1 Clinical College of Thoracic Medicine, Tianjin Medical University, 300070 Tianjin, China
2 Department of Cardiology, Tianjin Chest Hospital, 300222 Tianjin, China
†These authors contributed equally.
Academic Editors: Brian Tomlinson and Takatoshi Kasai
Abstract
Background and Aims: The incidence of diabetes mellitus has reached an
alarming level. Cardiovascular disease (CVD) is the leading cause of mortality in
diabetic patients. However, the association between ratio and survival outcomes
in patients with diabetes mellitus (DM) and new-onset acute coronary syndrome
(ACS) remains unknown. This study aimed to assess the association between the
TG/HDLC ratio and the risk of death in diabetic patients with new-onset acute
coronary syndrome in the Han Chinese population. Methods: Data in this
study were retrospectively collected from January 2016 to December 2016 from
patients with type 2 diabetes mellitus (T2DM) and new-onset ACS in Tianjin Chest
Hospital. Patients were classified according to the baseline TG/HDLC ratio.
Kaplan-Meier survival curves were used to demonstrate survival
outcomes. Univariate and multivariate Cox proportional risk regression analyses
were used to evaluate the hazard ratios and 95% confidence intervals (CIs) for
the risk of death. Subgroup analysis was used to determine the presence of any
interaction. Results: In total, 152 patients died, 98 of them from heart
disease. The Kaplan-Meier survival curve showed that there were no significant
differences for both all-cause and cardiac mortality between Median 1 and Median
2 in log-rank test. Multivariate Cox regression analyses revealed that the
adjusted hazard ratio increased significantly (p
Keywords
- triglycerides to high-density lipoprotein cholesterol
- type 2 diabetes mellitus
- acute coronary syndrome
- all-cause mortality
- cardiac death
- cardiovascular disease
- retrospective
Diabetes mellitus (DM) is a significant health problem. The prevalence of diabetes has constantly increased over the past few decades and has reached an alarming level [1]. The International Diabetes Federation (IDF) Diabetes Atlas 10th edition revealed more than 500 million people worldwide developed DM, and about one in ten adults was affected. Moreover, the number of diabetic patients has increased by 74 million in the last two years, highlighting the alarming increase in the global prevalence of diabetes [1]. The IDF speculated that this number would reach 783 million by 2045, and the proportion of adults with the disease could reach one in eight. Diabetes is also an important driver of global mortality [1]. The IDF also estimated that approximately 6.7 million adults would die from diabetes or its complications in 2021, accounting for more than one-tenth of the all-cause deaths worldwide and one in every five seconds due to diabetes [1].
Type 2 diabetes mellitus may affect more than 600 million people worldwide in the next 20 years [1]. It has a significant impact on survival and quality of life, especially in patients diagnosed at a younger age [1]. Although all complications of diabetes are significant, widespread cardiovascular disease remains the leading cause of morbidity and mortality in this population [2]. These amazing statistics highlight the urgent need for renewed attention to aggressive cardiovascular risk reduction in diabetic patients, especially those already suffering from acute coronary syndrome (ACS).
Although diabetic patients with ACS have high mortality, the relationship between the TG/HDLC ratio and the risk of death in patients with DM and new-onset ACS is unclear. Research on the TG/HDLC ratio has gradually increased, as this lipid parameter is closely related to many diseases. Several previous studies have indicated a positive correlation between the TG/HDLC ratio and hypertension [3, 4], insulin resistance [5, 6], metabolic syndrome [7, 8], and fatty liver [9, 10]. In addition, an elevated TG/HDLC ratio plays an important role in periodontal disease and renal insufficiency. Therefore, we carried out a retrospective cohort study to assess the association between the TG/HDLC ratio and the risk of death in diabetic patients with new-onset ACS.
