Academic Editor: Takatoshi Kasai
Background: Mitral valve (MV) morphology after MV repair affects
postoperative left ventricular (LV) blood flow pattern and long-term cardiac
function. Pilot data suggest that LV diastolic vortex flow pattern changes after
operation, but specific quantifiers remain unknown. We aimed to explore the role
of vector flow mapping (VFM) in LV diastolic vortex flow pattern in patients who
underwent MV repair. Methods: A total of 70 patients with degenerative
mitral regurgitation were consecutively enrolled and 30 age- and gender-matched
controls were recruited. 50 Patients who underwent MV repair were eventually
included in our study. LV average energy loss (EL-AVE) during diastole was
measured in the MV repair group by VFM one week before and one month after the
operation, and compared with that of controls using one-way analysis of variance.
The effect of surgical techniques and the extension of leaflet degeneration on
postoperative EL-AVE were analyzed using muti-way analysis of variance, and
patients were categorized into a resection subgroup (n = 29) and a non-resection
subgroup (n = 21). Results: The EL-AVE one month after operation in the
MV repair group was decreased (p
Mitral valve repair is an operative method for the treatment of degenerative mitral regurgitation. It has advantages over mitral valve replacement in terms of survival rate, valve complications, and valve durability [1, 2, 3, 4], and therefore is the first choice for the treatment of mitral regurgitation recommended by the Guide [5]. Currently, long-term function of the mitral valve and left ventricle (LV) after mitral valve repair is important in the management of patients. The description of cardiac flow patterns after surgical provides an intrinsic qualitative evaluation of therapeutic procedures, which is useful in assessing the potential risk of cardiac abnormalities in cardiac function analysis [6]. However, an effective index is still lacking in assessing cardiac fluid dynamics after mitral valve repair.
Echocardiography is typically used to clinically evaluate the surgery, but is difficult to observe the local and global movement of the myocardium in detail, as well as the changes in hemodynamics in the heart cavity. In most heart valve diseases, the hemodynamics in the heart cavity alter prior to the manifestations of clinical symptoms of cardiac dysfunction.
Vector flow mapping (VFM) is a safe, effective, and non-invasive new ultrasound technology to detect changes in hemodynamics in the heart cavity. It also provides visual observation and quantitative evaluation of the fluid dynamics of the cardiovascular system. At present, VFM technology has been applied to study and analyze energy loss. Studies have found that VFM does not only have value for the evaluation of heart function [7, 8, 9], but also has important clinical value for heart valve diseases, such as valve regurgitation or stenosis [10, 11]. VFM has been applied to evaluate surgical procedures and postoperative hemodynamics [12, 13]. The aim of this study was to apply the novel flow visualization echocardiographic technology VFM for the evaluation of the LV vortex flow patterns and average energy loss (EL-AVE) in patients who underwent mitral valve repair.
A retrospective review of VFM in the Mitral Valve Repair program database in our hospital identified patients with a diagnosis of degenerative mitral regurgitation between June 2019 and May 2021. A total of 70 consecutive patients with degenerative mitral regurgitation because of prolapse degeneration of the mitral valve involving single or two leaflet scallops were enrolled. Patients who were lost to follow up, with insufficient quality of images, or with mitral valve replacement were excluded. The final analysis included 50 patients who underwent mitral valve repair by a single surgeon in our hospital. Based on mitral leaflet resection, the patients were divided into two subgroups: 29 patients with mitral leaflet resection (resection subgroup) and 21 patients without mitral leaflet resection (non-resection subgroup) (Fig. 1). All subjects underwent echocardiography, one week before and one month after operation. There was no significant difference in postoperative drug treatment between patients.
 Fig. 1.
Fig. 1.Study flow chart.
To compare patients with degenerative mitral regurgitation with controls of similar age and gender, 30 healthy volunteers were selected as control group during the same period. All included volunteers were confirmed to be free of abnormalities by physical examination, electrocardiogram, X-ray, echocardiography and laboratory tests in a physical examination center. Data on height, blood pressure and weight were collected.
Inclusion criteria were as follows. The subjects were in sinus rhythm, had left
ventricular ejection fraction (LVEF)
Patients with atrial fibrillation, rheumatic mitral valve, myocardial infarction, cardiomyopathy, other severe valve diseases, hypertension, diabetes, chronic kidney disease, and previous heart surgery history were excluded.
