Academic Editor: Jerome L. Fleg
Background: Although ultrasound guidance for axillary vein (AV) access
(USGAVA) has been described as a reliable technique for cardiac implantable
electronic device (CIED) implantation, no data is available on the use of
handheld ultrasound devices (HUD) in such a setting. Objective: We
investigated the feasibility of using a HUD for USGAVA in patients referred to
our Institution for CIED implantation. Methods: The procedure details of
80 consecutive patients undergoing USGAVA (Group-1) from June 2020 to June 2021
were prospectively collected and compared to those of an age and sex-matched
cohort of 91 patients (Group-2) who had undergone AV access with the traditional
venipuncture guided by fluoroscopic landmarks. Results: The two groups
were comparable for the success rate of venous access (92.5% versus 93.4%,
p = 0.82), complication rate (1.3% versus 0.9%, p = 1.0), and
procedure time (71
Since the 1960s, the implantation of cardiac implantable electronic devices (CIEDs) through endovascular routes, including pacemakers, cardioverter-defibrillators, and devices for cardiac resynchronization therapy (CRT), has rapidly gained widespread acceptance by operators. In 2013, a European Heart Rhythm Association survey showed that cephalic vein dissection and subclavian vein puncture are the preferred venous access for CIED lead insertion [1]. While being very safe in preventing severe complications usually caused by traditional puncture of the subclavian or axillary vein, the cephalic vein has a lower success rate and longer procedural time [2]. Conversely, subclavian vein access is highly effective, quick to perform, and can accommodate multiple leads because of its large size [3]. Nevertheless, the approach to the subclavian vein, requiring venous access through an intrathoracic puncture, has feared complications that, although uncommon, may be potentially life-threatening, such as haemothorax or tensive pneumothorax and lead crush [4, 5].
Over the last years, axillary vein (AV) access has provided an alternative technique safer and more successful than the subclavian vein and cephalic vein, respectively [2, 6]. The standard approach to the AV is performed by an extrathoracic venipuncture under fluoroscopy guidance, with or without contrast venography [7]. However, ionizing radiation exposure to both operators and patients, as well as the risk of iodinated contrast medium allergy and nephropathy are non-negligible disadvantages of such a method. Nevertheless, the use of ultrasound as real-time guidance to puncture the AV may overcome these limits, as first described in the late ‘90s’ [8]. A growing amount of literature indicates ultrasound-guided AV access (USGAVA) as a safe, effective, and time-saving alternative to other traditional techniques for device implantation while avoiding x-ray exposure and contrast medium use [9, 10]. However, although the United States Agency for Healthcare Research and Quality has strongly recommended ultrasound guidance for central venous access [11], USGAVA has yet to achieve widespread acceptance from operators. Maneuvering bulky ultrasound machines near the operating field and the need for a second operator are perceived by operators as an impediment to the workflow. In the last few years, the progressive miniaturization of ultrasound machines with device sizes comparable to current smartphones may facilitate the spread of the technique.
This study aimed to assess the feasibility, efficacy, and safety of USGAVA performed as a single-operator maneuver using a pocket-sized handheld ultrasound device (HUD) in patients undergoing CIED implantation.
This study enrolled, from June 2020 to June 2021, all consecutive adult (
In our practice, USGAVA is a single operator maneuver with the non-dominant hand
holding the transducer and the dominant hand performing the venipuncture (Fig. 1). USGAVA is performed with Vscan Extend™ (GE Healthcare,
Waukesha, WI, USA), a handheld ultrasound system with a high-frequency 3.3–8 MHz
linear array transducer. Both the display unit and probe are covered in a single
sterile, transparent plastic sheath with sterile gel applied directly over the
probe inside the sheath. The skin is cleaned with 2% chlorhexidine solution for
antisepsis at the infraclavicular area, and a sterile disposable surgical
whole-body shaped drape with a preformed hole is applied to delimit the operation
area. Thanks to its light weight (321 gr) and small size (168
 Fig. 1.
Fig. 1.Operating field arrangement during a pacemaker implantation procedure. The entire ultrasound system is covered in a single sterile, transparent plastic sheath. The operator is carrying out the axillary vein puncture with ultrasound-guided freehand technique orienting the probe marker cranially. The needle is kept aligned to the probe’s centerline marker while imaging the longitudinal axis of the vessel.