This is a retrospective cohort study involving patients admitted to Tianjin
Chest Hospital between January 2016 and December 2016. A total of 1782 diabetic
patients with new-onset ACS were enrolled in the study. ACS was subdivided into
either non-ST-segment elevation myocardial infarction (MI), ST-segment elevation
MI, or unstable angina pectoris. Twenty-two patients with incomplete follow-up
data were excluded from the study. Based on a median TG/HDLC ratio, patients were
divided into the following two groups: Median 1 (n = 880, TG/HDLC
Clinical data, including sex, age, smoking status, history of hypertension, ACS
types and duration of diabetes, were collected by trained technicians. Blood
tests included total cholesterol (TC), high-density lipoprotein cholesterol
(HDLC), low-density lipoprotein cholesterol (LDLC), triglycerides (TG), fasting
plasma glucose (FPG), hemoglobinA1c (HbA1c), hypersensitive C-reactive protein
(hs-CRP), troponin T, serum creatinine, and N-terminal pro-brain natriuretic
peptide (NT-proBNP). All blood samples were collected intravenously and analyzed
by the laboratory of Tianjin Chest Hospital using standard automated
technologies. Cardiac ultrasound was used to measure left ventricular cardiac
ejection fraction (LVEF); all the ultrasound reports were from Tianjin Chest
Hospital. The glomerular filtration rate (eGFR) was derived using the MDRD
equation. Body mass index (BMI) was calculated as weight/height
The study’s endpoints included all-cause mortality and cardiac death. All-cause mortality was defined as death from any cause, including cardiac death and any other cause, such as cancerand stroke. Cardiac death was defined as MI, heart failure, and arrhythmia. Investigators were asked to follow up with patients at least once a year for the duration of the study which ended on February 23, 2021, except in the event of the patient’s death.
The Kolmogorov–Smirnov test was used to determine whether the continuous
variables conform to a normal distribution. If normally distributed, it was
expressed as mean
A total of 1760 diabetic patients with new-onset ACS were selected for analysis.
Table 1 summarizes the baseline characteristics of patients in the two groups,
which were based on a median split of the TG/HDLC ratio. Most variables were not
statistically different between groups, including age, sex, smoking,
hypertension, BMI, duration of Diabetes, LVEF, Lipoprotein(a) (Lp(a)), HbA1c,
FPG, eGFR, troponin T, treatment strategies, aspirin, statin,
| Variables | Total | Median 1 | Median 2 | p-value | |
| No. at risk | 1760 | 880 | 880 | ||
| Age, years | 66.0 |
66.1 |
66.0 |
0.774 | |
| Sex | 0.390 | ||||
| Female | 832 (47.3%) | 425 (48.3%) | 407 (46.3%) | ||
| Male | 928 (52.7%) | 455 (51.7%) | 473 (53.8%) | ||
| Smoking | 0.173 | ||||
| ever or current | 706 (40.1%) | 339 (38.5%) | 513 (58.3%) | ||
| never | 1054 (59.9%) | 541 (61.5%) | 367 (41.7%) | ||
| Hypertension | 1354 (76.9%) | 681 (77.4%) | 673 (76.5%) | 0.651 | |
| BMI, kg/m |
25.6 |
25.4 |
25.6 |
0.074 | |
| Duration of diabetes, months | 8.0 (3.0–14.0) | 8.0 (3.0–14.0) | 8.0 (3.0–14.0) | 0.540 | |
| LVEF, % | 60 (56–64) | 60 (56–63) | 60 (56–64) | 0.161 | |