Only patients who underwent mitral valve repair were included. The procedure frequently involves leaflet resection, use of annular rings and neochordae to reshape the annulus and support leaflet repair.
The Aloka F75 color Doppler ultrasound system and the UST-52105 heart probe with
a frequency of 1–5 MHz were used while the participant was in a left-side lying
position, and breathed calmly. An electrocardiogram was simultaneously recorded.
Height and weight were assessed to calculate the body surface area (BSA, unit
m
Dynamic color Doppler blood flow images of the LV chamber were collected from the apical four-chamber view in VFM mode. The probe emission frequency was adjusted to clearly display the endocardium. The maximum velocity range of the color Doppler (Nykist limit) was set at 60–80 cm/s, and the color baseline was kept at 0 cm/s. Under these conditions, the image frame rate was increased as much as possible.
Three cardiac cycles were continuously collected and VFM data was stored on the mobile hard disk for offline analysis. The VFM image data were entered in the DAS-RSI workstation, the analysis interface was entered. The time-flow curve was used to define the time period, and the electrocardiogram (ECG) R-R wave apex was selected as a complete cardiac cycle. Based on the ECG and valve opening and closing conditions, a complete cardiac cycle was divided in three periods: fast filling period (P1), slow filling period (P2), and atrial systolic period (P3) (Fig. 2). The LV diastolic EL-AVE was measured in energy loss mode, and the average EL-AVE value of these three time periods was calculated. The differences in EL-AVE between groups was compared (Fig. 3).
 Fig. 2.
Fig. 2.The LV cardiac cycle time-flow curve. Each point of the curve corresponds to the frame rate of the ECG. The three periods of the diastole: P1—fast filling period, P2—slow filling period, and P3—atrial systolic period.
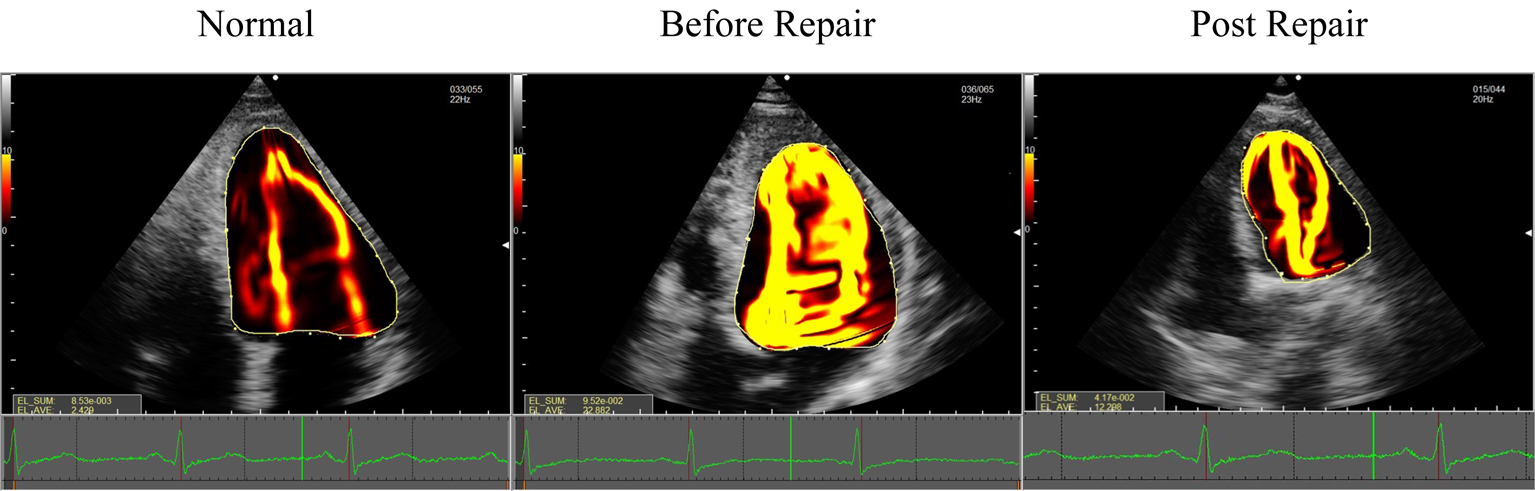 Fig. 3.
Fig. 3.EL-AVE in patients who underwent mitral valve repair and controls. EL-AVE of patients after mitral valve repair was higher than that of controls. EL-AVE, average energy loss.
