†These authors contributed equally.
Academic Editors: Ichiro Wakabayashi and Klaus Groschner
Background: Few studies have investigated the characteristics and long-term outcomes of type B aortic dissection (BAD) patients with simple renal cysts (SRC) after thoracic endovascular aortic repair (TEVAR). Methods: A multi-center retrospective cohort study was performed, including 718 BAD patients undergoing TEVAR from 2003 to 2016. The prevalence of SRC was 34.5% (n = 248). After propensity score matching, 214 matched pairs were selected for further analysis. Primary outcomes were long-term aortic-related adverse events (ARAEs). The effects of SRC in each subgroup of interest and their interactions were analyzed. Results: BAD patients with SRC were older and had a greater prevalence of comorbidities, including hypertension, coronary artery disease and chronic occlusive pulmonary disease. In addition, the SRC group presented a greater proportion of pleural effusion and aortic calcification. Compared with the non-SRC group, a significantly higher maximal diameter of ascending aorta was observed in the SRC group. Apart from the timing of the operation, no differences were found in the medication regime or intra-operative parameters. In the matched population, patients with SRC were at a higher risk of ARAEs in the long term. The multivariable Cox model indicated that SRC was an independent predictor of long-term ARAEs (hazard ratio: 1.84, 95% confidence interval: 1.13–3.00). The interaction between SRC and hypertension on rupture after TEVAR was statistically significant (p = 0.023). Conclusions: Compared with the non-SRC group, BAD patients with SRC experienced a higher risk of long-term ARAEs after TEVAR. Among the SRC subgroup, hypertensive patients had the highest risk of rupture after TEVAR.
Aortic dissection (AD) is a devastating aortic disease caused by an entry tear in the aortic intima or hemorrhage in the aortic media, leading to the separation of the aortic layers [1]. According to the Stanford classification, type B aortic dissection (BAD) originates distal to the ostium of the left subclavian artery [1]. With the development of thoracic endovascular aortic repair (TEVAR), the mortality rate of BAD patients has significantly reduced [2]. Nonetheless, patients can still suffer from diverse stent-graft-related complications, including endoleak, aortic dilation, retrograde type A aortic dissection (AAD) and new dissection [2]. Therefore, it is imperative to identify potential risk factors associated with aortic-related adverse events (ARAEs) in BAD patients undergoing TEVAR.
Recently published studies have found an association between simple renal cysts (SRC) and aortic dissection/aneurysm [3, 4]. However, the perioperative characteristics and long-term outcomes of BAD patients with SRC that undergo TEVAR remain unclear. In this context, a multi-center retrospective study was conducted. First of all, differences in perioperative characteristics and intraoperative details between SRC and non-SRC groups were compared. Moreover, a propensity score matching was performed to minimize selection bias. The differences in long-term outcomes between the two groups were compared in the overall and matched study populations. Finally, subgroup analysis was performed to identify which subpopulations of SRC patients sustained the highest risk of ARAEs after TEVAR.
A multi-center retrospective analysis was performed, including three Chinese tertiary referral centers (Supplementary Table 1). Consecutive BAD patients who underwent TEVAR from January 2003 to February 2016 were included in this study. A flowchart of the study population is shown in Fig. 1.
 Fig. 1.
Fig. 1.Flowchart of the study population. A multi-center retrospective cohort study was performed, including 718 BAD patients undergoing TEVAR from 2003 to 2016, consisting of 248 and 470 patients with and without SRC. After propensity score matching, 214 matched pairs were taken into further analysis.
The indications of TEVAR for complicated and uncomplicated BAD were reported in our previous studies [5]. All procedures were performed as previously described [5, 6].
