (12.8) | 11 (12.2) | 2 (4) | 0.264 |
| P2Y12 antagonists | 0.541 | |||||
| Clopidogrel, n (%) | 129 (34.7) | 26 (34.2) | 56 (35.9) | 34 (37.8) | 13 (26) | |
| Ticagrelor, n (%) | 243 (65.3) | 50 (65.8) | 100 (64.1) | 56 (62.2) | 37 (74) | |
| Data are expressed as the mean | ||||||
| Variables | Total (n = 372) | Group 1 (n = 76) | Group 2 (n = 156) | Group 3 (n = 90) | Group 4 (n = 50) | p value | |
| Lesion characteristics | |||||||
| Target vessel | |||||||
| LAD, n (%) | 292 (78.5) | 62 (81.6) | 124 (79.5) | 69 (76.7) | 37 (74) | 0.730 | |
| LCX, n (%) | 22 (5.9) | 4 (5.3) | 7 (4.5) | 5 (5.6) | 6 (12) | 0.300 | |
| RCA, n (%) | 58 (15.6) | 10 (13.2) | 25 (16) | 16 (17.8) | 7 (14) | 0.852 | |
| Ostial stenosis, n (%) | 95 (25.5) | 17 (22.4) | 41 (26.3) | 19 (21.1) | 18 (36) | 0.235 | |
| Proximal lesion, n (%) | 346 (93.0) | 71 (93.4) | 144 (92.3) | 85 (94.4) | 46 (92) | 0.920 | |
| Midcourse lesion, n (%) | 321 (86.3) | 66 (86.8) | 138 (88.5) | 73 (81.1) | 44 (88) | 0.423 | |
| Distal lesion, n (%) | 143 (38.4) | 34 (44.7) | 53 (34) | 31 (34.4) | 25 (50) | 0.111 | |
| Bifurcation, n (%) | 26 (7.0) | 1 (1.3) | 11 (7.1) | 8 (8.9) | 6 (12) | 0.071 | |
| Distortion, n (%) | 24 (6.5) | 2 (2.6) | 10 (6.4) | 8 (8.9) | 4 (8) | 0.352 | |
| Total lesion length (mm), Median (IQR) | 71.5 (57.0, 91.2) | 65.5 (54.8, 88.5) | 80.0 (60.0, 98.0) | 70.0 (56.0, 91.0) | 67.0 (56.2, 90.0) | 0.071 | |
| Procedural Characteristics | |||||||
| IABP suport, n (%) | 46 (12.4) | 7 (9.2) | 21 (13.5) | 12 (13.3) | 6 (12) | 0.812 | |
| Number of burrs used | 0.355 | ||||||
| 1 | 356 (95.7) | 72 (94.7) | 147 (94.2) | 87 (96.7) | 50 (100) | ||
| 2 | 16 (4.3) | 4 (5.3) | 9 (5.8) | 3 (3.3) | 0 (0) | ||
| Initial burr size (mm), n (%) | 0.125 | ||||||
| 1.25 | 87 (23.4) | 17 (22.4) | 29 (18.6) | 24 (26.7) | 17 (34) | ||
| 1.5 | 285 (76.6) | 59 (77.6) | 127 (81.4) | 66 (73.3) | 33 (66) | ||
| Maximum burr size (mm), n (%) | 0.096 | ||||||
