Academic Editors: Vincenzo Russo, Saverio Muscoli and Giuseppe Mascia
Sudden cardiac death (SCD) is responsible for approximately 6% of global mortality and 25% of cardiovascular (CV) deaths. SCD has been traditionally linked to coronary artery disease, valvular heart disease, cardiomyopathies, and genetic arrhythmia disorders. However, advancements in care for these diseases have not translated to a proportional reduction in SCD. This suggests an important role of underrecognized contributing pathologies. Neglected tropical diseases (NTDs) are a group of illnesses prevalent in tropical and sub-tropical regions which have been understudied partially due to their high prevalence in marginalized populations. The relationship between SCD and Chagas disease has been well-established, though emerging literature suggests that other NTDs with CV involvement may lead to fatal arrhythmias. Additionally, specific therapies for a subset of NTDs put patients at increased risk of malignant arrhythmias and other cardiac complications. This review aims to summarize the association between a group of selected NTDs and SCD.
Neglected tropical diseases (NTDs) predominantly impact tropical and sub-tropical regions of several low and low-middle-income countries (LMICs) (Fig. 1) [1]. Considering that the burden of traditional risk factors cannot fully explain the high rates of cardiovascular (CV) diseases in these regions, there is increasing recognition of the contribution of NTDs [2, 3].
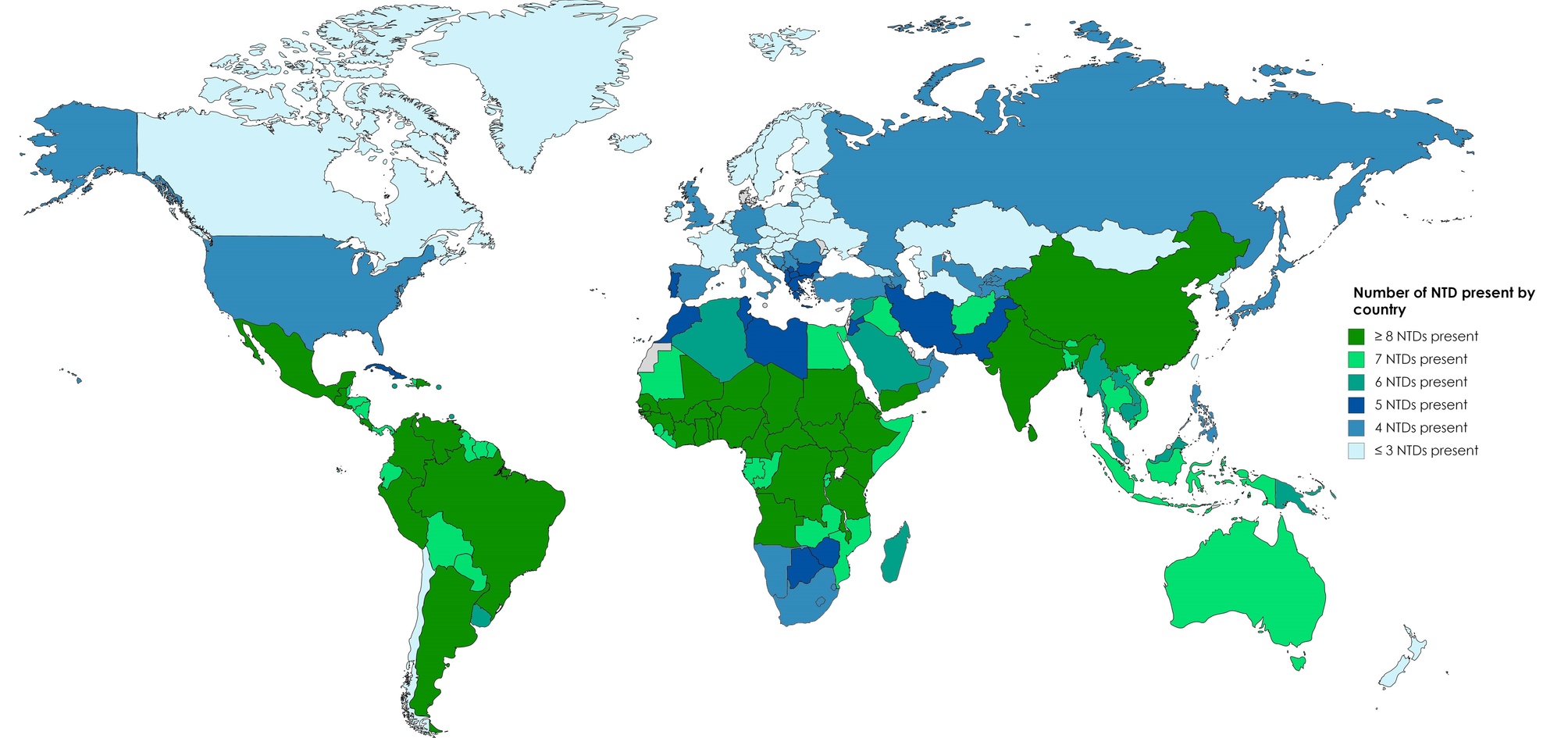 Fig. 1.