VFM uses blood flow velocity to determine energy loss caused by viscous friction [15]. Intracardiac energy loss is calculated using the following equation:
In which
As seen in the equation, energy loss is the total of squared differences between neighboring velocity vectors. It changes when the size and direction of velocity vector change.
Data were compared using SPSS version 22.0 (SPSS Inc., IBM, Chicago, IL, USA).
Continuous variables were presented as mean
The effect of postoperative EL-AVE one month after operation was investigated using muti-way analysis of variance, with subgroups of patients with leaflet resection and those without resection, patients with degeneration of mitral valve involving single or two leaflet scallops, and with or without neochordae. The patients in the subgroups were divided based on these factors.
Ten random individuals were selected for evaluation of intraobserver and interobserver agreement on EL-AVE using Bland-Altman analysis.
We screened 70 patients with degenerative mitral regurgitation and enrolled 50 patients in the study. The most common leaflet abnormality in the patients was prolapse involving the posterior mitral leaflet. The repair procedures included 50 patients with an O-shaped semi-rigid complete ring, 27 patients with neochordae, 29 patients with leaflet resection, and 21 patients with no leaflet resection. There were no statistically significant differences between patients who underwent mitral valve repair and the control group, including age (p = 0.64), sex distribution (p = 0.78), systolic blood pressure (p = 0.41), diastolic blood pressure (p = 0.35) and BSA (p = 0.38) (Table 1).
| Variable | Control group | Mitral valve repair group | p value | |
| (n = 30) | (n = 50) | |||
| Age, years | 53 (45–61) | 56 (47–66) | 0.64 | |
| Male (%) | 18 (60) | 29 (58) | 0.78 | |
| Body Surface Area, m |
1.62 |
1.60 |
0.38 | |
| Blood pressure, mm Hg | ||||
| Systolic | 118.35 |
115.3 |
0.41 | |
| Diastolic | 70.46 |
68.8 |
0.35 | |
| Extension of leaflet degeneration | ||||
| Single (%) | — | 32 (64) | — | |
| Two (%) | — | 18 (36) | — | |
| Location of leaflet degeneration | ||||
| Anterior leaflet (%) | — | 20 (40) | — | |
| Posterior leaflet (%) | — | 30 (60) | — | |
| Use of annular rings | ||||
| Use | — | 50 (100) | — | |
| No use | — | 0 (0) | — | |
| Leaflet resection | ||||
| Resection | — | 29 (58) | — | |
| No resection | — | 21 (42) | — | |
| Use of neochordae | ||||
| Use | — | 27 (54) | — | |
| No use | — | 23 (46) | — | |
| Values are n, mean | ||||
Compared with the control group, the left atrial and LV chamber were enlarged in
patients one week before operation with statistically significant differences in
LAD (p
| Variable | Control group | Mitral Valve Repair group (n = 50) | p value | |
| (n = 30) | Before op 1 week | Post op 1 month | ||
| LAD (mm) | 33.45 |
42.28 |
34.08 |
|
| LVEDD (mm) | 47.14 |
55.44 |
47.74 |
0.01 |
| LVEDV (mL) | 103.46 |
152.72 |
108.26 |
|
| LVESD (mm) | 32.54 |
35.98 |
32.84 |
0.01 |
| LVESV (mL) | 36.39 |
56.16 |
43.14 |
0.01 |
| LVEF (%) | 62.14 |
63.00 |
60.42 |
0.08 |
| EL-AVE(J/s·m) | 6.29 |
31.64 |
11.33 |
|
| Mean transmitral gradient | 3 (2–4) | — | 4 (3–5)* | |
| Values are mean LAD, left atrial dimension; LVEDD, left ventricular end-diastolic dimension; LVEDV, left ventricular end-diastolic volume; LVESD, left ventricular end-systolic dimension; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; EL-AVE, average energy loss. Note: Compared with control group, * p | ||||
Compared with one week before operation, the left atrial and LV chamber were
reduced one month after operation, and the difference in LAD (p
Compared with the control group, the EL-AVE before and after operation in the
mitral valve repair group was significantly increased (p
 Fig. 4.
Fig. 4.A statistically significant difference was observed when comparing EL-AVE of the LV during diastole in controls and patients before and after mitral valve repair. Green: EL-AVE of LV in controls. Orange: EL-AVE of LV in patients who underwent mitral valve repair one week before operation. Blue: EL-AVE of LV in patients who underwent mitral valve repair one month after operation. EL-AVE, average energy loss.