| Laboratory findings | |||||
| TG/HDLC ratio | 2.1 (1.8–2.9) | 1.0 (0.8–1.3) | 2.1 (1.8–2.9) | ||
| TC, mmol/L | 4.3 (3.5–5.0) | 4.5 (3.8–5.3) | 4.3 (3.5–5.0) | ||
| TG, mmol/L | 1.5 (1.1–2.1) | 1.1 (0.9–1.4) | 2.1 (1.7–2.7) | ||
| HDLC, mmol/L | 2.0 (0.9–4.8) | 1.2 (1.0–1.3) | 0.9 (0.8–1.1) | ||
| LDLC, mmol/L | 2.8 (2.1–3.5) | 2.9 (2.3–3.6) | 2.8 (2.1–3.5) | 0.024 | |
| VLDL, mmol/L | 0.5 (0.3–0.6) | 0.4 (0.2–0.5) | 0.5 (0.3–0.6) | ||
| Lp(a), mmol/L | 26.9 (10.7–75.3) | 30.1 (12.9–75.5) | 26.9 (10.7–75.3) | 0.265 | |
| HbA1c, % | 7.4 (6.1–9.3) | 7.3 (6.6–8.3) | 7.3 (6.6–8.3) | 0.475 | |
| FPG, mmol/L | 7.3 (6.1–9.3) | 7.2 (5.9–9.1) | 7.4 (6.1–9.3) | 0.103 | |
| Hcy, µmol/L | 12.7 (10.4–15.9) | 12.5 (10.2–15.9) | 12.8 (10.6–15.8) | 0.142 | |
| eGFR, mL/min | 92.5 |
93.1 |
92.5 |
0.975 | |
| hs-CRP, mg/L | 2.0 (0.9–4.8) | 1.7 (0.7–4.9) | 2.0 (0.9–4.8) | 0.007 | |
| troponin T, |
0.059 (0.025–0.095) | 0.061 (0.026–0.098) | 0.056 (0.023–0.092) | 0.054 | |
| NT-proBNP, pg/mL | 224.5 (99.9–631.6) | 204.4 (89.1–612.0) | 224.5 (99.9–631.6) | 0.049 | |
| Treatment strategies | 0.596 | ||||
| Medication only | 548 (31.3%) | 284 (32.3%) | 264 (30.0%) | ||
| PCI | 1009 (57.2%) | 496 (56.4%) | 513 (58.3%) | ||
| CABG | 203 (11.5%) | 100 (11.4%) | 102 (11.6%) | ||
| ACS types | 0.029 | ||||
| Unstable angina | 1379 (78.4%) | 667 (75.8%) | 712 (80.9%) | ||
| non-STEMI | 162 (9.2%) | 88 (10.0%) | 74 (8.4%) | ||
| STEMI | 219 (12.4%) | 125 (14.2%) | 94 (10.7%) | ||
| Medications at discharge | |||||
| Aspirin | 1697 (96.4%) | 845 (96.0%) | 852 (96.8%) | 0.369 | |
| Statin | 1667 (94.7%) | 836 (95.0%) | 831 (94.4%) | 0.881 | |
| Clopidogrel/Ticagrelor | 1386 (78.8%) | 676 (76.8%) | 710 (807%) | 0.048 | |
| 1129 (64.1%) | 563 (64.0%) | 566 (64.3%) | 0.881 | ||
| ACEI/ARB | 1007 (57.2%) | 506 (57.5%) | 501 (56.9%) | 0.810 | |
| CCB | 491 (27.9%) | 218 (24.8%) | 254 (28.9%) | 0.366 | |
| Nitrate | 905 (54.1%) | 450 (51.1%) | 455 (51.7%) | 0.812 | |
| Insulin | 688 (39.1%) | 342 (38.9%) | 346 (39.3%) | 0.845 | |
| Note: Continuous data are shown as mean standard deviation or median (interquartile range) and categorical data are shown as frequency (%). Abbreviations: BMI, body mass index; LVEF, left ventricle ejection fraction; TC, total cholesterol; TG, triglycerides; HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; TG/HDLC ratio, triglycerides to high-density lipoprotein cholesterol ratio; VLDL, very low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); HbA1c, hemoglobin A1c; Hcy, homocysteine; FPG, fasting plasma glucose; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal pro brain natriuretic peptide; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker. | |||||
Fig. 1 shows the Kaplan-Meier survival curve for the risk of death. Time referred to the interval between admission and the last follow-up visit or patient death. All-cause mortality and cardiac death increased gradually after 50 months and increased almost vertically at approximately 62.5 months. But there were no significant differences for both all-cause and cardiac mortality between Median 1 and Median 2 in log-rank test.