Fig. 1.Global burden of NTDs by country. NTDs, Neglected tropical diseases. Data from World Health Organization Control of Tropical Neglected Disease. Modified from Burgos LM, et al. Glob Heart. 2020; 15: 60 [1].
The incidence of sudden cardiac death (SCD) is higher in LMICs. Thus, socioeconomic status seems to be inversely correlated with SCD [4, 5, 6], and has become a critical public health problem in these populations. It has been established that patients coming from LMICs have a higher burden of CV risk factors, CV disease burden, and mortality than those in high-income countries [3, 7]. However, the burden of traditional risk factors and limited access to healthcare is insufficient to explain this socioeconomic gradient of SCD [3, 7]. Further, there is apaucity of high-quality health data collected in these regions, making it more difficult to delineate the contributors to SCD [8].
There is an urgent need to accurately understand the burden of SCD in already underserved regions. This requires the development of an in-depth understanding of the contribution of NTDs. The present review summarizes the literature on the relationship between a selected group of NTDs and SCD.
A narrative review of the literature was performed. Databases searched included PubMed, Scielo, and Google Scholar. Databases were reviewed without language or publication date restriction. Reference lists, abstracts, policies, international society guidelines, and governmental healthcare organizations’ statements were also reviewed. The search was performed independently by each coauthor with the criteria of studies describing NTDs and their relationship with SCD. The selection of relevant NTDs to discuss was based on previous systematic reviews carried out as part of the Interamerican Society of Cardiology (SIAC) NET-Heart Project. The conditions identified as having a potential increased risk of SCD were included in this review [9, 10, 11, 12, 13, 14, 15, 16].
Chagas disease (CD) is a protozoan infection caused by Trypanosoma cruzi (T. cruzi). It is transmitted to mammals via insects belonging to the Reduviidae family (triatomine) [17]. CD is endemic to the southern United States and most countries in Central and South America. Although 65 million people live in endemic areas, most infected individuals are undiagnosed or have significant limitations accessing medical care [14, 18]. Due to migration and globalization, CD has become a global health concern with an increasing presence of chronic complications in the United States, Europe, and Asia [19, 20, 21].
The most common clinical implications of chronic CD are related to CV compromise. These impacting 30% to 40% of patients after the acute infection, persisting for up to three decades [17]. CV involvement of CD includes bradycardia, atrioventricular (AV) block, intraventricular conduction abnormalities, autonomic dysfunction, syncope, heart failure (HF), left ventricular (LV) dysfunction, structural abnormalities, and SCD [17].
Approximately 50,000 people die annually due to CD. SCD represents the most common cause of death in these patients (60%), followed by HF (25%), and thromboembolic events (15%) [22]. Premature death in patients with CD is of particular concern. It affects a high proportion of young people, which contributes to economic impact and social burden. SCD can be the first manifestation of CV involvement. Ventricular arrhythmias (VAs) are thought to be the principal cause of death in this subset of patients [23]. Other arrhythmic conditions leading to SCD in CD include advanced AV block and pulseless electrical activity [24, 25].
The pathophysiology of SCD in CD is multifactorial. It includes myocardial inflammation, fibrosis, scarring, and remodeling caused by parasite invasion to the myocardium. Additionally, microvascular derangements, autonomic dysregulation, and autoimmunity may occur (Fig. 2) [26, 27]. Risk factors for the development of SCD in CD include male sex, previous VAs, LV dysfunction, syncope, bradycardia, late potentials on signal-averaged electrocardiography, and myocardial fibrosis [28]. Based on these factors, prediction scores have been developed to estimate the risk of SCD in this population (Table 1, Ref. [15]) [29, 30, 31, 32].
 Fig. 2.