While advancing an 18-gauge needle, the ultrasound transducer is tilted to image the AV in the longitudinal axis, and the needle is kept aligned to the probe’s centerline marker and in-plane of the ultrasound beam to visualize the needle tip until tenting the vessel wall. Then, when the needle tip-induced vessel indent is evident, the needle is advanced by performing short jabs until entering the lumen, as confirmed by aspiration of venous blood. A 0.035” j-tip guidewire is then inserted through the needle into the vein and advanced to the inferior vena cava under fluoroscopic guide. For CIEDs requiring more than a single lead, additional venous accesses are obtained with multiple punctures (one puncture per lead) by moving the puncture site by 0.5 cm proximally along the AV run. Alternatively, a single-puncture approach with a retained guidewire could be used for multiple lead implantation, based on the operator’s preference. A linear skin incision is made with a #11 surgical scalpel medially to the guidewires. Then, the device pocket is created by a manual detachment of subcutaneous tissue planes above the pectoralis major muscle fascia and the guidewires are reached using blunt dissecting scissors through the subcutaneous tissue and drawn under the skin into the device pocket. Finally, a peel-away dilator/introducer assembly is inserted over the guidewire into the central venous system and the leads are implanted following the standard fashion. If USGAVA fails, a skin incision is made, a device pocket prepared and an alternative venous approach without ultrasound guidance, based on the operator’s preference, was attempted using the standard technique as described below.
Group-1 was compared with a historical group represented by all patients who underwent conventional procedures—without ultrasound guidance—for transvenous CIED implantation at our Institution, from June 2019 to May 2020 (Group-2). In these patients, fluoroscopy-guided AV puncture, either with or without contrast-venography, was used following the consolidated standard techniques for CIEDs implantation. Patients who underwent cephalic access were excluded from the analysis. The AV puncture is routinely performed after making the skin incision and creating the device pocket; and contrast-venography is used only when the venipuncture attempt guided by the fluoroscopic landmarks fails.
As standard practice, in elective settings, oral anticoagulant therapy with vitamin K antagonists is not interrupted, targeting the international normalized ratio (INR) value between 2.0 and 3.0, preferably 2.0–2.5, on the procedure day. The CIED implantation is usually postponed if the INR value is over 3.0. Periprocedural bridging with either unfractionated heparin or low-molecular-weight heparin is not practiced at our Institution, but it might be considered for selected patients at very high risk of thromboembolism. Timing for both the holding and resumption of direct oral anticoagulants is based on the patient’s renal function according to the recommendations of the European Heart Rhythm Association, Heart Rhythm Society, and Asia Pacific Heart Rhythm Society [12]. Periprocedural either single or dual antiplatelet therapy is not interrupted.
Baseline demographics, biometric and clinical characteristics, procedural details, and complications occurring within 30 days after the procedure were collected prospectively in Group-1, either as continuous or categorical variables. The same parameters were retrospectively gathered from Group-2, reviewing patient electronic medical records and case-procedure logs via the official regional web-based registry RERAI (Registry of Emilia Romagna on Arrhythmia Interventions) for comparative data. In Group-1, ultrasound images of the AV were acquired by 4-second video clips and stored in the HUD. The captured images were then manually reviewed immediately after the end of the procedure to measure the vein depth and the maximum vein diameter (Fig. 2). To evaluate the reproducibility of the axillary vein measurements, a reliability analysis was performed as follows: the vein diameter was remeasured by the same operator in all 80 patients undergoing USGAVA for assessment of intraobserver variability; then, a second blinded operator measured the vein diameter in a group of 20 out of 80 patients to assess the interobserver variability. The overall procedural duration was measured from the performance of local anaesthesia to the completion of the skin suture. A venous access attempt was considered successful when all the leads to be implanted were imaged inside the inferior vena cava under fluoroscopy. In Group-1, if any other technique other than USGAVA was used, even for only one lead in the case of devices with multiple leads, the procedure was labelled as unsuccessful. In Group-2, when the operator had decided to change the initial chosen approach to gain central venous access with another strategy, including the additional use of contrast-venography, the procedure was considered unsuccessful. A chest X-ray was systematically performed the day after the procedure, with the patient standing whenever possible, to check the occurrence of either pneumothorax or lead dislodgement. The wound at the skin incision site was checked daily during the hospital stay and, subsequently, on removing the skin stitches, usually at day 12 from implantation.