Compared with one week before operation, the EL-AVE after operation in the
mitral valve repair group was significantly decreased (p
The effect of mitral valve resection on EL-AVE one month after operation was
significant (p
| EL-AVE (J/s·m) | ||
| Resection | ||
| with | 12.78 | |
| without | 9.33 | |
| p value | ||
| Extension | ||
| single | 10.97 | |
| two | 11.99 | |
| p value | 0.65 | |
| Neochordae | ||
| with | 11.57 | |
| without | 11.05 | |
| p value | 0.20 | |
| Values are mean EL-AVE, average energy loss. | ||
There were no statistically significant differences between the resection subgroup and the non-resection subgroup, including age (p = 0.67), sex distribution (p = 0.58), annuloplasty ring size (p = 0.39), systolic pressure (p = 0.37), diastolic pressure (p = 0.06) and BSA (p = 0.56).
Differences in the surgical procedure between the two subgroups were as follows.
The most common leaflet abnormalities in the resection subgroup were prolapse
involving single leaflet, posterior mitral leaflet and no use of neochordae. In
the non-resection subgroup, prolapse involving anterior mitral leaflet and use of
neochordae were the most common leaflet abnormalities. There was no significant
difference in the non-resection subgroup involving single or two leaflet
prolapse. Compared with the resection subgroup, the EL-AVE during diastole of the
non-resection subgroup was significantly decreased one month after operation
(p
| Resection subgroup | Non-Resection subgroup | p value | ||
| (n = 29) | (n = 21) | |||
| Age, years | 56.03 |
55 |
0.67 | |
| Male (%) | 16 (55) | 13 (62) | 0.58 | |
| Body Surface Area, m |
1.60 |
1.63 |
0.56 | |
| Annuloplasty ring size, mm | 30 |
30 |
0.39 | |
| Blood pressure, mm Hg | ||||
| Systolic | 117.2 |
115.6 |
0.37 | |
| Diastolic | 71.1 |
69.8 |
0.06 | |
| Extension of leaflet degeneration | ||||
| Single (%) | 22 (76) | 10 (47) | ||
| Two (%) | 7 (24) | 11 (53) | ||
| Location of leaflet degeneration | ||||
| Anterior leaflet (%) | 4 (14) | 16 (76) | ||
| Posterior leaflet (%) | 25 (86) | 5 (24) | ||
| Use of neochordae | ||||
| Use | 11 (38) | 18 (86) | ||
| No Use | 18 (62) | 3 (14) | ||
| EL-AVE, J/s·m | 12.78 |
9.33 |
||
| Values are n, mean EL-AVE, average energy loss. Note: Compared with resection group, * p | ||||
The Bland-Altman analysis for assessing the intraobserver (differences 4.62
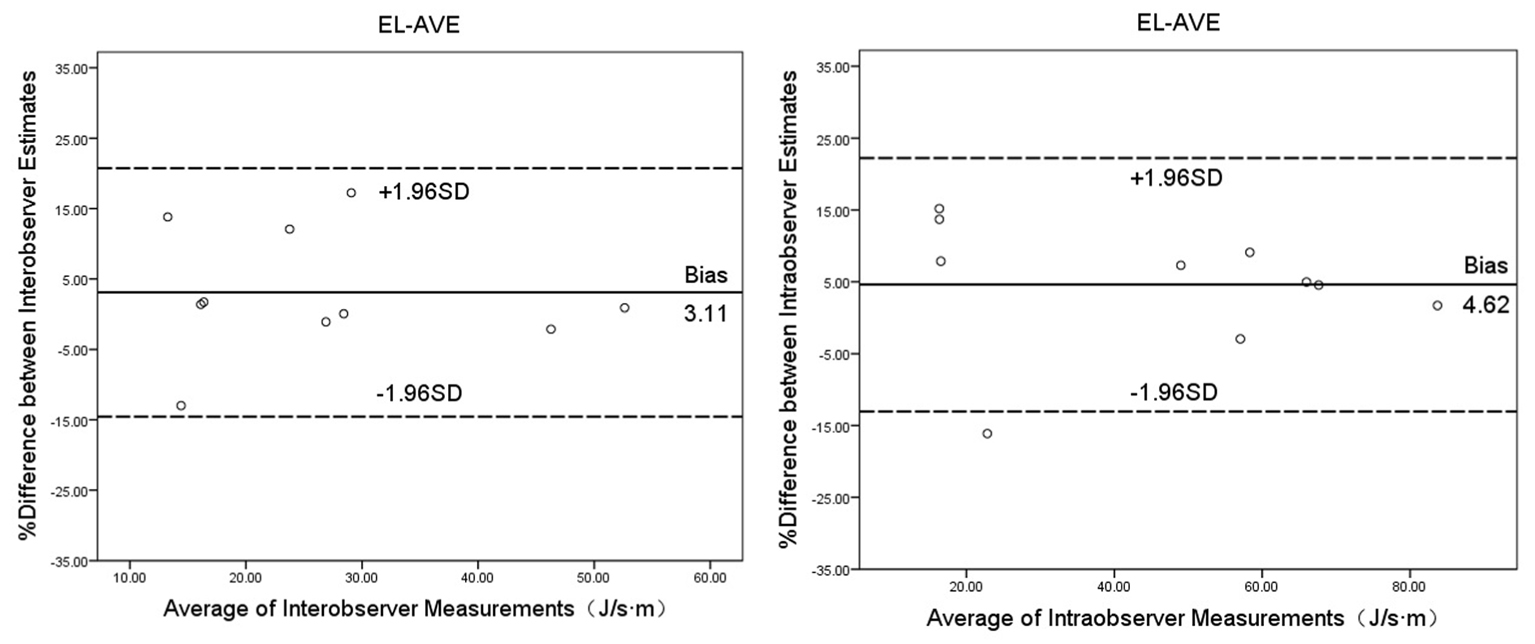 Fig. 5.
Fig. 5.Bland-Altman plots for interobserver and intraobserver agreement on EL-AVE during diastole. EL-AVE, energy loss.
Of the fifty patients who underwent mitral valve repair, one patient experienced
poor healing of the surgical incision in the resection subgroup, and one patient
had hoarseness in the non-resection subgroup, and the rest of the patients
recovered well after operation. Postoperative review by echocardiogram showed no