The duration of BAD was divided into acute (
The presence of SRC was confirmed by the CT or magnetic resonance imaging on
admission. The same definition was used to diagnose SRC in all participating
centers: a lesion characterized with round shape, thin wall, size
Primary outcomes were defined as any ARAEs, including endoleak (type I, II and III), new dissection, retrograde AAD, aortic dilation, rupture (of the false lumen) and aortic-related mortality at 5-year follow-up [2]. The in-hospital aortic-related mortality was evaluated by reviewing the inpatient record, death and autopsy reports, while the out-of-hospital mortality was determined by phone calls. Secondary outcomes included all-cause mortality and cardiovascular events at the five-year follow-up. A short-term outcome was defined as any clinical adverse event described at 30-day follow-up, while a long-term outcome was defined as any events occurring at the 5-year follow-up. The follow-up was performed until February 2021. The completeness of follow-up of our study was estimated using the Clark C index [10].
Data were presented as n (%) for categorical variables and
mean
To minimize selection bias and improve confounding variable balance, adverse
clinical outcomes were compared using propensity-matched data for the 214 pairs
of SRC and non-SRC patients. The propensity score matching was conducted using a
caliper width of
| Variables | Overall | Matched population | |||||||
| SRC group | Non-SRC group | SMD | p value | SRC group | Non-SRC group | SMD | p value | ||
| n = 248 | n = 470 | n = 214 | n = 214 | ||||||
| Baseline characteristics | |||||||||
| Age (y) | 61.1 |
56.3 |
0.38 | 59.5 |
60.3 |
0.06 | 0.494 | ||
| Male | 199 (80.2%) | 396 (84.3%) | 0.11 | 0.175 | 172 (80.4%) | 176 (82.2%) | 0.05 | 0.62 | |
| BMI | 24.4 |
24.7 |
0.1 | 0.254 | 24.6 |
24.6 |
0.01 | 0.893 | |
| Smoking | 135 (54.4%) | 245 (52.1%) | 0.05 | 0.556 | 116 (54.2%) | 120 (56.1%) | 0.04 | 0.697 | |
| Drinking | 47 (19.0%) | 99 (21.1%) | 0.05 | 0.504 | 44 (20.6%) | 45 (21.0%) | 0.01 | 0.905 | |
| Hypertension | 205 (82.7%) | 349 (74.3%) | 0.21 | 0.011 | 174 (81.31%) | 169 (78.97%) | 0.06 | 0.545 | |
| CAD | 22 (8.9%) | 18 (3.8%) | 0.21 | 0.005 | 12 (5.6%) | 14 (6.5%) | 0.04 | 0.686 | |
| Arrhythmia | 32 (12.9%) | 52 (11.1%) | 0.06 | 0.466 | 25 (11.7%) | 34 (15.9%) | 0.12 | 0.207 | |
| Stroke | 10 (4.03%) | 17 (3.62%) | 0.02 | 0.781 | 8 (3.7%) | 11 (5.1%) | 0.07 | 0.481 | |
| COPD | 46 (18.6%) | 27 (5.7%) | 0.40 | 23 (10.8%) | 26 (12.2%) | 0.04 | 0.649 | ||
| Diabetes mellitus | 21 (8.5%) | 37 (7.9%) | 0.02 | 0.781 | 16 (7.5%) | 24 (11.2%) | 0.13 | 0.184 | |
| CKD | 16 (6.5%) | 19 (4.0%) | 0.11 | 0.154 | 14 (6.5%) | 8 (3.7%) | 0.13 | 0.189 | |