Fig. 2.NTDs and their pathophysiological mechanisms related to SCD. AV, Atrioventricular; LV, Left ventricle; VAs, Ventricular arrhythmias.
| Rassi score (Prediction of mortality) | Ribeiro score (Prediction of mortality) | Sousa score (Prediction of SCD) | |||
| Variable | Points | Variable | Points | Variable | Points |
| Cardiomegaly | 5 | LVEF |
1 | QT dispersion | 3 |
| NYHA III or IV | 5 | Ventricular tachycardia (Holter or Stress test) | 1 | Syncope | 2 |
| LV wall motion abnormalities* | 3 | Prolonged QRS ( |
1 | LV function | 1 |
| Non-sustained ventricular tachycardia | 3 | Presence of PVC | 1 | ||
| Male sex | 2 | ||||
| Low QRS | 2 | ||||
| Risk categories | Points | Risk categories | Points | Risk categories | Points |
| Low | 0–6 | Low | 0–1 | Low | 0–2 |
| Intermediate | 7–11 | Intermediate | 2 | Intermediate | 3–4 |
| High | 12–20 | High | 3 | High | |
| 5-year risk of death | % | 5-year risk of death | % | 5-year risk of SCD | % |
| Low | 2 | Low | 1 | Low | 1.5 |
| Intermediate | 18 | Intermediate | 20 | Intermediate | 25 |
| High | 61 | High | 50 | High | 51 |
| 10-year risk of death | % | ||||
| Low | 10 | ||||
| Intermediate | 44 | ||||
| High | 88 | ||||
| * Accounts for segmental or global wall motion abnormalities.
ECG, Electrocardiogram; NYHA, New York Health Association; LV, Left ventricle; LVEF, Left ventricle ejection fraction; PVC, Premature ventricular contraction; SCD, Sudden cardiac death. Modified from reference [15]. | |||||
Treatment of VAs related to CD remains an area of controversy. Antiarrhythmic drugs successfully reduce arrhythmic burden, but they do not reduce mortality [24]. Further, there is limited evidence on the utility of amiodarone for the treatment of VAs in CD [33].
Catheter ablation is an alternative for patients who have recurrent VAs despite antiarrhythmic therapy or who are not able to tolerate medications [34]. The arrhythmia substrate in CD is complex because it frequently includes multiple foci, large scars, and epicardial circuits which influence procedure outcomes [24]. Recently published studies suggest that the endo/epicardial approach for catheter ablation improves results [35]. When VT recurs after ablation, neuraxial modulation and surgical cardiac denervation have shown effectiveness in reducing arrhythmia burden [34, 36]. Access to complex electrophysiology care is severely restricted for populations living in LMICs. As a result, invasive treatment options may be limited in the most affected regions.
Malaria is caused by the protozoa Plasmodium (P.) falciparum, knowlesi, vivax, and ovale. It is transmitted by infected female Anopheles mosquitoes [11]. Malaria is endemic to Asia and Oceania as well as South and Central America, with the highest risk of infection occurring in sub-Saharan Africa [11]. There is a considerable burden of disease associated with malaria, as it was responsible for approximately 627,000 deaths in 2020 [37].
The pathophysiology of CV consequences secondary to malaria is not well understood. It may involve a pro-inflammatory cytokine response, erythrocyte sequestration, and increased cytoadherence to endothelial linings [38, 39]. Additionally, the profound anemia produced by hemolysis and suppression of hematopoiesis in malaria may impair cardiac function. The proposed mechanism is the induction of cardiac stress leading to eccentric LV hypertrophy and associated volume overload, secondary tissue ischemia, and hypoxia. Sequestration of infected red blood cells in cardiac capillaries and subsequent blood flow obstruction may also cause LV dilation, ischemia, VAs, and SCD (Fig. 2) [11]. Patients with complicated malaria that progresses to severe sepsis or septic shock often have myocardial dysfunction, with ventricular dilation and reduced ejection fraction. This increasesthe risk of SCD [40].
Electrocardiographic changes have been reported in complicated malaria, such as ST-segment elevation secondary to ischemia and prolonged QTc intervals. These pathologies increase the risk of developing fatal VAs and SCD [40]. Notably, polymorphic ventricular tachycardia is a known life-threatening complication of antimalarial drugs that can lead to SCD [41, 42]. Tachycardia and tachyarrhythmias seen in malaria are also consequences of the anti-malarial drugs amodiaquine and halofantrine. Finally, quinine, another extensively used drug in treating severe malaria, is known to cause QT interval prolongation, Torsades des pointes, high degree AV block, and SCD [41].