significant abnormalities in mitral valve function according to the ASE
guidelines [14] with definitions EROA
Mitral valve repair has become the preferred surgical procedure for the treatment of patients with severe degenerative mitral regurgitation [16, 17]. The procedure involves partially resection of the posterior mitral leaflet and implantation of a mitral annuloplasty to reshape the annulus and support leaflet repair [18]. The changes in the spatial conformation of the annulus and elevated mitral gradients lead to a change of LV flow pattern and affects the prognosis of patients [19]. Morichi et al. [20] reported that energy loss after mitral valve repair was greater than that of healthy volunteers during early diastole, as measured by VFM. This may be due to a different type of annuloplasty ring that was used during mitral valve repair. The relatively small ring resulted in an abnormal LV flow pattern and increase in energy loss.
Our study has two main findings. First, the EL-AVE in patients after mitral valve repair was higher than that of controls, but lower than that before mitral valve repair. Second, mitral valve repair resulted in a higher EL-AVE in patients with resected leaflets than in those with unresected leaflets while the same type of annuloplasty ring was used.
Vortices play an important role in normal cardiac function by keeping blood in motion inside the cardiac chambers and preserving momentum. They create an ideal state of kinetic energy reserve, and accumulation and transport of blood in the early stage of ventricular contraction [21]. The biphasic vortex rings are formed in the early and late LV filling, which is a consequence of the LV chiral asymmetry and the interaction between the blood-filled jet, the wall and the mitral valve [22, 23]. The longer anterior leaflet generates a stronger anterior vortex, while the shorter posterior leaflet generates a weaker posterior vortex. The anterior vortex dominates the posterior vortex, thereby facilitating the transfer of blood and improving the filling efficiency of the LV. The asymmetry of this leaflet creates vortices with preservation of kinetic energy and no energy loss [24].
The energy loss equation shows that it is related to the size and direction of adjacent velocity vectors. Diastolic energy loss refers to the energy lost by shear friction of blood of that flows in the LV after opening of the mitral valve with the ventricular wall [25]. In our study, EL-AVE during mitral regurgitation increased with the severity of mitral regurgitation. This may be due to the change of the size and direction of the intraventricular velocity vectors as a result of the turbulence caused by the mitral regurgitation. EL-AVE increased due to powerful collisions with the ventricular wall.