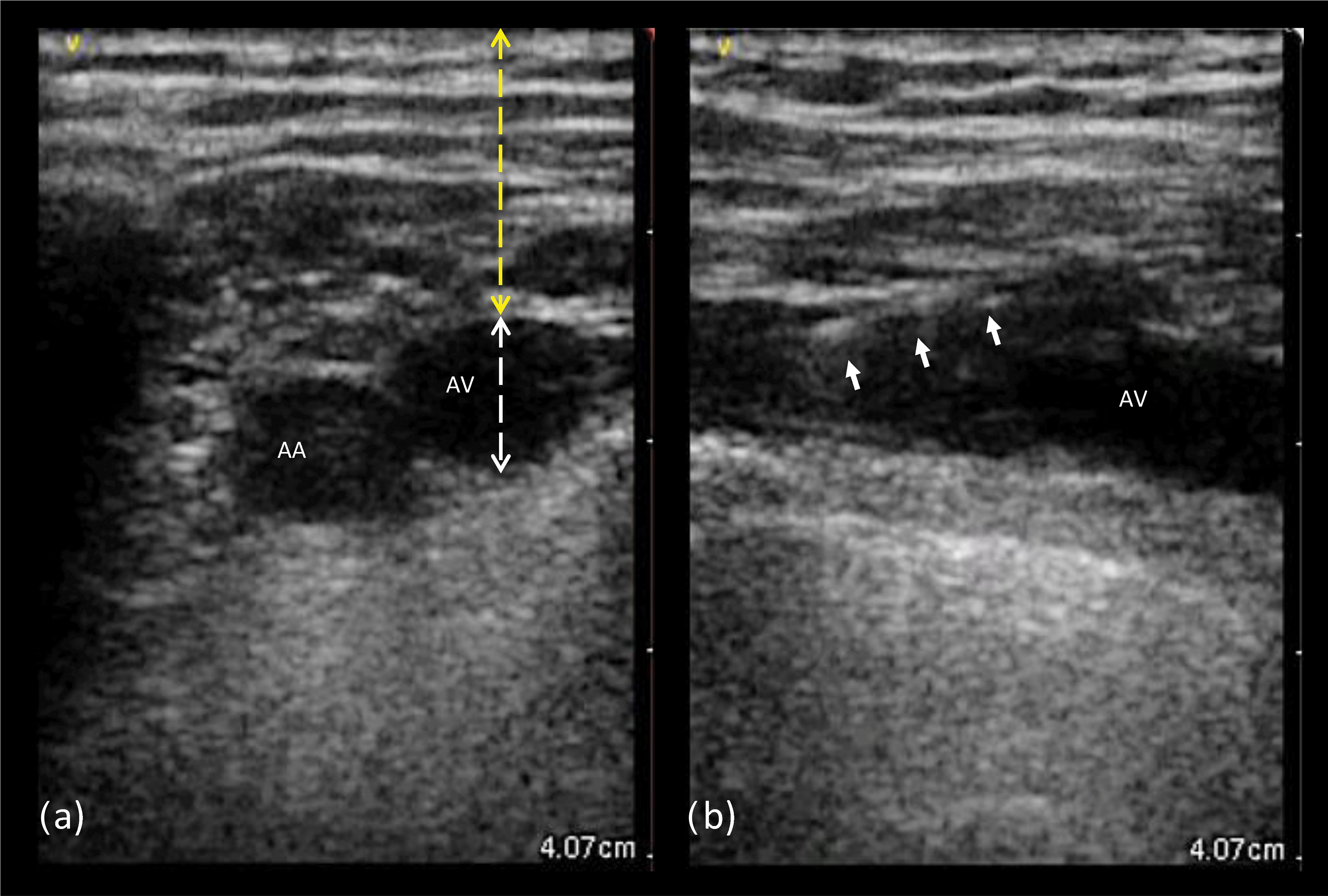 Fig. 2.
