1 School of Chinese Materia Medica, Beijing University of Chinese Medicine, 102488 Beijing, China
2 Department of Cardiovascular Diseases, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, 100053 Beijing, China
Academic Editor: Jerome L. Fleg
Abstract
Background: Homozygous familial hypercholesterolaemia (HoFH) patients have little or no low-density lipoprotein receptor (LDLR) function. HMG-CoA (3-hydroxy-3-methyl glutaryl coenzyme A) reductase inhibitors (statins) and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have limited lipid-lowering effects, therefore, there is an urgent need to develop new HoFH treatments. In 2012, the US Food and Drug Administration (FDA) approved the administration of lomitapide for lowering low-density lipoprotein cholesterol (LDL-C) levels. However, lomitapide is associated with various gastrointestinal disorders, elevated hepatic alanine aminotransferase (ALT) levels and other adverse reactions, thus, its long-term efficacy and safety in pediatrics and adults should be evaluated. A systematic review conducted in 2017 reported the efficacy and safety of lomitapide in Family hypercholesterolaemia (FH) patients. In this systematic review, we elucidate on the efficacy and safety of lomitapide in HoFH patients. Methods: A search was conducted in PubMed, Embase, Web of Science and Cochrane library databases to identify valid studies involving lomitapide-treated HoFH patients published before 11th August 2021. Results: A total of 18 clinical studies involving 120 lomitapide-treated HoFH patients were identified. Lomitapide significantly suppressed LDL-C levels in HoFH patients. Clinical manifestations for lomitapide in children were comparable to those in adults. The most common adverse events were gastrointestinal disturbances and elevated ALT levels. However, most patients tolerated the treatment-associated adverse reactions. Low-fat diets and drug dose adjustments were appropriate measures for controlling the treatment-associated adverse reactions. Conclusions: In pediatric and adult HoFH patients, lomitapide significantly suppresses LDL-C levels, therefore, it is an important option for HoFH treatment. The most common adverse events of lomitapide treatment include gastrointestinal disorders and elevated hepatic ALT levels. Despite the limitations, lomitapide is feasible for long-term treatment of HoFH patients, with dietary and safety monitoring. Registration Number in PROSPERO: CRD42021284425.
Keywords
- homozygous familial hypercholesterolemia (HoFH)
- lomitapide
- systematic review
- efficacy
- safety
Familial hypercholesterolaemia (FH), an autosomal dominant disorder of inherited cholesterol metabolism, was systematically described for the first time in 1937 [1, 2]. Homozygous familial hypercholesterolaemia (HoFH) is divided into simple homozygotes (each allele in the same gene carries the same mutation), compound heterozygotes (mutations on each allele in the same gene are different) and double heterozygotes (very rare, mutations on each allele come from different genes) [3, 4, 5].
In a previous study, 20% of patients were found to be administered with a
combination of lipid lowering therapy (LLT) and lipid-lowering drugs with 2.7%
of the patients had low-density lipoprotein cholesterol (LDL-C) levels below the
target value of 1.8 mmol/L [6]. Clinical incidences of HoFH are between 1/160,000
and 1/320,000 [7]. The mechanisms involved in HoFH occurrence are associated with
loss-of-function mutations of the two alleles of the low-density lipoprotein
receptor (LDLR) gene [8]. Untreated plasma total cholesterol (TC) levels in HoFH
patients are usually greater
Therapeutic options for HoFH include lipid-lowering drugs, lipoprotein plasma exchange, and liver transplantation among other surgical treatments. The mechanisms through which HMG-CoA (3-hydroxy-3-methyl glutaryl coenzyme A) reductase inhibitors (statins) and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors suppress plasma TC levels rely on LDLR [11, 12]. It is difficult for a number of patients with HoFH to achieve the recommended LDL-C level through drug treatment [13].
Lipoprotein apheresis (LA) is the main approach for the treatment of LLT in HoFH patients [14]. However, LDL-C kinetics makes plasma cholesterol levels of patients rebound to baseline levels within 2 weeks [15]. Liver transplantation, which is high risk, is a curative treatment approach for HoFH [16]. Therefore, there is an urgent need for new treatment approaches for HoFH.