Although there is no specific treatment to reduce the risk of SCD in malaria, the complicated forms of the infection can be treated with intravenous quinidine. Patients require continuous intensive care monitoring, including serial ECG in those treated with cardiotoxic antimalarials. Hence recognizing the CV complications of these drugs and their electrocardiographic manifestations facilitates the use of the ECG as a monitoring tool. Severe anemia and high levels of parasitemia can be managed with blood transfusions and supplemental oxygen to reduce the risk of arrhythmia, HF, and secondary myocardial injury [11].
Toxoplasmosis is caused by the parasite Toxoplasma gondii which spreads to humans through the consumption of undercooked meat, contaminated water, or contact with mammal feces [16]. Toxoplasmosis is typically mild and self-resolving in healthy hosts but may lead to severe disease in immunocompromised patients [16]. The CV involvement in toxoplasmosis typically manifests as myocarditis, where inflammatory cellular infiltrates occur with or without myocyte necrosis [43]. Though uncommon and often subclinical, toxoplasmic myocarditis is associated with progressive cardiac dysfunction (Fig. 2) [16]. To date, 12 cases of cardiac toxoplasmosis with SCD have been reported in the literature. All patients were immunocompromised, and 11 suffered from human immunodeficiency virus and acquired immunodeficiency syndrome. The remaining patient underwent a heart transplant [43, 44, 45, 46].
Reports of cardiac toxoplasmosis may be uncommon due to the requirement of invasive diagnostic strategies. Extensive tissue sampling of the heart might be needed to confirm the diagnosis as lesions often localize in the myocardium of the LV [43]. Thus, the connection between SCD and toxoplasmosis has not been extensively studied and requires further investigation.
Acquired toxoplasmosis in immunocompetent patients with mild symptoms does not require specific treatment. Though, intervention is necessary when severe systemic compromise occurs. CV toxoplasmosis has been successfully treated with a combination of pyrimethamine, sulfadiazine, and folinic acid [16].
Based on the currently available information, immunocompromised patients with toxoplasmic myocarditis are at a considerable risk of related SCD. Clinicians should take appropriate measures to prevent this fatal outcome. These may include managing symptoms and promptly treating CV complications such as arrhythmias and LV dysfunction. In highly endemic areas, providing prophylaxis for organ transplant recipients is recommended [47].
Tuberculosis is the leading global cause of mortality due to infectious diseases and is among the top 10 leading causes of death worldwide [48]. Though it is predominantly a pulmonary disease, cardiac involvement is not infrequent in tuberculosis and contributes to morbidity and mortality [13].
CV involvement in tuberculosis occurs due to compromise of the pericardium, myocardium, and aorta. Pericardial disease is present in up to 5% of all cases of pulmonary disease and may manifest as acute pericarditis, pericardial effusion, or chronic constrictive pericarditis [13]. Myocarditis is rare, with an estimated incidence of less than 1% in these patients [49, 50]. Mycobacterium tuberculosis can spread to the myocardium in three ways: direct extension, retrograde spread via lymphatics, or via the hematogenous route [13, 50].
Once the myocardium is affected, CV involvement may progress to fulminant myocarditis, HF, conduction system disturbances, AV block, prolonged QT, and/or SCD [49, 51]. SCD in tuberculosis may be secondary to VAs induced by myocardial necrosis and damage of the interventricular septum, leading to electro-mechanical uncoupling. VT and ventricular fibrillation resulting in asystole may also occur (Fig. 2). Septal conduction system disturbances due to direct tuberculosis infiltration have also been proposed to cause death [52]. Likewise, HF leading to dilated cardiomyopathy can predispose patients to SCD [51, 53].
Typically, a four-drug conjugated regimen (isoniazid, rifampicin, ethambutol, and pyrazinamide) is the mainstay of anti-tuberculosis therapy [54]. Adverse CV events secondary to tuberculosis medications are rare. Notably, isoniazid treatment has been associated with QT interval prolongation and polymorphic ventricular tachycardia [54]. No link between other anti-tuberculosis medications and SCD has been reported.
There is insufficient data to guide a specific treatment for myocarditis in tuberculosis or prevent its complications [55]. Thus, prevention strategies for SCD should be prioritized. A useful and applicable algorithm has been published previously, suggesting that early diagnosis of cardiac tuberculosis, improved access to diagnostic tests, and combined antituberculosis medications with steroids are the basis for reducing associated mortality [13].