| SBP at admission (mmHg) | 138.7 |
138.0 |
0.03 | 0.669 | 137.9 |
137.6 |
0.01 | 0.956 | |
| DBP at admission (mmHg) | 82.9 |
82.3 |
0.05 | 0.509 | 83.0 |
81.2 |
0.15 | 0.115 | |
| Pleural effusion | 104 (41.9%) | 127 (27.0%) | 0.32 | 84 (39.3%) | 76 (35.5%) | 0.08 | 0.424 | ||
| Malperfusion | 18 (7.26%) | 23 (4.89%) | 0.1 | 0.194 | 16 (7.5%) | 9 (4.2%) | 0.14 | 0.149 | |
| Echocardiography parameters | |||||||||
| Diameters of ascending aorta (cm) | 3.4 |
3.4 |
0.06 | 0.643 | 3.4 |
3.4 |
0.06 | 0.677 | |
| LVEF (%) | 61.8 |
62.2 |
0.08 | 0.434 | 61.9 |
62.5 |
0.12 | 0.482 | |
| Medication in hospital | |||||||||
| ACEI/ARB | 140 (56.5%) | 239 (50.9%) | 0.11 | 0.153 | 111 (51.87%) | 112 (52.34%) | 0.01 | 0.923 | |
| 96 (38.7%) | 187 (39.8%) | 0.02 | 0.779 | 81 (37.9%) | 86 (40.2%) | 0.05 | 0.62 | ||
| 167 (67.3%) | 339 (72.1%) | 0.1 | 0.181 | 143 (66.8%) | 146 (68.2%) | 0.03 | 0.757 | ||
| CCB | 187 (75.4%) | 352 (74.9%) | 0.01 | 0.881 | 160 (74.8%) | 147 (68.7%) | 0.14 | 0.163 | |
| Diuretic | 33 (13.3%) | 60 (12.8%) | 0.02 | 0.838 | 27 (12.6%) | 35 (16.4%) | 0.11 | 0.272 | |
| Anatomical characteristics | |||||||||
| Arch type | 0.07 | 0.641 | 0.12 | 0.459 | |||||
| I | 83 (33.5%) | 157 (33.4%) | 68 (31.8%) | 71 (33.2%) | |||||
| II | 32 (12.9%) | 50 (10.6%) | 27 (12.6%) | 19 (8.9%) | |||||
| III | 133 (53.6%) | 263 (56.0%) | 119 (55.6%) | 124 (57.9%) | |||||
| Diameters of maximum ascending aorta (mm) | 40.2 |
38.3 |
0.35 | 0.012 | 38.9 |
38.2 |
0.12 | 0.413 | |
| Diameters of maximum descending aorta (mm) | 44.1 |
44.1 |
0.01 | 0.97 | 44.3 |
44.2 |
0.01 | 0.772 | |
| Calcification | 0.66 | 0.13 | 0.595 | ||||||
| None | 93 (37.5%) | 319 (67.9%) | 87 (40.65%) | 95 (44.39%) | |||||
| Mild | 105 (42.3%) | 117 (24.9%) | 89 (41.59%) | 91 (42.52%) | |||||
| Moderate | 40 (16.1%) | 26 (5.5%) | 31 (14.49%) | 23 (10.75%) | |||||
| Severe | 10 (4.0%) | 8 (1.7%) | 7 (3.27%) | 5 (2.34%) | |||||
| Thrombosis | 0.15 | 0.331 | 0.08 | 0.879 | |||||
| Patent | 113 (45.6%) | 182 (38.7%) | 95 (44.4%) | 90 (42.1%) | |||||
| Partial | 83 (33.5%) | 174 (37.0%) | 74 (34.6%) | 72 (33.6%) | |||||
| Complete | 34 (13.7%) | 79 (16.8%) | 29 (13.6%) | 34 (15.9%) | |||||
| ULP | 18 (7.3%) | 35 (7.5%) | 16 (7.5%) | 18 (8.4%) | |||||
| Values are reported as n (%), mean | |||||||||
| Variables | Overall | Matched population | |||||||
| SRC group | Non-SRC group | SMD | p value | SRC group | Non-SRC group | SMD | p value | ||
| n = 248 | n = 470 | n = 214 | n = 214 | ||||||
| Operation time (min) | 120.0 (79.0–156.3) | 115.0 (80.0–160.0) | 0.07 | 0.448 | 120.0 (80.0–160.0) | 115.0 (80.0–150.0) | 0.07 | 0.629 | |