Fig. 2.Ultrasound images from left axillary vein. (a) Transverse view: the probe is aligned perpendicular to the axis of both the axillary artery and vein, which appear as anechoic circular images; the anatomic landmarks to measure the axillary vein depth (yellow) and diameter (white) are shown. (b) Longitudinal view: the probe is aligned with the axillary vein course, creating a tubular image of the vessel; the white arrows indicate the guidewire entering the target vein lumen. AA, axillary artery; AV, axillary vein.
The study endpoints were procedural-related outcomes including successful venous access, total procedure time, and total X-ray exposure time. Also, we collected the complications occurring within 30 days from CIED implantation including pneumothorax, pocket haematoma requiring any intervention (e.g., halting antithrombotic therapy, drainage positioning, etc.), infection, venous thrombosis of the accessed vein, or other procedural-related complications.
Statistical analyses were performed using IBM SPSS version 26.0 software (SPSS
Inc, Chicago, IL, USA). Continuous variables are expressed as means
The study involved 171 participants, including 80 patients (Group-1) who
underwent CIED implantation with USGAVA as the first-choice strategy for lead
insertion. Baseline characteristics of the overall study population are listed in
Table 1. The mean patient age was 78
| Variable | USGAVA | Standard access | p-value | |
| (Group-1, n = 80) | (Group-2, n = 91) | |||
| Age, years ( |
77 |
78 |
0.20 | |
| Male gender, n (%) | 57 (71) | 62 (68) | 0.66 | |
| Body mass index, Kg/m |
27.2 |
28.9 |
0.44 | |
| Body surface area, m |
1.87 |
1.84 |
0.25 | |
| Success rate of the initial strategy for venous access, n (%) | 74/80 (92.5) | 85/91 (93.4) | 0.82 | |
| Use of contrast venography, n (%) | 1 (1) | 6 (7) | 0.12 | |
| Device type, n (%) | ||||
| Single-chamber pacemaker | 14 (17) | 20 (22) | 0.57 | |
| Dual-chamber pacemaker | 35 (44) | 39 (43) | 1.00 | |
| Biventricular pacemaker | 15 (19) | 12 (13) | 0.40 | |
| Single-chamber cardioverter-defibrillator | 9 (11) | 15 (16) | 0.38 | |
| Dual-chamber cardioverter-defibrillator | 0 (0) | 0 (0) | - | |
| Biventricular cardioverter-defibrillator | 7 (9) | 5 (6) | 0.55 | |
| Average number of leads per patient, n ( |
1.9 |
1.7 |
0.08 | |
| Right sided implantation site, n (%) | 2 (2.5) | 0 (0) | 0.23 | |
| Left ventricular ejection fraction, % ( |
50.2 |
51.1 |
0.64 | |
| Diabetes mellitus, n (%) | 19 (24) | 26 (29) | 0.49 | |
| Coronary artery disease, n (%) | 24 (30) | 33 (36) | 0.42 | |
| Chronic obstructive pulmonary disease, n (%) | 11 (14) | 16 (18) | 0.53 | |
| Hypertension, n (%) | 59 (74) | 60 (66) | 0.32 | |
| History of cardiac surgery, n (%) | 10 (13) | 10 (11) | 0.81 | |
| Creatinine, mg/dL ( |
1.01 |
1.14 |
0.13 | |
| Antithrombotic therapy, n (%) | 62 (78) | 71 (78) | 1.00 | |
| Vitamin K antagonists | 14 (18) | 14 (15) | 0.84 | |
| Direct oral anticoagulants | 24 (30) | 17 (19) | 0.11 | |
| Single antiplatelet therapy | 18 (23) | 27 (30) | 0.30 | |
| Dual antiplatelet therapy | 2 (3) | 6 (7) | 0.29 | |
| Anticoagulant plus single antiplatelet therapy | 2 (3) | 6 (7) | 0.29 | |
| Anticoagulant plus dual antiplatelet therapy | 2 (3) | 1 (1) | 0.60 | |
| Total procedure duration time, min ( |
71 |
70 |
0.90 | |
| Total fluoroscopy exposure time, min ( |
5.7 |
7.6 |
0.03 | |
| Complications, n (%) | 1 (1.3) | 1 (0.95) | 1.00 | |
Both the success rate of the initial strategy for venous access and the 30-day complication rate showed no difference between the study groups. In Group-1, successful USGAVA was obtained in 74 of 80 patients (92.5%), resulting in a total of 142 out of 152 leads implanted (94%). A single-puncture approach with a retained guidewire for the insertion of multiple leads was used in 4 patients who received a dual-chamber pacemaker. In two other patients, the operator, after an unsuccessful first attempt with a standard 0.035” j-tip guidewire, used a thinner 0.028” guidewire for advancing through the AV. In the remaining patients, USGAVA followed the standard technique as described above. In 6 patients (7.5%) USGAVA was unsuccessful as the operator failed to enter the lumen of the vein with the needle tip. All these patients underwent successful CIED implantation via AV cannulation by conventional fluoroscopy-guided approach, with (1 patient) or without (5 patients) contrast-venography. Following our definition, complications occurred in only one patient who had an upper extremity deep venous thrombosis, ipsilateral to the implantation site, that was diagnosed 2 weeks after dual-chamber pacemaker implantation with successful USGAVA, while taking single antiplatelet therapy.