| 1.25 | 77 (20.7) | 14 (18.4) | 24 (15.4) | 22 (24.4) | 17 (34) | ||
| 1.5 | 275 (73.9) | 57 (75) | 121 (77.6) | 66 (73.3) | 31 (62) | ||
| 1.75 | 20 (5.4) | 5 (6.6) | 11 (7.1) | 2 (2.2) | 2 (4) | ||
| Initial burr-to-artery ratio | 0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.520 | |
| Maximum burr-to-artery ratio | 0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.173 | |
| Maximum burr-to-artery ratio | 0.590 | ||||||
| 360 (96.8) | 73 (96.1) | 152 (97.4) | 88 (97.8) | 47 (94) | |||
| 12 (3.2) | 3 (3.9) | 4 (2.6) | 2 (2.2) | 3 (6) | |||
| Initial rotational speed (× 1000 rpm), n (%) | |||||||
| 13 | 17 (4.6) | 17 (22.4) | 0 (0) | 0 (0) | 0 (0) | ||
| 14 | 59 (15.9) | 59 (77.6) | 0 (0) | 0 (0) | 0 (0) | ||
| 15 | 168 (45.2) | 0 (0) | 156 (100) | 0 (0) | 12 (24) | ||
| 16 | 117 (31.5) | 0 (0) | 0 (0) | 90 (100) | 27 (54) | ||
| 18 | 11 (3.0) | 0 (0) | 0 (0) | 0 (0) | 11 (22) | ||
| Higher speed than initial speed (× 1000 rpm), n (%) | |||||||
| 0 | 323 (86.8) | 76 (100) | 156 (100) | 90 (100) | 1 (2) | ||
| 1 | 7 (1.9) | 0 (0) | 0 (0) | 0 (0) | 7 (14) | ||
| 2 | 38 (10.2) | 0 (0) | 0 (0) | 0 (0) | 38 (76) | ||
| 3 | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | ||
| 4 | 3 (0.8) | 0 (0) | 0 (0) | 0 (0) | 3 (6) | ||
| Maximum rotational speed (× 1000 rpm) | 15.4 |
13.8 |
15.0 |
16.0 |
18.2 |
||
| TIMI flow grade following RA, n (%) | 0.006 | ||||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 1 | 3 (0.8) | 0 (0) | 0 (0) | 0 (0) | 3 (6) | ||
| 2 | 82 (22) | 17 (22.4) | 27 (17.3) | 22 (24.4) | 16 (32) | ||
| 3 | 287 (77.2) | 59 (77.6) | 129 (82.7) | 68 (75.6) | 31 (62) | ||
| Procedural success, n (%) | 370 (99.5) | 76 (100) | 156 (100) | 89 (98.9) | 49 (98) | 0.239 | |
| RA + drug-eluting stent, n (%) | 368 (98.9) | 76 (100) | 156 (100) | 89 (98.9) | 47 (94) | 0.008 | |
| RA + drug-coated ballon, n (%) | 2 (0.5) | 0 (0) | 0 (0) | 0 (0) | 2 (4) | 0.019 | |
| Emergent CABG, n (%) | 2 (0.5) | 0 (0) | 0 (0) | 1 (1) | 1 (2) | 0.237 | |