| Oversize (%) | 15.8 (7.4–23.5) | 16.1 (7.4–33.5) | 0.45 | 0.074 | 16.1 (8.0–27.1) | 12.5 (6.6–28.7) | 0.05 | 0.792 | |
| Length of proximal landing zone (mm) | 24.0 (17.3–32.8) | 20.6 (14.1–30.3) | 0.22 | 0.239 | 22.4 (15.0–32.3) | 22.0 (15.8–34.8) | 0.02 | 0.802 | |
| Timing of operation | 0.22 | 0.02 | 0.05 | 0.856 | |||||
| Acute phase | 153 (61.7%) | 239 (50.9%) | 128 (59.8%) | 127 (59.4%) | |||||
| Subacute phase | 64 (25.8%) | 160 (34.0%) | 59 (27.6%) | 63 (29.4%) | |||||
| Chronic phase | 31 (12.5%) | 71 (15.1%) | 27 (12.6%) | 24 (11.2%) | |||||
| Hybrid approach | 1 (0.4%) | 10 (2.1%) | 0.15 | 0.074 | 1 (0.5%) | 4 (1.9%) | 0.13 | 0.372 | |
| Chimney technique | 51 (20.6%) | 77 (16.4%) | 0.11 | 0.164 | 45 (21.0%) | 36 (16.8%) | 0.11 | 0.267 | |
| Adjunctive procedure | 44 (17.7%) | 97 (20.6%) | 0.07 | 0.353 | 40 (18.7%) | 45 (21.0%) | 0.06 | 0.545 | |
| Era | 0.03 | 0.697 | 0.03 | 0.771 | |||||
| 2003–2010 | 106 (42.7%) | 208 (44.3%) | 98 (45.8%) | 95 (44.4%) | |||||
| 2011–2016 | 142 (57.3%) | 262 (55.7%) | 116 (54.2%) | 119 (55.6%) | |||||
| Values are reported as n (%) or median (interquartile range). | |||||||||
To identify potential risk factors for ARAEs, Cox hazard analysis was used in
the matched population. Variables that had a significant correlation (p
From January 2003 to February 2016, 718 patients were enrolled in the study, of
whom 595 were males (82.9%), and 248 presented with at least one SRC (34.5%)
(Table 1). Patients with SRC were older (61.1
Table 2 shows the differences in intra-operative details between the SRC and non-SRC groups.
Short-term outcomes in the overall and matched population were shown in the Supplementary Table 2.
The median length of follow-up in the matched population was 3.6 (IQR: 1.7–5.9) years and 3.1 (IQR: 1.2–5.7) years for patients with and without SRC, respectively. The completeness of follow-up for the primary outcomes was 67.3% for the SRC group and 68.1% for the non-SRC group. In the matched population, ARAEs were significantly lower in the non-SRC group than in the SRC group (Gray’s test p = 0.006 and log-rank p = 0.007) (Fig. 2A and Fig. 3A). In terms of 5-year aortic rupture, the cumulative incidence was 5.9% in the SRC group and 2.1% in the non-SRC group (p = 0.04) (Fig. 2B). Cumulative incidence of 5-year aortic dilation was also higher in patients with SRC (8.2% vs. 2.1%, p = 0.006) (Fig. 2C). No difference was observed in the aortic-related mortality between the two groups (log-rank p = 0.163) (Fig. 3B). Supplementary Figs. 1–4 show the cumulative incidences of aortic-related mortality, endoleak, retrograde AAD and new dissection in the matched population. Long-term outcomes in the total population are presented in the Supplementary Table 3.