In Group-2, venous access was successful in 85 out of 91 patients (93.4%). In the remaining 6 patients, the operator, after failing the venous cannulation with the initial strategy, decided to use contrast venography for guiding the AV puncture. The complications included one haemothorax in a patient undergoing cardioverter-defibrillator implantation on dual antiplatelet therapy, which was successfully managed with a chest tube insertion.
While the overall procedure time did not differ between the two study groups,
the total fluoroscopy exposure time during the procedure was significantly higher
in Group-2 compared to Group-1 (7.6
To investigate predictors of failed ultrasound-guided AV cannulation for CIED
implantation in our study population, we compared the measured variables between
Group-1 patients with successful and unsuccessful USGAVA (Table 2). Patients with
a failed attempt of USGAVA exhibited a trend towards a higher body mass index
(30.1
| Variable | Successful USGAVA | Unsuccessful USGAVA | p-value | |
| (n = 74) | (n = 6) | |||
| Age, years ( |
77.3 |
70.8 |
0.23 | |
| Male gender, n (%) | 52 (70) | 5 (83) | 0.50 | |
| Body mass index, Kg/m |
26.9 |
30.1 |
0.07 | |
| Body surface area, m |
1.87 |
1.94 |
0.60 | |
| Device type, n (%) | ||||
| Single-chamber pacemaker | 13(18) | 1 (17) | 0.96 | |
| Dual-chamber pacemaker | 32 (43) | 3 (50) | 0.75 | |
| Biventricular pacemaker | 15 (20) | 0 (0) | 1.00 | |
| Single-chamber cardioverter-defibrillator | 7 (9) | 2 (33) | 0.08 | |
| Dual-chamber cardioverter-defibrillator | 0 (0) | 0 (0) | - | |
| Biventricular cardioverter-defibrillator | 7 (10) | 0 (0) | 0.43 | |
| Average number of leads per patient, n ( |
1.9 |
1.7 |
0.40 | |
| Right sided implantation site, n (%) | 2 (2.5) | 0 | 0.68 | |
| Left ventricular ejection fraction, % ( |
50.6 |
45.7 |
||
| Diabetes mellitus, n (%) | 17 (23) | 2 (33) | 0.57 | |
| Coronary artery disease, n (%) | 19 (26) | 2 (33) | 0.68 | |
| Chronic obstructive pulmonary disease, n (%) | 10 (14) | 1 (17) | 0.83 | |
| Hypertension, n (%) | 54 (76) | 5 (83) | 0.58 | |
| History of cardiac surgery, n (%) | 10 (14) | 0 (0) | 1.00 | |
| Creatinine, mg/dL ( |
1.02 |
0.81 |
0.17 | |
| Antithrombotic therapy, n (%) | ||||
| Vitamin K antagonists | 13 (18) | 1 (17) | 0.83 | |
| Direct oral anticoagulants | 23 (31) | 1 (17) | 0.66 | |
| Single antiplatelet therapy | 17 (23) | 1 (17) | 0.62 | |
| Dual antiplatelet therapy | 1 (4) | 1 (17) | 0.27 | |
| Anticoagulant plus single antiplatelet therapy | 2 (3) | 0 (0) | 1.00 | |
| Anticoagulant plus dual antiplatelet therapy | 2 (3) | 0 (0) | 1.00 | |
| Total procedure duration time, min ( |
71 |
69 |
0.66 | |
| Total fluoroscopy exposure time, min ( |
5.6 |
6.1 |
0.55 | |
| Complications, n (%) | 1 (1.3) | 0 (0) | 1.00 | |
| Axillary vein depth, mm ( |
22.2 |
20.6 |
0.55 | |
| Axillary vein diameter, mm ( |
9.2 |
1.8 |
||
To the best of our knowledge, this is the first report on the use of a pocket-sized handheld ultrasound system for real-time image-guided vascular access during transvenous CIED implantation. The results of our study enrich the growing amount of data indicating ultrasound-guided access of the AV for pacemaker or cardioverter-defibrillator leads implantation as a feasible and comparable alternative technique to the traditionally used AV puncture guided by fluoroscopy landmarks.