| Mean diameter of stents (mm), Median (IQR) | 2.9 (2.8, 3.1) | 3.0 (2.8, 3.2) | 2.9 (2.8, 3.1) | 2.9 (2.8, 3.1) | 3.0 (2.8, 3.1) | 0.622 | |
| Number of stents used, n (%) | 0.172 | ||||||
| 0 | 4 (1.1) | 0 (0) | 0 (0) | 1 (1.1) | 3 (6) | ||
| 1 | 25 (6.7) | 3 (3.9) | 9 (5.8) | 9 (10) | 4 (8) | ||
| 2 | 137 (36.8) | 36 (47.4) | 52 (33.3) | 32 (35.6) | 17 (34) | ||
| 3 | 139 (37.4) | 25 (32.9) | 60 (38.5) | 36 (40) | 18 (36) | ||
| 4 | 53 (14.2) | 10 (13.2) | 29 (18.6) | 9 (10) | 5 (10) | ||
| 5 | 11 (3.0) | 1 (1.3) | 5 (3.2) | 3 (3.3) | 2 (4) | ||
| 6 | 3 (0.8) | 1 (1.3) | 1 (0.6) | 0 (0) | 1 (2) | ||
| Mean diameter of stents (mm), Median (IQR) | 2.9 (2.8, 3.1) | 3.0 (2.8, 3.2) | 2.9 (2.8, 3.1) | 2.9 (2.8, 3.1) | 3.0 (2.8, 3.1) | 0.622 | |
| Total stent length (mm), Median (IQR) | 75.0 (59.0, 95.0) | 69.0 (56.5, 91.5) | 84.0 (62.0, 102.0) | 74.0 (58.5, 95.0) | 69.0 (58.2, 94.8) | 0.090 | |
| Data are expressed as the mean | |||||||
In addition, we analyzed the incidence of complications during PCI as shown in
Table 3. A total of 19 patients (38%) in group 4 experienced slow flow after RA,
compared to 22.4%, 17.3% and 24.4% in group 1, group 2 and group 3,
respectively (p = 0.025). A generalized additive model was used to
assess the nonlinear relationship between the maximum rotational speed and
incident of slow flow. A curvilinear relationship between the rotational speed
and the probability of slow flow was developed as shown in Fig. 1. After
adjusting for confounding factors such as age, sex or CVD history, the
probability of slow flow was intensified with the increase in rotational speed
(p for non-linearity = 0.131; adjusted model). A total of 48 patients
(12.9%) developed vasospasm, among which 15 cases were in group 1, which was
higher compared to the other three groups. However, there was no significant
difference in the development of vasospasm among the four groups (p =