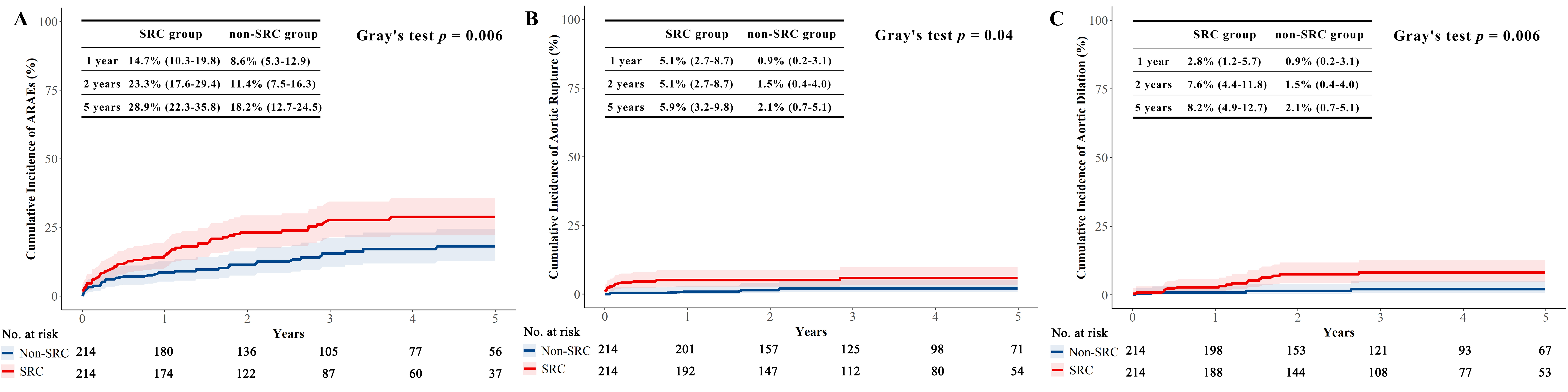 Fig. 2.
Fig. 2.The cumulative incidences of the ARAEs, rupture and aortic dilation, with all-cause death as the competing risk in the matched population. (A)The cumulative incidence of ARAEs in the SRC group was significantly greater than in the non-SRC group (p = 0.006). (B) The cumulative incidence of rupture in the SRC group was significantly greater than in the non-SRC group (p = 0.04). (C) The cumulative incidence of aortic dilation in the SRC group was significantly greater than in the non-SRC group (p = 0.006). The differences were assessed with Gray’s test.
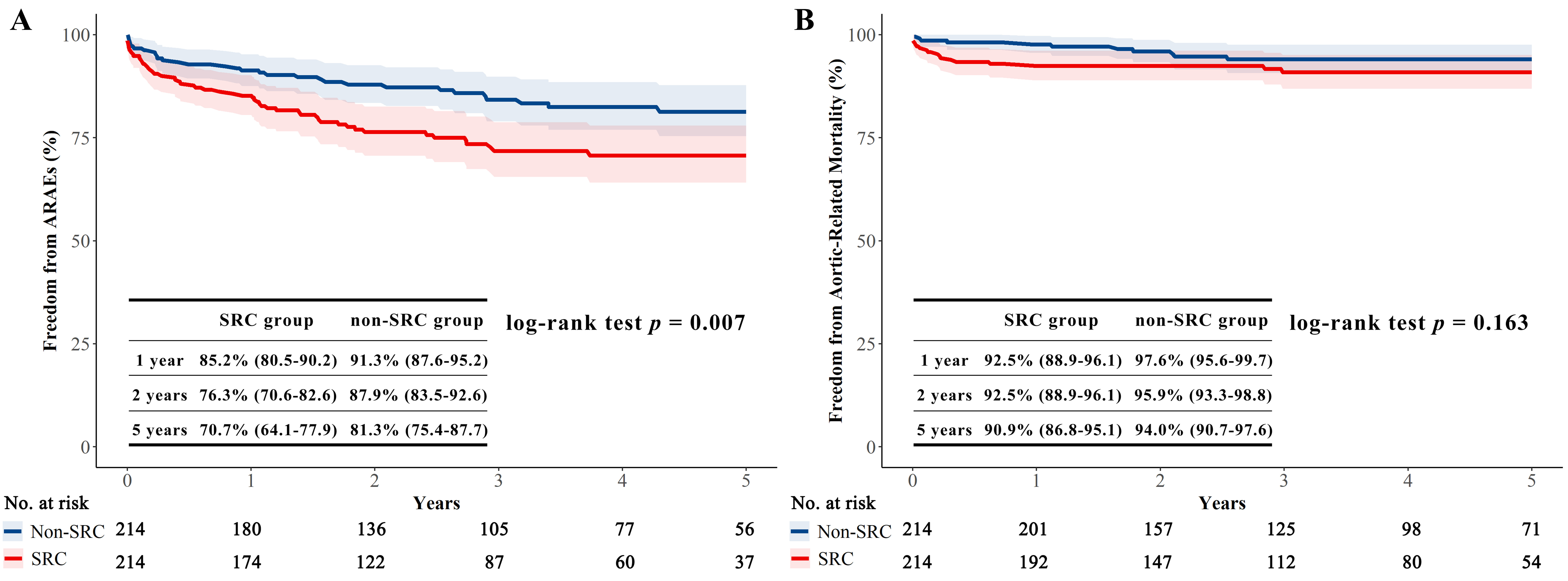 Fig. 3.
Fig. 3.Kaplan-Meier survival analysis of ARAEs and aortic-related mortality in the matched population. (A) Freedom from ARAEs between the SRC and non-SRC groups. Freedom from ARAEs in the SRC group was significantly lower than that of the non-SRC group (p = 0.007). (B) Freedom from aortic-related mortality between the two groups. Freedom from aortic-related mortality in the SRC group was similar with that of the non-SRC group (p = 0.163). The differences between the SRC and non-SRC groups were assessed with log-rank test.
Univariate Cox hazard analysis indicated that SRC, hypertension, chronic
occlusive pulmonary disease, operation time, stroke, malperfusion, chronic aortic
dissection, maximum diameters of descending aorta were potential risk factors for
ARAEs (p
| Variables | Hazard ratio (95% CI) | p value | |
| SRC | 1.84 (1.13, 3.00) | 0.014 | |
| Stroke | 2.62 (1.31, 5.24) | 0.006 | |
| Timing of operation | |||
| Acute phase | Reference | ||
| Subacute phase | 1.56 (0.92, 2.64) | 0.098 | |
| Chronic phase | 2.16 (1.16, 4.02) | 0.015 | |