In our study, USGAVA, while requiring less x-ray exposure (5.7
Like most of the literature concerning the performance of USGAVA, our results
refer to a comparison with fluoroscopy-guided venipuncture techniques. We
excluded from the analysis patients undergoing cephalic vein access because it is
rarely used at our Institution. A recent multicenter randomized clinical trial
indicated USGAVA as superior in terms of success rate (97.7% versus 54.5%;
p
As shown in Table 3 (Ref. [9, 10, 14, 15, 16, 17, 18, 19]), our results paralleled the most recent literature on ultrasound-guided AV access for the same procedure type. Indeed, although the studies were somewhat different in ultrasound-guided venipuncture techniques and ultrasound system machines (i.e., on-cart, portable, or wireless), the success rate proved similar across the patient groups with USGAVA, including our procedures performed by a handheld device [9, 10, 14, 15, 16, 17, 18, 19]. Conversely, the complication rate was quite different between studies, probably affected either by the different follow-up duration or the broad definition of procedure-related complications, as some of these were unlikely related to the venous cannulation technique. However, in our study, the high percentage of patients on antithrombotic therapy (78%) highlights the safety of the technique, especially with regard to the risk for hematoma. Similarly, ElJamili and coll. showed no hematoma in 180 patients under antithrombotic therapy undergoing CIED implantation with USGAVA, including cardioverter-defibrillators and CRT [17].
| Our data | Esmaiel [14] | Franco [15] | Lin [16] | Liccardo [10] | Tagliari [9] | Eljamili [17] | De Sensi [18] | Chandler [19] | ||
| Study design | Single center, prospective, observational | Single center, retrospective | Single center, prospective, observational | Single center, retrospective | Single center, randomized | Multicenter, randomized | Multicenter, prospective, observational | Single center, retrospective | Single center, retrospective | |
| Number of patients | 80 | 403 | 50 | 137 | 116 | 44 | 200 | 119 | 187 | |
| Mean age, year |
77 |
N/A | 74 |
68 |
74 |
67.5 (55–76) |
78 |
79 |
69 | |
| Male gender, % | 71 | N/A | 56 | 63 | 57 | 59 | 58 | 63 | 67 | |
| Number of leads | 142 | 648 | 86 | 207 | 75 | 360 | 204 | 396 | ||
| Ultrasound system machine | handheld ultrasound device | on-cart ultrasound machine | wireless ultrasound transducer | portable laptop ultrasound systems | on-cart ultrasound machine | on-cart ultrasound machine | portable laptop ultrasound systems | on-cart ultrasound machine | wireless ultrasound transducer | |
| Ultrasound section to image axillary vein | longitudinal | transverse | transverse | transverse | transverse | longitudinal/transverse | transverse | longitudinal | longitudinal | |
| Ultrasound-guided venipuncture | before skin incision | after skin incision, inside the device pocket | before skin incision | before skin incision | before skin incision | before skin incision | before skin incision | before skin incision | before skin incision | |
| Success rate for USGAVA, % | 92.5 | 99.3 | 98 | 100 | 91 | 97.7 | 91 | 95 | 95 | |
| Device types, n (%) | ||||||||||
| Pacemaker | 49 (61) | 403 (100) | 36 (72) | N/A | 46 (40) | 29 (66) | 134 (67) | 93 (78) | 75 (40) | |
| Cardioverter-defibrillator | 9 (11) | 0 | 10 (22) | N/A | 70 (60) | 15 (34) | 12 (6) | 13 (11) | 69 (37) | |
| CRT | 22 (28) | 0 | 4 (8) | 27(20) | 0 | 0 | 34 (27) | 12 (11) | 43 (23) | |
| Upgrade | 0 | 0 | 0 | N/A | 0 | 0 | 14 (7) | 4 (3) | 8 (4) | |
| Total fluoroscopy time, min | 6.1 |
N/A | N/A | N/A | N/A | N/A | 8.5 |
N/A | 3.6 (2.0–5.5) | |