During diastole, the left intraventricular pressure is reduced by active relaxation of the myocardium and dilatation of the LV. This maximizes the pressure gradient between left atrium and LV, causing withdrawal of blood from the atria and acceleration of blood into the LV. Recent data suggest that functional mitral stenosis may occur following valve repair [26]. Increases in transmitral flow after mitral valve repair leads to turbulent flow above and below the mitral valve, resulting in an increase of energy loss. When the anterior and posterior leaflets of the mitral valve have the same size or the posterior leaflet is short, an increase in energy loss is observed as the blood flow collides on the ventricular wall and the stability of vortices is destroyed (Fig. 6). The aim during mitral valve repair is to preserve the vortex pattern, resulting in a lower energy loss. In our study, the resection subgroup consisted mainly of patients with prolapse of the posterior mitral leaflet. The rigidity of the posterior mitral leaflet after resection restricts the opening of the posterior mitral leaflet, and the transmissive inflow tends to collide on the ventricular wall, resulting in an elevated energy loss. In addition, the transition of the mitral annulus from a saddle D-shape during systole of the cardiac cycle to a flat D-shape during diastole has been confirmed [27]. Compared with the D-shaped mitral annulus morphology, the use of an O-shaped semi-rigid complete ring resulted in more energy loss because of the strength of the dominant vortical structure that was formed and the energy dissipation [28].
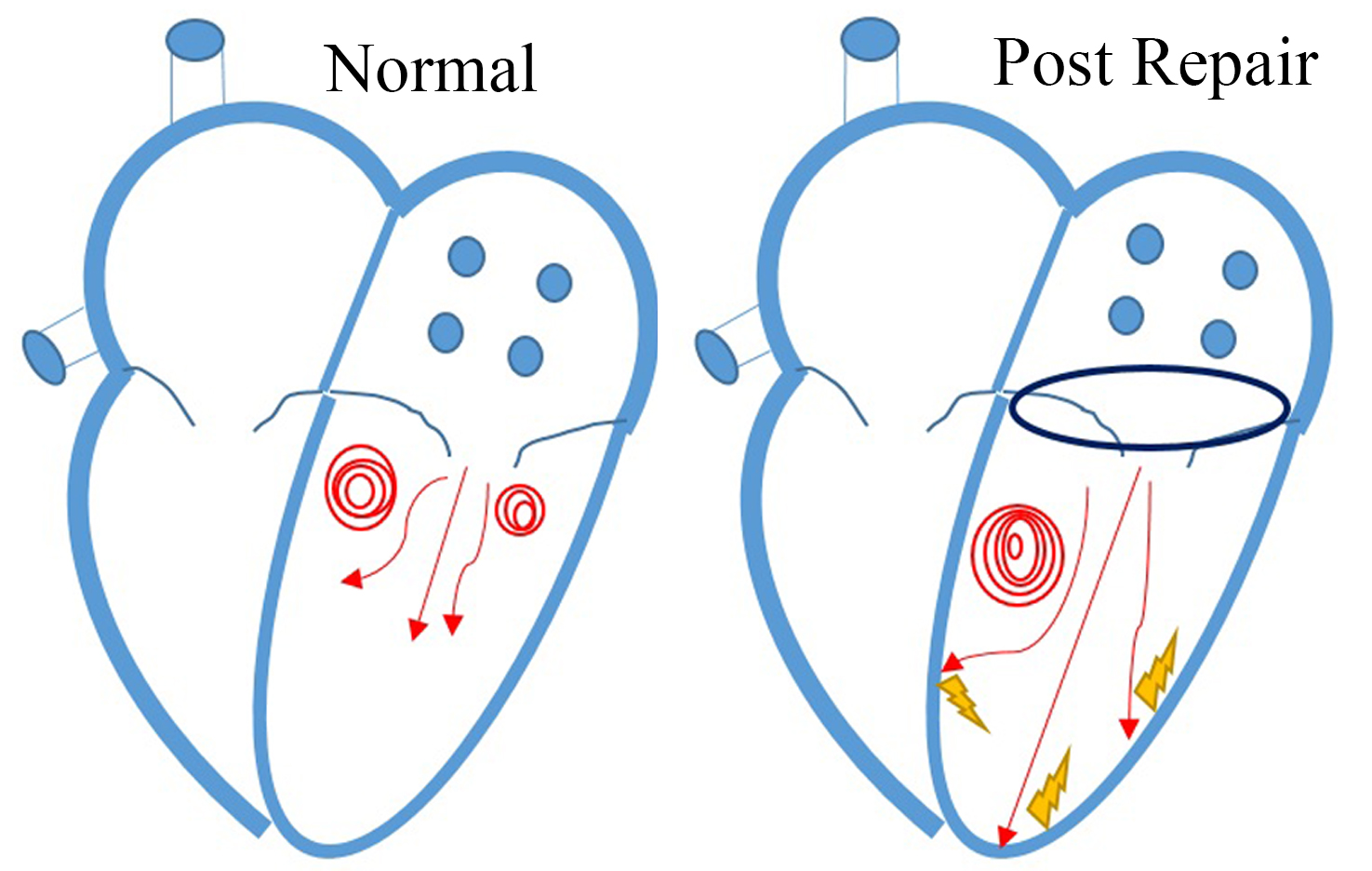 Fig. 6.