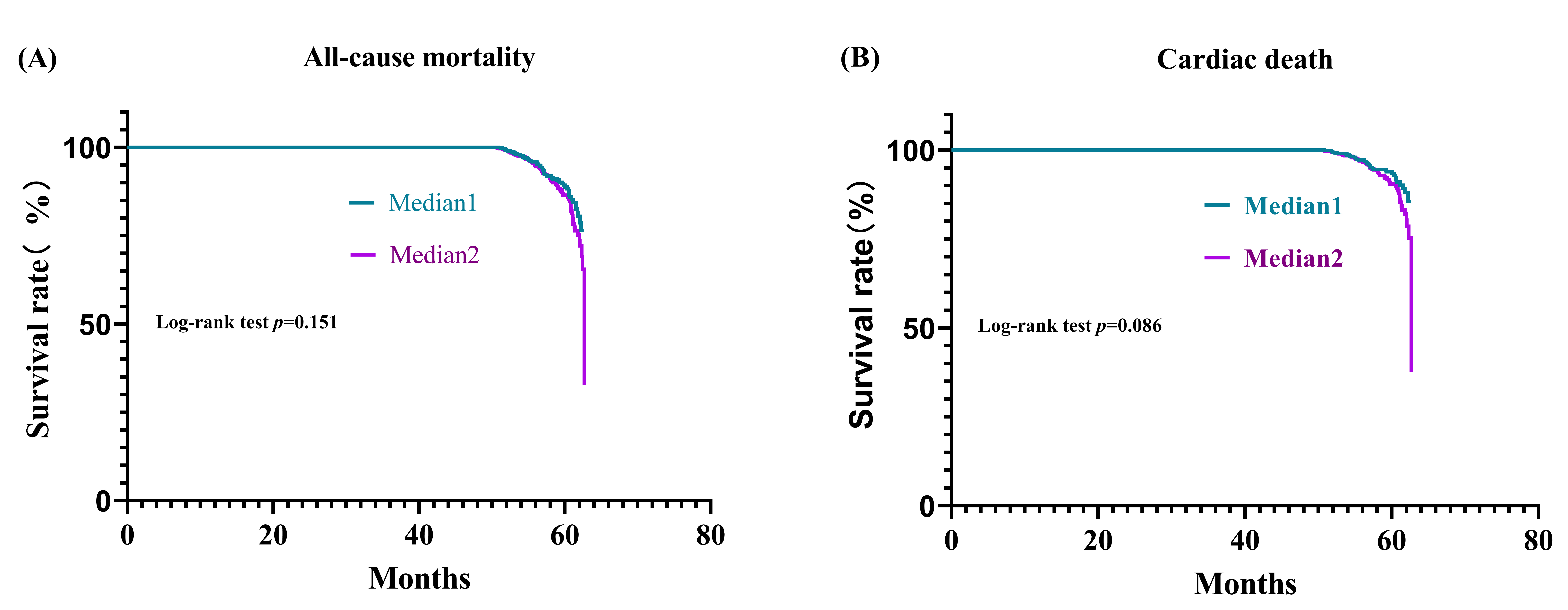 Fig. 1.
Fig. 1.Survival analyses. (A) Kaplan-Meier survival curve for all-cause mortality across TG/HDLC ratio median. (B) Kaplan-Meier survival curve for cardiac mortality across TG/HDLC ratio median.