Dengue virus (DENV) is a flavivirus transmitted by the vector Aedes
aegypti which is predominantly found in tropical and subtropical regions [56].
Most patients infected with DENV are asymptomatic or have a mild illness. A
minority of patients develop severe disease and subsequent CV complications [9].
Risk factors for CV involvement include age
CV complications of DENV are seen in up to 12.5% of patients [9]. Manifestations of CV compromise include bradyarrhythmias, tachyarrhythmias, myocarditis, LV dysfunction, and pericarditis [9, 57].
DENV infection may lead to three interrelated pathophysiological processes associated with SCD: LV systolic dysfunction, myocarditis, and arrhythmias (Fig. 2) [57]. These processes act as triggers and modulators of cardiac electrical instability. Arrhythmias can progress to VF (with or without previous VT) [58], and less frequently severe bradyarrhythmia with consequent SCD [59].
There is no specific treatment for DENV infection. In general, early identification of CV involvement and timely hemodynamic support should be initiated to treat patients with severe cardiac affection [9, 57, 59].
Zika virus (ZIKV) is an arbovirus that is part of the Flaviviridae family. It is mainly transferred to humans by Aedes aegypti and Aedes Albopictus mosquitoes [60]. It is also transmissible by asymptomatic hosts via sexual intercourse, blood transfusion, and transplacentally [61]. Currently, the ZIKV is mainly found in the Americas, Africa, the West Pacific, and Southeast Asia. Most patients with ZIKV disease do not present with any symptoms. Approximately 20% of patients with ZIKV have mild to moderate symptoms that usually resolve after two to seven days [15, 61].
CV involvement in ZIKV disease includes pericarditis, myocarditis, arrhythmias, and HF which may lead to SCD (Fig. 2). Specifically, the virus invades the cell through tyrosine-protein kinase 3 and ICAM-3 receptors, prompting pro-inflammatory cytokine release. Subsequently, inflammatory or autoimmune damage triggers cellular apoptosis [15]. Myocarditis caused by ZIKV may only present as nonspecific chest pain or no symptoms at all and is usually undiagnosed. Thus, myocardial damage may go unnoticed. This can develop into HF, myocardial infarction, cardiogenic shock, and even SCD during the acute stage of infection [15, 62, 63].
Early diagnosis and treatment of CV complications are the cornerstone to reduce the risk of sudden death in ZIKV disease patients. Acute symptoms, such as fever and fatigue may resolve with adequate hydration, rest, and antipyretics. However, severe infection may require intravenous polyvalent immunoglobulins [15].
Chikungunya virus (CHIKV) is an enzootic ribonucleic acid virus that is transmitted by female Aedes albopictus and Aedes aegypti mosquitoes to humans [10]. CHIKV disease commonly presents in patients with acute fever and severe arthralgia. Systemic involvement, such as CV complications, is only seen in severe cases [10]. Patients who have comorbidities or are elderly are at a greater risk of systemic compromise [10].
Those with severe CHIKV are at elevated risk of developing myocarditis, arrhythmias, and HF (Fig. 2). CHIKV directly damages muscle fibers by puncturing myocytes, thereby augmenting inflammation, and producing secondary necrosis [10]. The most common CV complication related to CHIKV is myocarditis, which may contribute to further developing arrhythmias, myocardial necrosis, and SCD [10, 64, 65].
The early recognition and treatment of CHIKV are critical to preventing CV manifestations [10]. Myocarditis does not always present with symptoms before evolving to SCD [64]. Supportive measures such as cardiac monitoring, inotropic drugs, and oxygenation, can be used in severe cases [10]. Currently, there is no treatment or vaccine against CHIKV disease. However, there are certain proposed treatments for CHIKV cardiac complications. Notably, intravenous hydrocortisone may be administered to treat myocarditis. Although there is uncertainty regarding its benefit to mortality [10].
Implementing preventative measures is the first step in reducing the burden of SCD in NTDs. Further, raising awareness will aid in early diagnosis and recognition of the CV involvement. Additionally, the pathophysiology of SCD in NTDs must be elucidated to improve evidence-based care [22, 51].