0.052). Moreover, the incidence of hypotension, dissection, perforation and
bradyarrhythmia were comparable among the four groups (p
| Variables | Total | Group 1 | Group 2 | Group 3 | Group 4 | p |
|---|---|---|---|---|---|---|
| (n = 372) | (n = 76) | (n = 156) | (n = 90) | (n = 50) | ||
| Hypotension, n (%) | 6 (1.6) | 1 (1.3) | 5 (3.2) | 0 (0) | 0 (0) | 0.244 |
| Vasospasm, n (%) | 48 (12.9) | 15 (19.7) | 22 (14.1) | 5 (5.6) | 6 (12) | 0.052 |
| Dissection, n (%) | 35 (9.4) | 11 (14.5) | 12 (7.7) | 7 (7.8) | 5 (10) | 0.368 |
| Slow flow, n (%) | 85 (22.8) | 17 (22.4) | 27 (17.3) | 22 (24.4) | 19 (38) | 0.025 |
| Perforation, n (%) | 7 (1.9) | 2 (2.6) | 3 (1.9) | 1 (1.1) | 1 (2) | 0.905 |
| Bradyarrhythmias, n (%) | 11 (3.0) | 2 (2.6) | 4 (2.6) | 2 (2.2) | 3 (6) | 0.566 |
| Burr entrapment, n (%) | 1 (0.3) | 0 (0) | 1 (0.6) | 0 (0) | 0 (0) | 1.000 |
| RotaWire fracture, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Heart failure, n (%) | 139 (37.4) | 24 (31.6) | 59 (37.8) | 35 (38.9) | 21 (42) | 0.648 |
| Stent thrombosis, n (%) | 3 (0.8) | 0 (0) | 1 (0.6) | 2 (2.2) | 0 (0) | 0.563 |
| Cardiac death, n (%) | 8 (2.2) | 0 (0) | 4 (2.6) | 3 (3.3) | 1 (2) | 0.459 |
| Data are expressed as the number (percentage). | ||||||
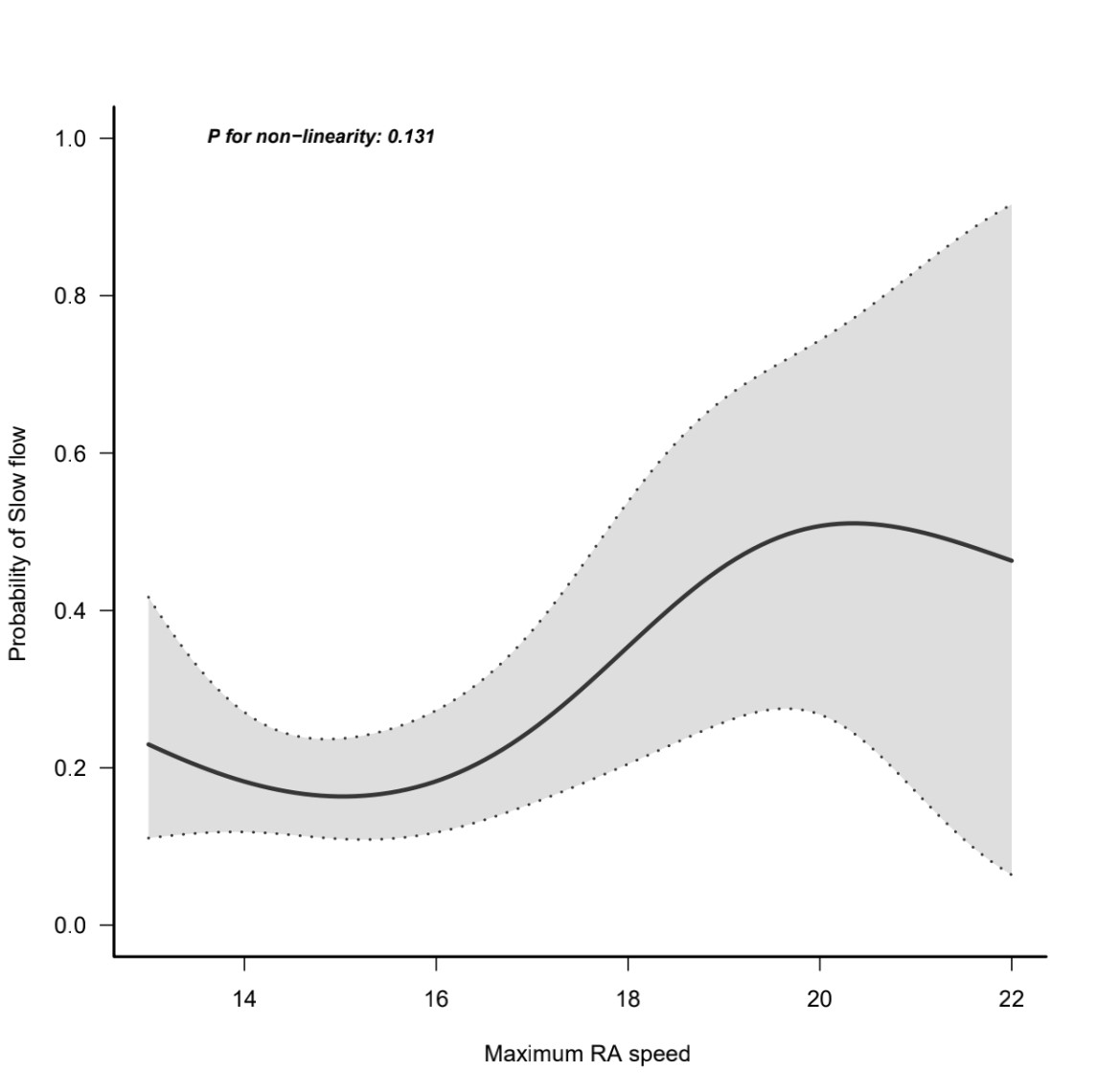 Fig. 1.
Fig. 1.The curvilinear relationship between the rotational speed and the probability of slow flow.
We analyzed the incidence of long-term endpoints in the four group as shown in Table 4. The data showed that there were 34 patients (9.1%) who experienced MACCE, among which 17 cases were in group 2, which was higher compared to the other three groups. However, no significant difference was observed among the four groups (p = 0.452). After adjustment for confounding factors, the probability of MACCE was intensified with the increase in rotational speed (p for non-linearity = 0.183; adjusted model) (Fig. 2). Analysis of mortality rate showed comparable six-months mortality