Table 2 shows the results of the Cox regression analysis. The TG/HDLC ratios
were statistically significant after adjusting for confounders and for all-cause
mortality, cardiac death, nonfatal stroke, fatal stroke, fatal MI and some
non-cardiac death. However, the ratios were not statistically significant for
nonfatal MI, sudden death and major adverse cardiovascular events after adjusting
for confounders. Before adjustment, the risks of all-cause mortality and cardiac
death between the two groups were similar. After adjusting for confounders, an
increase in the TG/HDLC ratio was associated with an increased risk of cardiac
death (p
| Endpoint | Events, n/total (%) | Crude HR (95% CI) | Crude p-value | Adjusted HR (95% CI) | Adjusted p-value | |
| All-cause mortality | 152/1760 (8.6%) | 0.152 | 0.002 | |||
| Median 1 | 68/880 (7.7%) | 1.00 (reference) | 1.00 (reference) | |||
| Median 2 | 84/880 (9.5%) | 1.26 (0.92–1.74) | 1.90 (1.27–2.86) | |||
| Cardiac death | 98/1760 (5.6%) | 0.088 | ||||
| Median 1 | 41/880 (4.7%) | 1.00 (reference) | 1.00 (reference) | |||
| Median 2 | 57/880 (6.5%) | 1.42 (0.95–2.12) | 2.44 (1.45–4.10) | |||
| Nonfatal MI | 77/1760 (4.4%) | 0.714 | 0.513 | |||
| Median 1 | 40/880 (4.5%) | 1.00 (reference) | 1.00 (reference) | |||
| Median 2 | 37/880 (4.2%) | 0.92 (0.58–1.46) | 1.23 (0.67–2.26) | |||
| Nonfatal stroke | 435/1760 (24.7%) | 0.498 | 0.004 | |||
| Median 1 | 227/880 (25.8%) | 1.00 (reference) | 1.00 (reference) | |||
| Median 2 | 208/880 (23.6%) | 0.94 (0.78–1.13) | 1.34 (1.10–1.64) | |||
| Cardiac death plus nonfatal MI or nonfatal stroke | 502/1760 (28.5%) | 0.471 | 0.055 | |||
| Median 1 | 262/880 (29.8%) | 1.00 (reference) | 1.00 (reference) | |||
| Median 2 | 240/880 (27.3%) | 0.94 (0.79–1.12) | 1.20 (1.00–1.44) | |||