Myocarditis is the primary CV manifestation of most NTDs. It is frequently associated with the development of acute HF, arrhythmias, and SCD [9, 10, 11, 13, 15, 16, 17]. In general, the standard treatment for each NTD leads to the resolution of myocarditis [42, 66, 67]. In severe cases, steroid or mechanical circulatory support may be considered in addition to conventional therapy [42, 68, 69, 70, 71]. If HF develops, management is based on current HF guidelines (Fig. 3) [72, 73].
 Fig. 3.
Fig. 3.General approach for specific therapies to prevent SCD in NTDs. AAD, antiarrhythmic drugs; CA, Catheter ablation; CV, cardiovascular; ICD, implantable cardioverter defibrillator; GDMT, guideline-directed management and therapy; HF, heart failure; LVEF, left ventricular ejection fraction; TB, tuberculosis; VT, ventricular tachycardia; VF, ventricular fibrillation.
Risk stratification tools for SCD have been established for patients with chronic Chagas cardiomyopathy (Table 1) [30]. Similar tools have not been developed for other NTDs due to the limited number of cases reported in the literature and limited understanding of the mechanisms of SCD [2, 3, 12].
The indications for preventative Implantable Cardioverter Defibrillators (ICDs) in NTD patients have been extrapolated from international guidelines [74]. However, these recommendations are limited to patients with an LV ejection fraction less than 35% and New York Heart Association HF class II-III (Fig. 3) [72, 73]. In this setting, cardiac magnetic resonance (CMR) with late gadolinium enhancement could contribute to risk stratification. CMR is useful, as the presence of fibrosis is associated with an increased risk of SCD regardless of the ejection fraction [75].
Patients with CD cardiomyopathy have received specific attention in the literature [76]. Observational data suggests that in patients with CD with a LV ejection fraction less than 41% and life-threatening arrhythmias the treatment with a combination of an ICD and amiodarone reduces all-cause mortality by 72% and rates of SCD in 95% [77]. ICD is also indicated in the secondary prevention of SCD in patients with previous aborted cardiac arrest attributable to VAs (Fig. 3) [72, 73].
Antiarrhythmic drugs and catheter ablation can be considered ancillary interventions in patients with VAs after ICD implant to reduce the risk of appropriate and inappropriate shocks, which are associated with an increase in mortality and a significant reduction in quality of life (Fig. 3) [78, 79].
The relationship between NTDs and SCD is still poorly understood. To give a broad overview of this complex topic, we propose the NET Heart’s deadly quartet of SCD in NTDs (Fig. 4) which highlights four cornerstones:
(1) Social factors: Poor access to optimal and timely healthcare, late recognition of CV involvement in NTDs, and limited recognition of the link between NTDs and SCD.
(2) Systemic factors: Pro-inflammatory cytokine response, hypoxia, ischemia, autoimmunity, and autonomic dysregulation.
(3) Cardiac factors: Inflammation, necrosis, apoptosis, microvascular occlusion, fibrosis, scarring, and remodeling.
(4) Drugs: Some medications required for the treatment of NTDs have cardiotoxic effects that can be related to SCD such as quinine, quinidine, antimonials, and isoniazid.
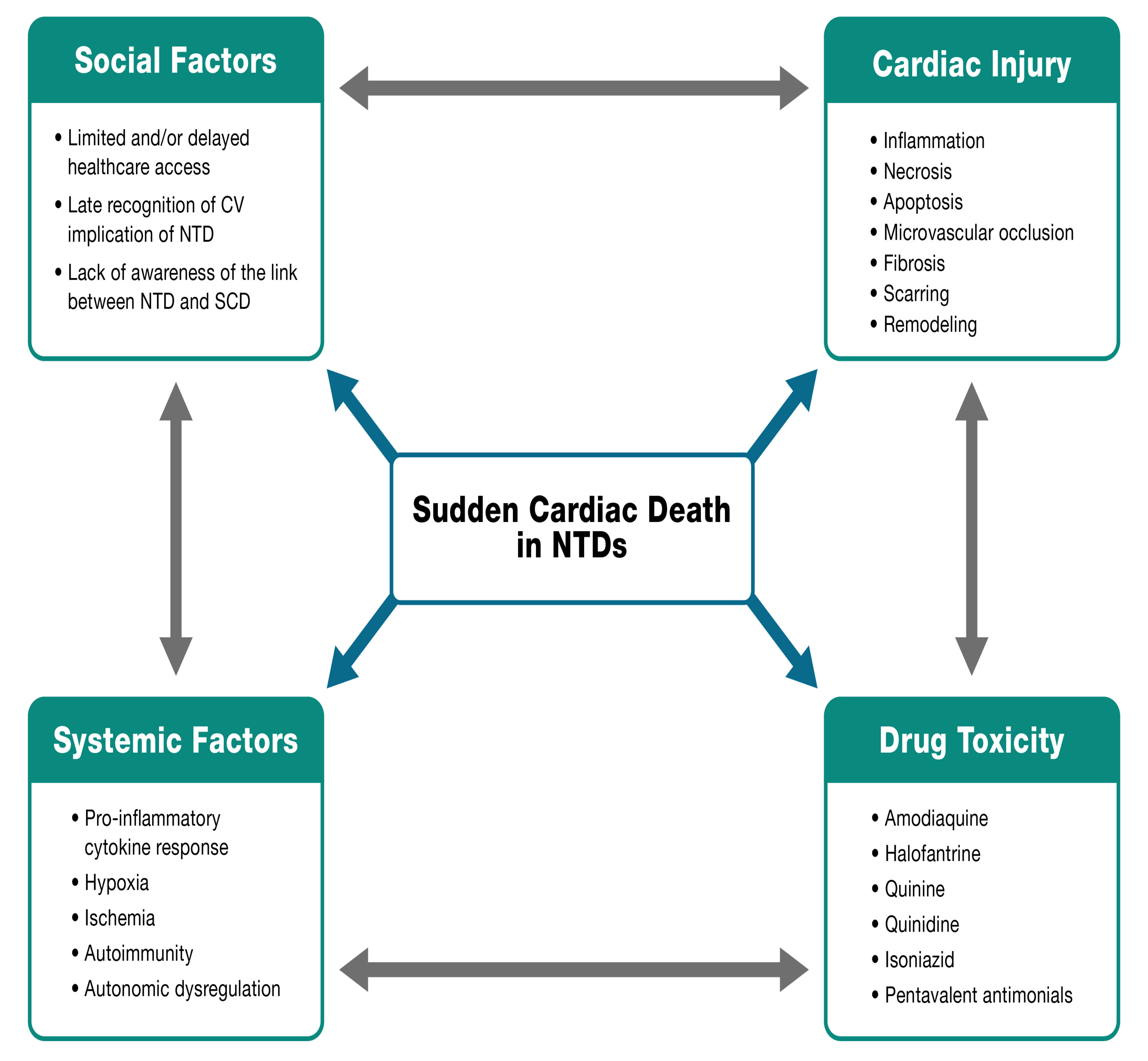 Fig. 4.
Fig. 4.NET Heart’s deadly quartet of SCD in NTDs. CV, cardiovascular.
These considerations will allow clinicians to recognize the interconnection between NTDs and SCD in regions with the highest prevalence. Thus, allowing to focus on developing multimodal approaches that reduce the impact generated by these unrecognized and neglected causes of SCD.
Populations living in poverty are at a higher risk of SCD associated with NTDs identifying several roadblocks to accessing optimal care. These considerations impact clinical decisions and require the development of approaches tailored to low-resource settings.
The main limitation of the present review is that it was not systematic. Additionally, it was based on a topic with limited associated literature. Further, NTDs encompass an extensive and heterogeneous group of diseases. Discussing in the proposed CV involvement of every NTD is beyond the scope of this document
The relationship between NTDs and SCD is complex, and the literature continues to be scarce on this topic. Some NTDs share pathophysiological mechanisms that can contribute to the development of SCD. However, multiple factors trigger and perpetuate the risk of death in this heterogeneous group of patients. We propose a holistic view of this public health problem entitled the NET Heart’s deadly quartet of SCD in NTDs aiming to facilitate the understanding and the implementation of strategies to reduce the impact generated by these conditions.
AFMA and AB conceived the initial idea of the project and planned the structure of the manuscript. AFMA, LGGB, JF, ZZ, RAR, JPLL, JPJL, and SG participated in the redaction of the first draft of the manuscript. AFMA, LGGB, JF, and KL made the initial corrections, style adjustments. and correction of the first draft. AFMA and KL designed the figures and tables. EJZ, CS, ÁSL, DP, RKK, and AB reviewed and corrected the final draft. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Adrian Baranchuk is serving as one of the Editorial Board members of this journal. We declare that Adrian Baranchuk had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Vincenzo Russo, Saverio Muscoli and Giuseppe Mascia.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