among the four groups. However, there was no six-month follow-up cardiac deaths in group 1 (0%), and a lower rate of death from any reason (2.6%), which had no significant difference among the four groups (p = 0.288 and p = 0.832, respectively). Kaplan–Meier curves were plotted to assess survival data for patients with different rotational speed of the RA (Fig. 3). The log-rank test demonstrated that there was no significant differences in the survival rate between the four groups, with only borderline trend in favor of patients using lower rotational speed (p = 0.25). In addition, a total of 44 patients (11.8%) developed heart failure, with comparable incidence among the four groups (group 1 vs. group 2 vs. group 3 vs. group 4: 15.8% vs. 10.9% vs. 10% vs. 12%, p = 0.668).
| Variables | Total | Group 1 | Group 2 | Group 3 | Group 4 | p |
|---|---|---|---|---|---|---|
| (n = 372) | (n = 76) | (n = 156) | (n = 90) | (n = 50) | ||
| Primary endpoints | ||||||
| MACCE, n (%) | 34 (9.1) | 4 (5.3) | 17 (10.9) | 7 (7.8) | 6 (12) | 0.452 |
| MI, n (%) | 3 (0.8) | 0 (0) | 1 (0.6) | 0 (0) | 2 (4) | 0.100 |
| Stent thrombosis, n (%) | 8 (2.2) | 0 (0) | 7 (4.5) | 1 (1.1) | 0 (0) | 0.097 |
| TVR, n (%) | 8 (2.2) | 0 (0) | 5 (3.2) | 1 (1.1) | 2 (4) | 0.280 |
| Cardiac death, n (%) | 10 (2.7) | 0 (0) | 5 (3.2) | 4 (4.4) | 1 (2) | 0.288 |
| Death from any reason, n (%) | 17 (4.6) | 2 (2.6) | 8 (5.1) | 5 (5.6) | 2 (4) | 0.832 |
| Stroke, n (%) | 11 (3.0) | 5 (6.6) | 4 (2.6) | 2 (2.2) | 0 (0) | 0.204 |
| Secondary endpoints | ||||||
| Heart failure, n (%) | 44 (11.8) | 12 (15.8) | 17 (10.9) | 9 (10) | 6 (12) | 0.668 |
| Data are expressed as the number (percentage). MI, myocardial infarction; TVR, target vessel revascularization. | ||||||
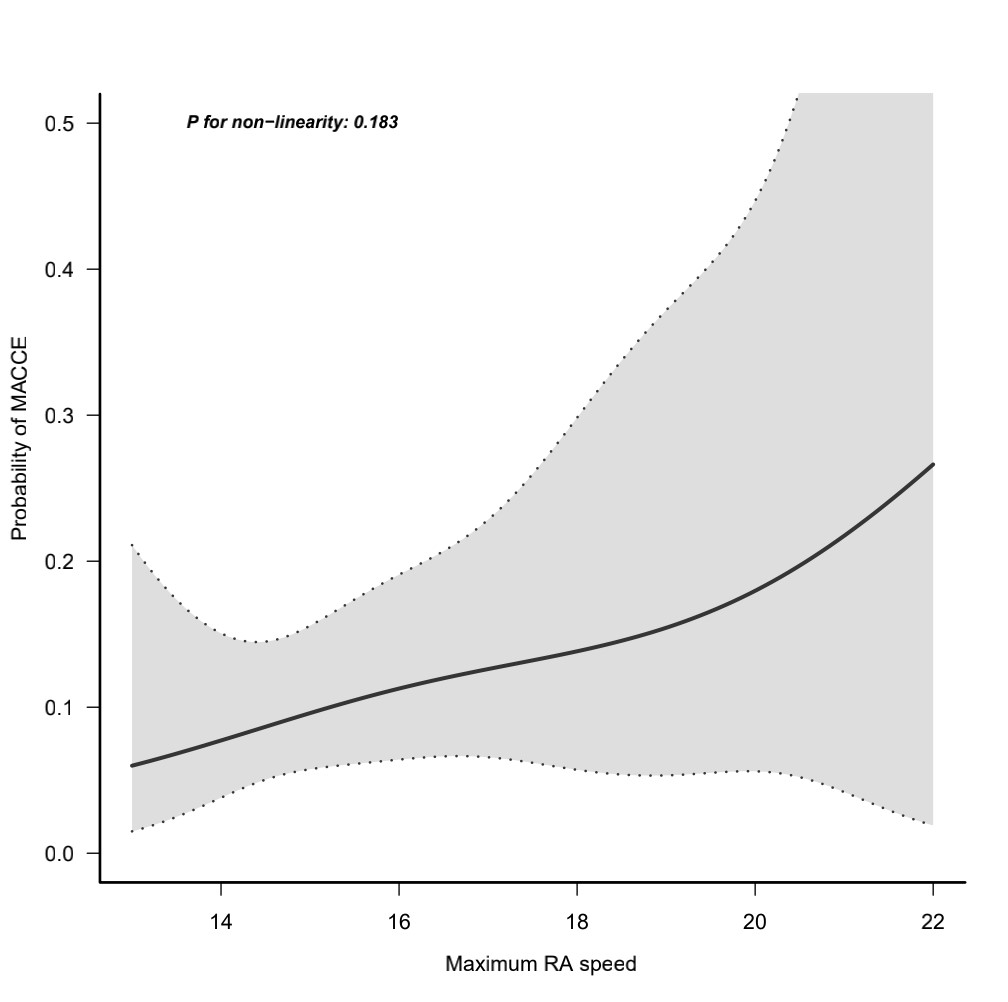 Fig. 2.
Fig. 2.The curvilinear relationship between the rotational speed and the probability of MACCE. MACCE, major cardiovascular and cerebrovascular events.