| Anticoagulation therapy at implant, % | 53 | N/A | 42 | N/A | 48 | N/A | 52 | 34 | 42 | |
| Single antiplatelet therapy at implant, % | 25 | N/A | 24 | N/A | N/A | N/A | 37 | 35 | 71 | |
| Combined antithrombotic therapy at implant, % | 10 | N/A | 0 | N/A | N/A | N/A | 9 | N/A | N/A | |
| Complications, n (%) | 1 (1.3) | 2 (0.5) | 1 (2) |
3 (2.2%) | 4 (3) |
1 (2.3) | 0 (0) | 4 (3.4) | 7 (4) | |
| Comparison refers to patient groups approached by ultrasound guided technique;
combined antithrombotic therapy at implant means dual antiplatelet therapy, dual
antithrombotic therapy, or triple antithrombotic therapy. | ||||||||||
All published studies on USGAVA in CIED implantation used, initially, stationary high-end ultrasound machines and, more recently, mobile on-cart or portable laptop ultrasound systems. The manual handling of such bulky ultrasound systems in the operating room, the transducer wires over the surgical field, and the need for an additional operator at the console for tuning the echo imaging while the primary operator is attempting the venipuncture might endanger the maintenance of the sterility and hinder the procedure workflow. These conditions likely contributed to hampering the spread of USGAVA in clinical practice. Recently, Franco and coll. showed that USGAVA, performed with a portable laptop ultrasound system with a wireless transducer, proved highly effective and safe in CIED implantation [15]. As underlined by the authors, not having to deal with transducer wires over the operating field and the possibility to tune the image from the probe by the same operator who is attempting the venipuncture (without the need for a second operator) both represented advantages compared to the traditional ultrasound systems.
In the last years, technological improvements have engendered a progressive miniaturization of ultrasound machines, with device sizes comparable to current smartphones. In 2019, a position statement of the European Association of Cardiovascular Imaging highlighted the potentials of using HUD in different clinical settings, including vascular invasive procedures such as central venous catheter insertion [20]. A randomized study of performance on a simulation model showed that the imaging qualities were similar between pocket-sized and standard ultrasound devices to guide internal jugular venipuncture [21]. Recently, in a prospective randomized clinical trial by Yamamoto and coll., the use of a HUD for internal jugular venipuncture proved not inferior to a standard ultrasound on-cart system, despite differences in visibility because of the lower device performance of the pocket-sized devices [22]. In our experience, despite the inherent technological limitations and restricted functions of a pocket-sized device, the use of a HUD to guide vascular access during CIED implantation proved comparable in efficacy and safety to standard ultrasound systems with higher technological capabilities used in previous studies (Table 3). Given the small size and handiness of the ultrasound system, performing the USGAVA procedure with HUD placed over the operating field did not impede the efficacy of the maneuver, resulting in feasibility in 74 out of 80 patients. Finally, technical limits, including image resolution and a small screen, go along with miniaturized portable devices. In our experience, such technical issues did not negatively impact the operators’ performance for AV puncture. However, most of the currently available HUBs, including the one used in our study, allow the display to be mirrored onto a larger wireless monitor nearby, such as the screen for fluoroscopy. This capability could be helpful to overcome some technical limits related to the device’s small size.