| fatal MI | 37/1760 (2.1%) | 0.211 | 0.006 | |||
| Median 1 | 15/880 (1.7%) | 1.00 (reference) | 1.00 (reference) | |||
| Median 2 | 22/880 (2.5%) | 0.92 (0.58–1.46) | 3.22 (1.41–7.40) | |||
| fatal stroke | 15/1760 (8.5%) | 0.013 | 0.013 | |||
| Median 1 | 2/880 (0.2%) | 1.00 (reference) | 1.00 (reference) | |||
| Median 2 | 13/880 (0.5%) | 6.56 (1.48–29.05) | 8.67 (1.58–47.50) | |||
| sudden death | 20/1760 (1.1%) | 0.967 | 0.850 | |||
| Median 1 | 10/880 (1.1%) | 1.00 (reference) | 1.00 (reference) | |||
| Median 2 | 10/880 (1.1%) | 1.02 (0.42–2.45) | 1.13 (0.33–3.88) | |||
| fatal stroke plus fatal MI or sudden death | 72/1760 (4.1%) | 0.028 | 0.004 | |||
| Median 1 | 27/880 (3.1%) | 1.00 (reference) | 1.00 (reference) | |||
| Median 2 | 45/880 (5.1%) | 1.71 (1.06–2.76) | 2.47 (1.34–4.54) | |||
| Note: Abbreviations: HR, hazard ratio; CI, confidential interval; MI, myocardial
infarction. Ajusted variables for all-cause mortality: smoking stauts, age, TG, NT-proBNP, eGFR, Hcy, FPG, LVEF, ACS types. Ajusted variables for cardiac death: smoking stauts, duration of diabetes, age, TG, NT-proBNP, eGFR, Hcy, FPG. Ajusted variables for fatal MI: smoking stauts, age, TG, NT-proBNP, LVEF, Hcy, FPG, troponin T, ACS types. Ajusted variables for fatal stroke: NT-proBNP, eGFR, troponin T, insulin, statin, Clopidogrel/Ticagrelor. Ajusted variables for fatal stroke plus fatal MI or sudden death: smoking stauts, age, TG, NT-proBNP, Hcy, troponin T, insulin, statin, ACS types. | ||||||
Fig. 2 illustrates the results of the subgroup analysis for all-cause and
cardiac mortality. The TG/HDLC ratio was not statistically different in
evaluating all-cause and cardiac death risks regarding age, sex, smoking status,
hypertension, LDLC, and HbA1c (all of p values
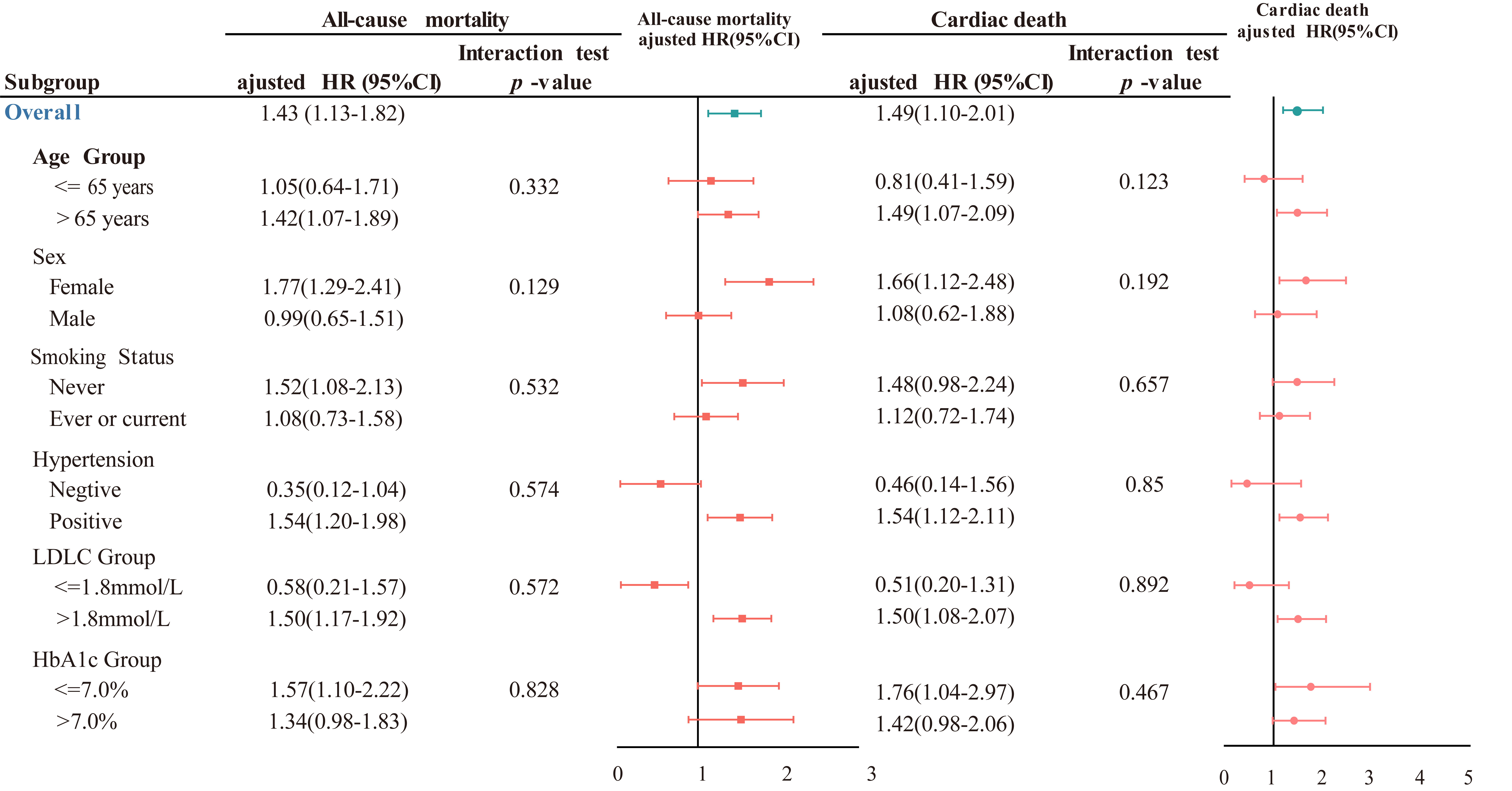 Fig. 2.