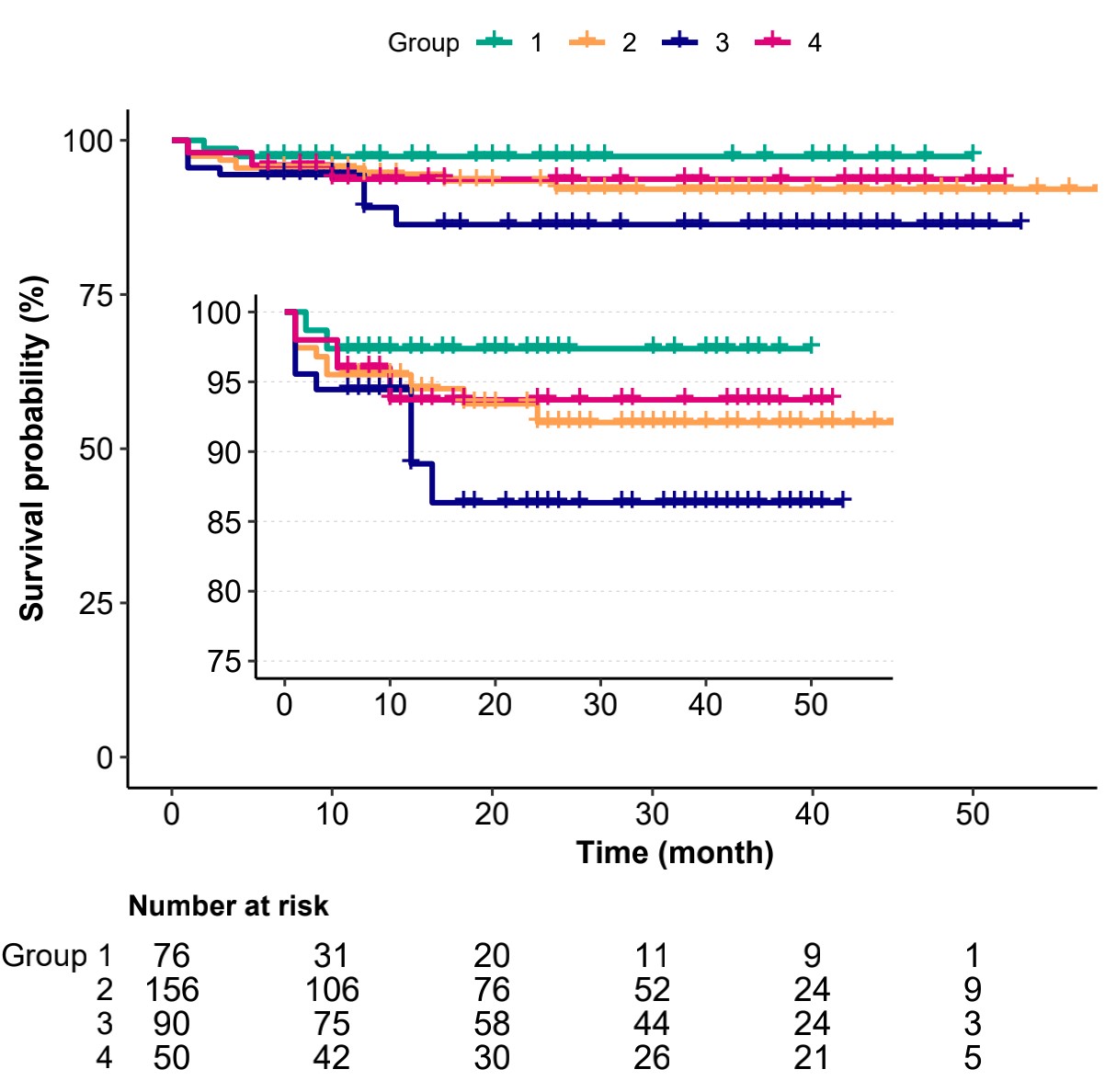 Fig. 3.
Fig. 3.Kaplan–Meier death from any reason-free survival curves. Log
rank p = 0.25. group 1 (
For the multivariable logistic regression analysis, we selected variables that
commonly affect clinical cardiovascular outcomes such as age, sex or CVD history,
as regression models (Table 5). After adjusting for age, sex, CVD history, FPG,
glutamic-pyruvic transaminase (GPT), triglyceride (TG) and low density
lipoprotein C (LDL-C), LVEF, maximum burr to artery ratio, as well as type of
CHD, including SCAD, UA, ST-segment elevation myocardial infarction (STEMI), and
NSTEMI in the analysis of vasospasm events, individuals with lower rotational
speed (
| Variable | No. of total | No. of event (%) | Crude OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| Vasospasm | rotational speed | 372 | 48 (12.9) | 0.79 (0.62 |
0.077 | 0.85 (0.66 |
0.214 |
| 76 | 15 (19.7) | 4.18 (1.44 |
0.008 | 3.3 (1.08 |
0.036 | ||
| 150,000 rpm | 156 | 22 (14.1) | 2.79 (1.02 |
0.046 | 2.2 (0.77 |
0.138 | |
| 160,000 rpm | 90 | 5 (5.6) | 1 (Ref) | 1 (Ref) | |||
| 50 | 6 (12) | 2.32 (0.67 |
0.184 | 2.11 (0.58 |
0.255 | ||
| Slow flow | rotational speed | 372 | 85 (22.8) | 1.27 (1.07 |
0.005 | 1.25 (1.05 |
0.010 |
| 76 | 17 (22.4) | 1.38 (0.7 |
0.357 | 1.61 (0.79 |
0.187 | ||
| 150,000 rpm | 156 | 27 (17.3) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||
| 160,000 rpm | 90 | 22 (24.4) | 1.55 (0.82 |
0.179 | 1.56 (0.81 |
0.184 | |
| 50 | 19 (38) | 2.93 (1.45 |
0.003 | 3.18 (1.52 |
0.002 | ||
| MACCE | rotational speed | 372 | 34 (9.1) | 1.19 (0.95 |
0.131 | 1.14 (0.9 |
0.291 |
| 76 | 4 (5.3) | 0.66 (0.19 |
0.519 | 0.9 (0.24 |
0.877 | ||
| 150,000 rpm | 156 | 17 (10.9) | 1.45 (0.58 |
0.429 | 1.89 (0.71 |
0.201 | |
| 160,000 rpm | 90 | 7 (7.8) | 1 (Ref) | 1 (Ref) | |||
| 50 | 6 (12) | 1.62 (0.51 |
0.413 | 1.9 (0.57 |
0.296 | ||
| MI | rotational speed | 372 | 3 (0.8) | 1.84 (1.13 |
0.014 | 1.88 (0.82 |
0.139 |
| 76 | 0 (0) | 1 (0 |
0.999 | Inf (Inf |
|||
| 150,000 rpm | 156 | 1 (0.6) | 14986245.91 (0 |
0.996 | Inf (Inf |
||
| 160,000 rpm | 90 | 0 (0) | 1 (Ref) | 1 (Ref) | |||
| 50 | 2 (4) | 96786171.49 (0 |
0.995 | Inf (Inf |
|||
| Stent thrombosis | rotational speed | 372 | 8 (2.2) | 0.84 (0.47 |
0.549 | 0.8 (0.41 |
0.526 |
| 76 | 0 (0) | 0 (0 |
0.994 | 0 (0 |
|||
| 150,000 rpm | 156 | 7 (4.5) | 4.18 (0.51 |
0.184 | 4.89 (0.48 |
0.182 | |
| 160,000 rpm | 90 | 1 (1.1) | 1 (Ref) | 1 (Ref) | |||
| 50 | 0 (0) | 0 (0 |
0.995 | 0 (0 |
0.912 | ||
| TVR | rotational speed | 372 | 8 (2.2) | 1.32 (0.9 |
0.152 | 1.5 (0.92 |
0.107 |
| 76 | 0 (0) | 0 (0 |
0.994 | 0 (0 |
0.953 | ||
| 150,000 rpm | 156 | 5 (3.2) | 2.95 (0.34 |
0.327 | 3.92 (0.28 |
0.312 | |
| 160,000 rpm | 90 | 1 (1.1) | 1 (Ref) | 1 (Ref) | |||
| 50 | 2 (4) | 3.71 (0.33 |
0.29 | 4.6 (0.25 |
0.305 | ||