Seto and coll. estimated USGAVA-related additional professional reimbursement costs similar to venography, although with higher technical fees [23]. At our Institution, we roughly estimated an additional cost associated with USGAVA of €1.8/procedure comprising the sterile plastic sleeve (€0.8/unit) and disposal gel (€1/unit). The initial cost of the HUD with dual probe (phased-array and linear) should also be considered in the final estimate if not yet available in the operating room. Of note, the cost of the entire HUD is approximate to the sole cost of the vascular probe of high-end, portable on-cart or laptop ultrasound systems.
To investigate predictors of failed USGAVA in CIED implantation, we compared
patients with successful and unsuccessful USGAVA. A failed attempt of USGAVA was
more likely in patients with higher body mass index; however, unlike other USGAVA
reports, such correlation resulted in a trend without reaching a statistical
significance [18, 19, 23]. On the other hand, a positive relationship between
successful USGAVA and AV size was shown, with a 3-fold increase of probability of
success per each 1 mm increase in the AV diameter. Finally, based on the ROC curve
analysis, an AV diameter
Based on our experience, USGAVA for CIED implantation is similar in efficacy, safety, and total procedure time but significantly lower in ionizing radiation exposure compared to consolidated techniques using fluoroscopic anatomical landmarks. We reported 1.9 minutes less fluoroscopy with USGAVA than with traditional non-USGAVA access techniques. This is important in patients who may-require multiple procedures during their lifetime (e.g., device upgrade, lead revision) or for laboratory staff who perform many procedures per year. Thus, considering the guiding principle of radiation safety (ALARA principle), which states that ionizing radiations applied to humans and animals should be as low as reasonably achievable, we believe our results in minimizing radiation exposure to be worthy of emphasis. A recent study, which collected more comprehensive radiation exposure data (including Air-Kerma and Dose Area Product), showed similar results [25]. Therefore, in our current practice, USGAVA is the first-choice technique, reserving either axillary venipuncture guided by fluoroscopic landmarks or cephalic vein cutdown to the unsuccessful USGAVA cases. Though less frequently used at our Institution, cephalic vein cutdown may be preferred by the operator in selected cases (e.g., single lead pacing). Finally, contrast medium injection for venography is considered only when venipuncture guided by the fluoroscopy landmarks failed. At our Institution, USGAVA is not a routine technique in device upgrades because the operator might decide to check the patency of the venous route proximal to the AV with preprocedural venography.
Some limitations have to be addressed in the present study. First, this is a single-center, retrospective, and non-randomized study; therefore, further prospective, or randomized multicenter studies are needed to confirm our results. Secondly, as a historical group of patients was used for comparison, the skills of the operators and techniques/safeguards may have improved since this historical cohort. Thirdly, as lead revisions and device upgrades have been excluded from the analysis, our results cannot be extrapolated to such procedures. Fourthly, since no mid- and long-term complications have been collected, we cannot provide safety data for comparison over a 30-day follow-up. Fifthly, while having identified a cut-off value of the AV diameter to predict successful USGAVA, we are cautious in recommending this as the sole criterion when deciding whether to proceed with USGAVA since the venous diameter is a dynamic variable whose measurement may be affected by several factors (e.g., dehydration, fluid administration). Finally, as USGAVA was performed by long-standing experienced operators in ultrasound-guided venous access in electrophysiology, our results might not be reproducible with unskilled operators.
The use of a HUD to guide the insertion of pacemaker or cardioverter-defibrillator leads into the axillary vein was shown to be feasible, proving similar in efficacy and safety to the traditional AV puncture guided by fluoroscopic landmarks. Furthermore, the maneuver facilitates a potential reduction in ionizing radiation exposure for both the operator and patient without lengthening the CIED implantation procedure time. Our results, in line with those of other published experiences, may facilitate the spread of the technique.
All the authors participated substantially in the work and meet the following conditions. BS and GP—contributions to the conception and design; GS and SV—acquisition and analysis of data; DM—responsible for methodology and software; BS—drafting the original manuscript. All authors have read and approved the final version of the manuscript to be submitted.
This study was approved by the local Ethics Committee (identifier: 759/2021/Oss/AUSLFe) and conformed to the principles of the Declaration of Helsinki.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Daniele Muser was serving as Guest Editor of this journal. We declare that Daniele Muser had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Jerome L. Fleg.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