Fig. 2.Subgroup analyses. Test the interactions in prespecified subgroups. The p values for interaction in all subgroups were more than 0.05. HR, hazard ratio; CI, confidential interval.
This study analyzed the association between the TG/HDLC ratio and the risks of
all-cause mortality and cardiac death in diabetic patients with new-onset ACS. An
elevated TG/HDLC ratio (TG/HDLC
There is an advantage to the TG/HDLC ratio to assess the risk of death in diabetic patients. High TG is a cardiovascular risk factor and has been associated with all-cause mortality and the incidence of coronary artery disease (CAD) events [11]. Several epidemiological studies have shown a significant relationship between serum HDLC concentration and CAD risk. The typical lipid profile of diabetes was high TG and low HDLC [11]. TG and HDLC were independent of each other, and in the absence of insulin resistance, a single lipid parameter did not reflect the actual status of plasma atherosclerosis and the risk of CAD. However, the TG/HDLC ratio combining both plasma atherosclerosis and CAD could better predict the risk of death in diabetic patients with new-onset ACS. It also appears to be a better indicator for primary and secondary prevention of cardiovascular diseases (CVDs) [12, 13, 14]. A previous study suggested that the TG/HDLC ratio had a better predictive value for mortality than individual lipid parameters [15]. In addition, a high TG/HDLC ratio was a good predictor of the extent of CAD [16, 17]. An elevated TG/HDLC ratio was an independent predictor of the long-term all-cause mortality in patients undergoing coronary angiography and was strongly associated with long-term risk of major adverse cardiovascular events [18]. Therefore, the TG/HDLC ratio assessment is of clinical value in diabetic patients with new-onset ACS.
The TG/HDLC ratio is associated with a residual risk of cardiovascular disease.
In a certain proportion of patients taking oral statins, however, the risk of
cardiovascular disease remains increased despite LDLC compliance. Both remnant
lipoprotein particle cholesterol (RLPC) and LDLC are associated with the risk of
ischemic heart disease (IHD) and MI [19]. A previous study showed that a residual
cholesterol level
The predictive value of the TG/HDLC ratio for cardiovascular events in diabetic patients is controversial. However, insulin resistance (IR) may be responsible for this controversy because it plays a critical role in cardiovascular events in diabetic patients. One study found that high TG and low HDLC levels were significant risk factors for coronary heart disease (CHD) only in the presence of IR [29]. Another study showed that the risk of major cardiovascular events was significantly greater in the presence of IR, regardless of whether triglyceride and HDL cholesterol levels were high or low [30]. Other studies have shown that IR at any level of obesity exacerbated the risk of developing CHD and T2DM [31]. The mechanisms by which insulin resistance promotes cardiovascular events in diabetic patients are as follows. (1) Triglyceride-enriched VLDL particles are hydrolyzed by lipoprotein lipase or hepatic lipase to produce small dense LDLC (sdLDLC) particles [32]; (2) In the presence of IR and high secretion of VLDL particles, these sdLDLC particles are usually present in high concentrations [33]; (3) Whereas sdLDLC particles are highly atherogenic, compared to normal LDL particles, they are more easily oxidized, have a higher affinity for the extracellular matrix, and have a higher degree of retention in the arterial wall [32]. In addition, the smaller the LDL, the less it binds to the LDL receptor, and the longer it resides in the circulation [32].
Summarizing the findings of the previous literatures, we found that the relationship between the TG/HDLC ratio and the risk of death in diabetic patients with new-onset ACS is unclear. Clarifying this relationship is extremely important to assess the prognosis of this high-risk population. This relationship has yet to be established in the published literature. Therefore, to clarify the relationship between the TG/HDL ratio and the risk of death in diabetic patients with new-onset ACS, we used Cox regression analysis and subgroup analysis to explore this relationship. We found that the TG/HDLC ratio was positively associated with the risk of death in diabetic patients with new-onset ACS.