| Cardiac death | rotational speed | 372 | 10 (2.7) | 1.09 (0.72 |
0.674 | 1.13 (0.68 |
0.628 |
| 76 | 0 (0) | 0 (0 |
0.989 | 0 (0 |
0.981 | ||
| 150,000 rpm | 156 | 5 (3.2) | 0.71 (0.19 |
0.62 | 0.68 (0.13 |
0.635 | |
| 160,000 rpm | 90 | 4 (4.4) | 1 (Ref) | 1 (Ref) | |||
| 50 | 1 (2) | 0.44 (0.05 |
0.467 | 0.48 (0.04 |
0.577 | ||
| Death from any reason | rotational speed | 372 | 17 (4.6) | 1.09 (0.79 |
0.605 | 1.13 (0.79 |
0.497 |
| 76 | 2 (2.6) | 0.46 (0.09 |
0.361 | 0.48 (0.08 |
0.43 | ||
| 150,000 rpm | 156 | 8 (5.1) | 0.92 (0.29 |
0.885 | 0.96 (0.27 |
0.951 | |
| 160,000 rpm | 90 | 5 (5.6) | 1 (Ref) | 1 (Ref) | |||
| 50 | 2 (4) | 0.71 (0.13 |
0.687 | 0.95 (0.16 |
0.958 | ||
| Stroke | rotational speed | 372 | 11 (3) | 0.55 (0.3 |
0.049 | 0.5 (0.26 |
0.036 |
| 76 | 5 (6.6) | 3.1 (0.58 |
0.184 | 3.53 (0.92 |
0.065 | ||
| 150,000 rpm | 156 | 4 (2.6) | 1.16 (0.21 |
0.867 | 1.49 (0.43 |
0.529 | |
| 160,000 rpm | 90 | 2 (2.2) | 1 (Ref) | 1 (Ref) | |||
| 50 | 0 (0) | 0 (0 |
0.992 | 0 (0 |
0.568 | ||
| Heart failure | rotational speed | 372 | 44 (11.8) | 0.91 (0.72 |
0.467 | 0.9 (0.71 |
0.369 |
| 76 | 12 (15.8) | 1.69 (0.67 |
0.267 | 1.73 (0.64 |
0.277 | ||
| 150,000 rpm | 156 | 17 (10.9) | 1.1 (0.47 |
0.826 | 1.04 (0.43 |
0.931 | |
| 160,000 rpm | 90 | 9 (10) | 1 (Ref) | 1 (Ref) | |||
| 50 | 6 (12) | 1.23 (0.41 |
0.714 | 1.22 (0.39 |
0.731 | ||
| Multifactor models adjusted for SCAD, UA, NSTEMI, STEMI, sex, age, CVD history, FPG, GPT, TG, LDL-C, LVEF and maximum burr to artery ratio. SCAD, stable coronary heart disease; UA, unstable angina; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non ST-segment elevation myocardial infarction; CVD Cardio Vascular Diseases; FPG, fasting plasma glucose; TG, triglyceride; LDL-C, low density lipoprotein C; GPT, glutamic-pyruvic transaminase; MI, myocardial infarction; TVR, target vessel revascularization. | |||||||
In our study, we investigated interventional outcomes of RA at different
rotational speeds and compared clinical outcomes associated with RA in CHD
patients. Our findings demonstrated that CHD patients treated with high
rotational speeds (
RA speed remains an important issue because of its standardization challenges among different RA surgeons. Expert consensus, including in China, Japan and Europe, has differential recommendations for RA speed [9, 10, 11, 12, 13]. For instance, the European RA expert consensus recommends a safe range of rotablation speed between 135,000 and 180,000 rpm [9]. On the other hand, the consensus of Chinese RA experts recommended a starting speed of 135,000~180,000 rpm. However, for lesions that cannot completely pass through the stenosis after repeated operations, the speed can be increased to a maximum of 220,000 rpm [13]. However, the expert consensus on RA does not refer to whether speed of rotablation is associated with the long-term prognosis. Moreover, data on long-term effect of RA remain scant. Here, we performed a targeted study to evaluate the outcomes of RA speed in CHD patients.