As seen in Table 4, a statistically significant interaction between SRC and hypertension on the risk of rupture after TEVAR was found (p = 0.023).
| Outcomes | Hazard ratio (95% CI) | p value | p for interaction* | |
| ARAEs | 0.917 | |||
| Overall | 1.84 (1.13–3.00) | 0.014 | ||
| Normotension | 1.64 (0.55–4.89) | 0.372 | ||
| Hypertension | 1.77 (1.09–2.85) | 0.02 | ||
| Aortic-related mortality | 0.472 | |||
| Overall | 1.74 (0.82–3.69) | 0.147 | ||
| Normotension | 0.7 (0.06–7.75) | 0.773 | ||
| Hypertension | 1.78 (0.79–4.00) | 0.161 | ||
| Rupture | 0.023 | |||
| Overall | 3.13 (1.01–9.72) | 0.048 | ||
| Normotension | 0.92 (0.15–5.51) | 0.926 | ||
| Hypertension | 5.58 (1.25–24.95) | 0.024 | ||
| Endoleak | 0.398 | |||
| Overall | 1.19 (0.43–3.28) | 0.739 | ||
| Normotension | 2.81 (0.26–31.0) | 0.399 | ||
| Hypertension | 0.96 (0.31–2.98) | 0.946 | ||
| Aortic dilation | 0.918 | |||
| Overall | 4.17 (1.39–12.48) | 0.011 | ||
| Normotension | 4.05 (0.42–38.98) | 0.226 | ||
| Hypertension | 4.27 (1.21–15.00) | 0.023 | ||
| New dissection | NA | |||
| Overall | 1.11 (0.07–17.8) | 0.942 | ||
| Normotension | NA | NA | ||
| Hypertension | 1.07 (0.07–17.21) | 0.961 | ||
| Retrograde AD | 0.602 | |||
| Overall | 1.29 (0.53–3.12) | 0.573 | ||
| Normotension | 0.73 (0.07–8.01) | 0.794 | ||
| Hypertension | 1.39 (0.53–3.64) | 0.509 | ||
| *Interactions between SRC and hypertension on outcomes were investigated. | ||||
When patients were stratified according to potential impact factors for ARAEs, no significant interactions with SRC were found for age, hypertension, stroke, operation timing, chimney technique, adjunctive procedure and hybrid approach (Supplementary Table 5).