Fig. 6.Vortex change after mitral valve repair during diastole. Normal vortex patterns in a normal LV (left) and after mitral valve repair (right). Blood flow dissipated due to the collision of transmitral inflow on the ventricular wall after mitral valve repair.
The ultimate aim of heart valve surgery is to reduce the cardiac dysfunction by stopping regurgitation or reducing pressure gradients, which are the factors contributing to hemodynamic abnormalities. The superiority of mitral valve repair over replacement for short-term and long-term survival is due to the subvalvular apparatus that is preserved in mitral valve repair. This maintains left ventricular geometry and allows for a reduction in the left ventricular radius. Nevertheless, Chan et al. [19] found that elevated mitral gradient correlates with prognosis in patients after mitral valve repair for degenerative mitral valve regurgitation. The intraventricular vortex and intraventricular energy loss are key factors affecting the prognosis after mitral valve surgery [29]. In our study, different surgical techniques resulted in different postoperative EL-AVE. This was especially observed with relatively small effective orifice area that induced abnormal LV flow patterns and increased EL-AVE. A long-term follow-up study is needed to study the effect of EL-AVE increase on cardiac function after mitral valve repair.
First, mitral valve repair has been widely accepted, due to its superiority over valve replacement regarding long-term survival, fewer valve-related complications, and preservation of the LV function [30]. Second, VFM is safe, effective and non-invasively detects hemodynamic changes in the heart cavity. The reproducibility and generalizability of VFM technology for the evaluation of LV flow patterns in different types of mitral valve surgery have been confirmed in this study [31].
First, the apex of the heart cannot be completely enclosed in patients with significant LV enlargement due to two-dimensional angulation. When there is a defect in the ventricular wall, the EL-AVE may not be accurately measured. Second, when the area of the reflux beam is greater than 50% of the area of the left atrium, the blood flow in the LV cavity may result in aliasing twice, which affects the accuracy of the EL-AVE measurement; Third, the postoperative follow-up time of this study is short, and there is a lack of long-term postoperative sample data. Fourth, the number of samples in this study is small, and further research is necessary to collect more relevant data. Fifth, EL-AVE is only applicable to patients in sinus rhythm in this study. Whether it is applicable to all patients regardless of rhythm will require further studies to determine.
In summary, the LV flow patterns of patients with mitral valve repair can be quantitatively evaluated. Moreover, a greater energy loss was observed in patients after mitral valve repair than in healthy volunteers. The mitral leaflet resection and complete rings changed LV flow patterns, resulting in changed energy loss distribution. Different surgical techniques can affect the changes of energy loss after operation, especially in patients with a relatively small effective orifice area. A potential role for VFM in clinical decision-making merits further investigation.
YW conceived the present study, participated in the design, collected and assembled all data, conducted data analysis, and drafted the manuscript. YNL and CYC commented on the manuscript drafts. ZWG provided material and technical support. YYL, YBH and DQH commented on the manuscript drafts. CZW and LL aided the interpretation of data, commented on this study design, and provided acritical review. All authors have read and approved the manuscript.
This study was conducted following the Declaration of Helsinki (as revised in 2013) and was approved by Fuwai Central China Cardiovascular Hospital Ethics Committee (IRB#2019042). Individual consent for this retrospective analysis was waived.
The authors are grateful to the patients and families who participated in this study.
This work was supported by the National Natural Science Foundation of China (82071950, 81800287), National Natural Science Foundation of Henan Provincial for Excellent Young Scientists (202300410364), Medical Science and Technology Project of Henan Province (LHGJ20200084).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