There may be several potential mechanisms for the association between the TG/HDLC ratio and the risk of death in patients with DM and new-onset ACS: (1) Elevated TG level and reduced HDLC play a vital role in the progression of atherosclerosis, which may be related to the TG/HDLC ratio as a marker of LDL particle size [34]. Previous studies have reported that a high TG/HDLC ratio was strongly associated with elevated levels of small, dense LDLC, which is considered to be very atherogenic [35, 36, 37]. (2) The TG/HDLC ratio is significantly associated with insulin resistance in diabetic patients [38, 39, 40]. Furthermore, insulin resistance is associated with increased vulnerability of atherosclerotic plaque rupture resulting in ACS [41]. (3) The TG/HDLC ratio is related to the severity of atherosclerosis because the total plaque area is positively correlated with the TG/HDLC ratio [40]. (4) Hyperglycemia contributes to systemic macrovascular and microvascular disease in diabetic patients, including diabetic nephropathy, CAD, and ischemic stroke, which may be an additional risk for all-cause and cardiac death [42, 43, 44].
Several limitations of this study should be acknowledged: (1) Follow-up
information was collected by telephone or electronic medical records. This
information mainly included survival information. Baseline data after four years
of follow-up were not collected. Because blood lipid levels varied by race, it
was unclear whether these findings also apply to other races. (2) The
complications and severity of new-onset ACS and DM differed, affecting the risks
of all-cause mortality and cardiac death. (3) In this study, we found that
overweight patients account for about half (54.83%), but obese patients account
for smaller proportion (18.01%). The patients (LVEF
Therefore, the overweight patients account for a large proportion, but the obese
patients account for a small proportion. Besides, the patients (LVEF
There are some reasons why non-obese patients develop diabetes as follows: (1) A genetic defect can lead to mitochondrial dysfunction. People with this genetic defect are unable to burn glucose or fatty acids efficiently, which can contribute to lipotoxicity and fat accumulation in muscle cells. (2) Non-alcoholic fatty liver is an independent predictor of type 2 diabetes, and a cause of insulin resistance and type 2 diabetes. (3) Chronic inflammation is an important mechanism that leads to insulin resistance in muscle, liver and fat cells.
As a new lipid-lowering drug, the proprotein convertase subtilisin/Kexin type 9 (PCSK9) inhibitor is gaining attention [45]. Therefore, we intend to study whether PCSK9 inhibitor can affect the TG/HDLC ratio to decreasethe risk of all-cause and cardiac death in diabetic patients with new-onset ACS.
An elevated TG/HDLC ratio (TG/HDLC
The data related to the study findings can be requested from the corresponding author for appropriate reasons.
ACS, acute coronary syndrome; BMI, Body mass index; CAD, coronary artery disease; CVD, cardiovascular disease; CI, confidence interval; DM, diabetes mellitus; T2DM, type 2 diabetes mellitus; EGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, hemoglobinA1c; HR, hazard ratio; hs-CRP, hypersensitive C-reactive protein; IDF, International Diabetes Federation; IHD, ischemic heart disease; Lp(a), Lipoprotein(a); LVEF, left ventricular cardiac ejection fraction; MI, myocardial infarction; NT-proBNP, N-terminal pro brain natriuretic peptide; eGFR, PCSK9, proprotein convertase subtilisin/Kexin type 9; RLPC, remnant lipoprotein particle cholesterol; sdLDLC, small dense LDLC; TC, total cholesterol; TG, triglycerides; HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; VLDL, very low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); TG/HDLC, triglycerides to high-density lipoprotein cholesterol.
HC contributed to the conception and design of the study; LW collected data; DS analyzed data and wrote the manuscript. All authors read and approved the final manuscript.
This study was approved by the Institutional Review Board of the Tianjin Chest Hospital (2022LW-010). Consent to participate is not applicable. Consent to participate is not applicable.
We sincerely appreciate the favors from all of the participants in this study and Tianjin Chest Hospital.
This research was funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJKMDCP2021).
The authors declare no conflict of interest.