Although previous studies indicated that SRC is a significant risk factor for BAD patients undergoing TEVAR [4], the relatively short follow-up periods and small sample size in these studies could be a source of selection and observational bias. To our best knowledge, this is the largest and most contemporary report providing comprehensive analyses of SRC-related disparities in baseline characteristics, anatomical patterns, intra-operative details and long-term outcomes of BAD patients after TEVAR.
In our study, BAD patients with SRC were significantly older than those without,
consistent with the literature [13]. In comparison with the general population,
patients with BAD were found to be older [14]. Clinical evidence from the
international registry of acute aortic dissection (IRAD) showed that the mean age
of BAD patients was 63.5 years (the mean age in our study is 57.9 years) [15].
Moreover, we found that the SRC group was significantly older than the non-SRC
group (mean age, 61.1 years vs. 56.3 years; p
A significant gender imbalance was found in SRC patients in our study, with a
male-to-female ratio of 4.8:1. Moreover, female patients were significantly older
than male patients (62.1
In our study, a difference was found in the prevalence of hypertension between the two groups in the total population (Table 1). Consistently, previous clinical studies have shown that SRC is associated with a greater prevalence of hypertension [17]. Although we found that hypertension is not an independent predictor of ARAEs, the subgroup analysis showed that patients with SRC and hypertension led to the highest risk of rupture after TEVAR (Table 4). The attenuation of the power to detect a direct association between hypertension and ARAEs may also result from relatively small sample (n = 214) after matching. Recently, Lu et al. [4] found that lower diastolic blood pressure at admission could predict ARAEs in BAD patients after TEVAR, which emphasized the important role of hemodynamic stability in aortic remodeling. Whether hypertensive patients with SRC suffer from severer blood pressure fluctuation than that without after onset of BAD warrants further study.
Contrary to the previous belief that SRC is a benign disease, an increasing body of evidence suggests that patients with SRC experience a higher risk of long-term ARAEs after TEVAR [3, 4]. What’s more, no difference in 5-year all-cause mortality or cardiovascular events (shown in Supplementary Figs. 5,6) was found between the two groups, implying that SRC might play an exclusive role in the adverse aortic remodeling after TEVAR.
We noticed that the difference of ARAEs between the two groups was mostly accumulated in the first 2 years (Fig. 2). Therefore, a landmark analysis was performed by dividing the 5-year follow-up into the first two years and the remaining three years. Results indicated that during the first two years, patients with SRC were at a higher risk of ARAEs (HR: 1.93, 95% CI: 1.17–3.19, p = 0.009). However, for the remaining three years, the cumulative hazard ratios in the two groups were similar (HR: 1.02, 95% CI: 0.37–2.82, p = 0.97). One explanation for this trend is that the patients initially without SRC could have developed new cysts, increasing the probability of ARAEs at follow-up. Another explanation is that all patients receiving TEVAR could benefit from positive aortic remodeling in the long term, irrespective of the presence of SRC [18]. In any case, further studies should be conducted to identify the role of SRC on long-term patient outcomes.
According to current clinical and laboratory evidence, there are several possible underlying correlations between SRC and BAD.