The rotational speed used during RA was between 130,000–220,000 rpm for all the
patients in this study, which is in line with recommendation of most of the
current guidelines. Our analysis showed that the overall incidence of slow flow
was 21%, which was relatively higher than that observed in the randomized
ROTAXUS and PREPARE-CALC trials [14, 15]. This may be due to the low proportion of
ACS in ROTAXUS and PREPARE-CALC trials, and their target lesion length was
significantly shorter (27.7
Like the risk of slow flow during PCI, highest six-month MACCE occurrence in CHD
patients with rotational speed (
The incidence of vasospasm in the group with a rotational speed of
Although our study highlights important findings, it still has some limitations that need to be considered. Firstly, it is a single-center, retrospective analysis, which cannot represent a randomized controlled trial. Secondly, the sample size was relatively small, with a short follow-up time which is underpowered for all clinical outcomes especially for the long term outcomes. In addition, there was also lack of total duration time of RA, lack of routine use of intravascular ultrasound or optical coherence tomography to optimize interventional procedures. Prospective randomized controlled trials with larger sample sizes are needed to further confirm the findings.
In conclusion, CHD patients treated with RA at a rotational speed of
Not applicable.
All data used or analysed during the current study are available from the corresponding author on reasonable request.
LKM designed the study and edited the manuscript. JWW helped with the study design, planned, organized and conducted participant recruitment and performed medical screening. GQQ and HH planned and performed data analyses. JWW drafted the manuscript. All authors provided useful input to data interpretation and contributed to drafting and finalizing the manuscript. All authors read and approved the final manuscript.
This study was approved by the Medical Research Ethics Committee of the First Affiliated Hospital of USTC (Anhui Provincial Hospital) (No. 2022-RE-126) and all participants signed the informed consent prior to study enrolment.
The authors would like to express their gratitude to all students involved in the study for invaluable assistance during intervention follow-up and data sampling. Moreover, we would like to thank all study participants for their effortful and dedicated contributions.
This study was supported by the National Key Research and Development Program of China (2021YFA0804904).
The authors declare no conflict of interest.