First, both SRC and BAD are related to increased matrix metalloproteinases (MMPs). It has been established that the structural integrity of the aorta depends on the expression of extracellular matrix (ECM) proteins, which are regulated by proteolytic enzymes. Zhang et al. [19] found that MMP-1, MMP-9, and active MMP-9 levels were higher in aortic dissection tissue than control tissue. Interestingly, MMP overexpression and an elevated ratio of MMPs to tissue inhibitors of metalloproteinases (TIMPs) in aortic tissue could induce the degradation of multiple components in the ECM, making the blood vessel more vulnerable to adverse clinical events [20]. Similarly, Harada et al. [21] found massive accumulation of MMP-2 and MMP-9 in human benign cystic fluids. Obermuller et al. [22] found the upregulation of MMP-14 in a rat model of autosomal-dominant polycystic kidney disease and advocated that TIMPs are promising biomarkers for treating polycystic kidney. In summary, the imbalance of MMPs and TIMPs may persist in patients with SRC and BAD, making them susceptible to ARAEs after TEVAR.
Moreover, our study indicated that the patients with SRC had a greater aortic
calcification prevalence than the non-SRC group in the total population. It has
been established that multiple factors can affect arterial calcification,
including calcium and phosphorus imbalances [23]. Calcium ions are key mediators
that regulate inflammation, apoptosis and calcification in vascular smooth muscle
cells (VSMC) [24]. Consistently, decreased calcium concentration has been
documented in AD patients [25], which could be attributed to calcium loss
resulting from the administration of diuretics for blood pressure control [26].
Interestingly, clinical studies demonstrated that Ca
Overall, the results of our study suggested that SRC may be an independent high-risk predictor of long-term ARAEs in BAD patients after TEVAR. According to our findings, BAD patients with SRC should be followed-up by CTA at 1-month, 1-year and 2-year follow-ups and blood pressure should be closely monitored within two years after TEVAR. Annual CTA follow-up and routine blood pressure monitor should be recommended in patients with SRC, two years after TEVAR.
Due to the retrospective design of our study, it was impossible to randomly allocate the patients.
Compared with the non-SRC group, BAD patients with SRC experienced a higher risk of long-term ARAEs after TEVAR. Subgroup analysis showed that the presence of SRC and hypertension led to the highest risk of rupture after TEVAR.
All authors agreed with the publication of this study.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
AD, aortic dissection; BAD, type B aortic dissection; SRC, simple renal cysts; TEVAR, thoracic endovascular aortic repair; AAD, type A aortic dissection; Retrograde AAD, Retrograde type A aortic dissection; ARAEs, aortic-related adverse events; PSM, propensity score matching; CAD, coronary artery disease; COPD, chronic occlusive pulmonary disease; CKD, chronic kidney disease; MMPs, matrix metalloproteinases; CTA, computed tomographic angiogram; DSA, digital subtraction angiography; IQR, interquartile range; SMD, standardized mean difference; HR, hazard ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; IRAD, international registry of acute aortic dissection; ECM, extracellular matrix; TIMPs, tissue inhibitors of metalloproteinases; VSMC, vascular smooth muscle cells; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ULP, ulcer-like projection.
HQZ—Investigation and Writing. KWZ—Writing and Data curation. GKW—Writing and Providing data. JJL—Providing data. YFP—Editing and Supervision. JZ—Writing, Review, Editing and Supervision. ZPJ—Conceptualization, Project administration. All authors read and approved the final version of the manuscript.
This retrospective study was approved by the Institutional Review Board of Shanghai Changhai Hospital (CHEC-Y2020-042, April 21, 2020). Individual informed consent was waived due to the retrospective design of the study.
Not applicable.
The study, and collection, analysis, interpretation of data, and preparation of the manuscript were supported by the source of funding as follows: the National Natural Science Foundation of China (81870366) and Science and Technology Innovation Action Plan in Shanghai (20JC1418700).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