The microsomal triglyceride transfer protein (MTP) inhibitor, lomitapide (Juxtapid), which was approved by the US Food and Drug Administration (FDA) in 2012 [17]. However, it does not rely on the expressions of LDLR to reduce LDL-C levels [18]. In addition, the first ANGPTL3 inhibitor, evinacumab, as an adjuvant to other LDL-C reduction therapies for children over 12 years old and adult HoFH patients by FDA in February 2021. MTP is expressed in hepatocytes and intestinal cells where it mediates the triglycerides (TGs) transfer to Apo B particles to form very low-density lipoprotein (VLDL) and chylomicrons. Therefore, inhibition of this reaction reduces VLDL particle foramtion and LDL-C [14]. Experimentally, MTP inhibitors significantly suppressed LDL-C levels in LDLR-deficient Watanabe hereditary hyperlipidemia rabbits (animal models of HoFH) [15, 19]. In adult HoFH patients, lomitapide combined with other LLTs are effective therapeutic options for reducing LDL-C levels, which enabled patients to reach EAS-recommended target levels of LDL-C [20].
Evaluation of the risk profile of lomitapide in clinical trials has confirmed its remarkable efficacy. However, in actual clinical applications and patient management, the benefits and/or risk profiles of lomitapide have not been clearly elucidated. A previous systematic review evaluated the efficacy and safety of lomitapide in hypercholesterolemia. Lomitapide is suitable for improving lipid indices in HoFH patients and severe hypertriglyceridemia recurrent acute pancreatitis [21]. However, the number of included studies and cases in the study was small, while the efficacy and safety of lomitapide in pediatrics were not reported. Moreover, long-term efficacies and safety of lomitapide have not been conclusively determined. Therefore, we elucidate on the long-term efficacy of lomitapide with regards to lipid levels, adverse reactions (gastrointestinal reactions and elevated liver ALT levels) in HoFH patients, as well as on the role of diet in adverse reaction management.
The inclusion criteria for studies in this systematic review were: (i) studies whose main objective was assessing oral lomitapidefor HoFH and (ii) clinical cases, case series, retrospective, or prospective studies.
The exclusion criteria for studies in this systematic were: (i) review-type studies or systematic reviews and (ii) studies whose methodology did not mention positivity to HoFH in study participants.
Independently, two reviewers (GL and SL) performed the literature search in PubMed, Embase, Web of Science and Cochrane Library databases on 11th August 2021. There were no restrictions on publication dates. In case of disagreements between the two reviewers, a third reviewer (NW) was contacted to make the final decision. Key search words were: (‘lomitapide’ OR ‘Juxtapid’ OR ‘AEGR 733’ OR ‘BMS 201038’) AND (Hypercholesterolemia OR Hypercholesterolemias OR High Cholesterol Levels OR Cholesterol Level, High OR Cholesterol Levels, High OR High Cholesterol Level OR Level, High Cholesterol OR Levels, High Cholesterol OR Elevated Cholesterol OR Cholesterol, Elevated OR Cholesterols, Elevated OR Elevated Cholesterols OR Hypercholesteremia OR Hypercholesteremias). The PubMed search strategy is shown in Supplementary Table 1.
For study selection, the titles and abstracts, were filtered to identify the keywords used in the search strategy. Selected studies were placed in a Document Management software (EndNote) to identify duplicate studies. Last, full texts were reviewed to identify studies that met the inclusion criteria. Study selection was independently performed by two reviewers (GL and SL); In case of disagreements between the two reviewers, a third reviewer (NW) was contacted to make the final decision.
Data on patient characteristics, including age/age range, number of participants and gender, baseline LDL-C levels, HoFH Type, xanthoma, cardiovascular diseases (CVD) events, and disease severity, as well as on lipid-lowering program after lomitapide treatment from the included studies were extracted by two reviewers. Treatment modalities and efficacies of lomitapide therapy, including LDL-C levels before lomitapide administration, LDL-C levels during lomitapide administration, whether it was discontinued, safety, and management of adverse reactions were also recorded.
Methodological indices for non-randomized studies (MINORS) tool was used to access the quality of single-arm studies [22]. The Joanna Briggs Institute (JBI) Checklists were used to evaluate the quality of retrospective case series and case reports [23].
Efficacy outcomes included changes in LDL-C levels after treatment, compared to baseline levels and lowest LDL-C levels after treatment. Safety outcomes included gastrointestinal symptoms, abnormally elevated liver transaminase levels, and adverse reaction management.
A total of 489 articles met the initial inclusion criteria. After eliminating duplicates and screening with the exclusion criteria, data analysis was performed on 18 studies with 120 patients. Studies (2 single-arm studies, 2 retrospective case series and 14 case reports) reporting on the changes in lipid levels after lomitapide treatment were selected for analysis. Fifteen studies involving 106 patients reported on adult HoFH [19, 21, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36]. Moreover, 4 studies involved children (14) as study participants [33, 37, 38, 39]. One study included adults and minors as participants [33]. One study did not define the specific age for each patient, and only reported the overall age range: 8–62 years [25]. The study selection processes in this systematic review is presented in Fig. 1 while baseline characteristics of patients are presented in Table 1 (Ref. [19, 21, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39]).
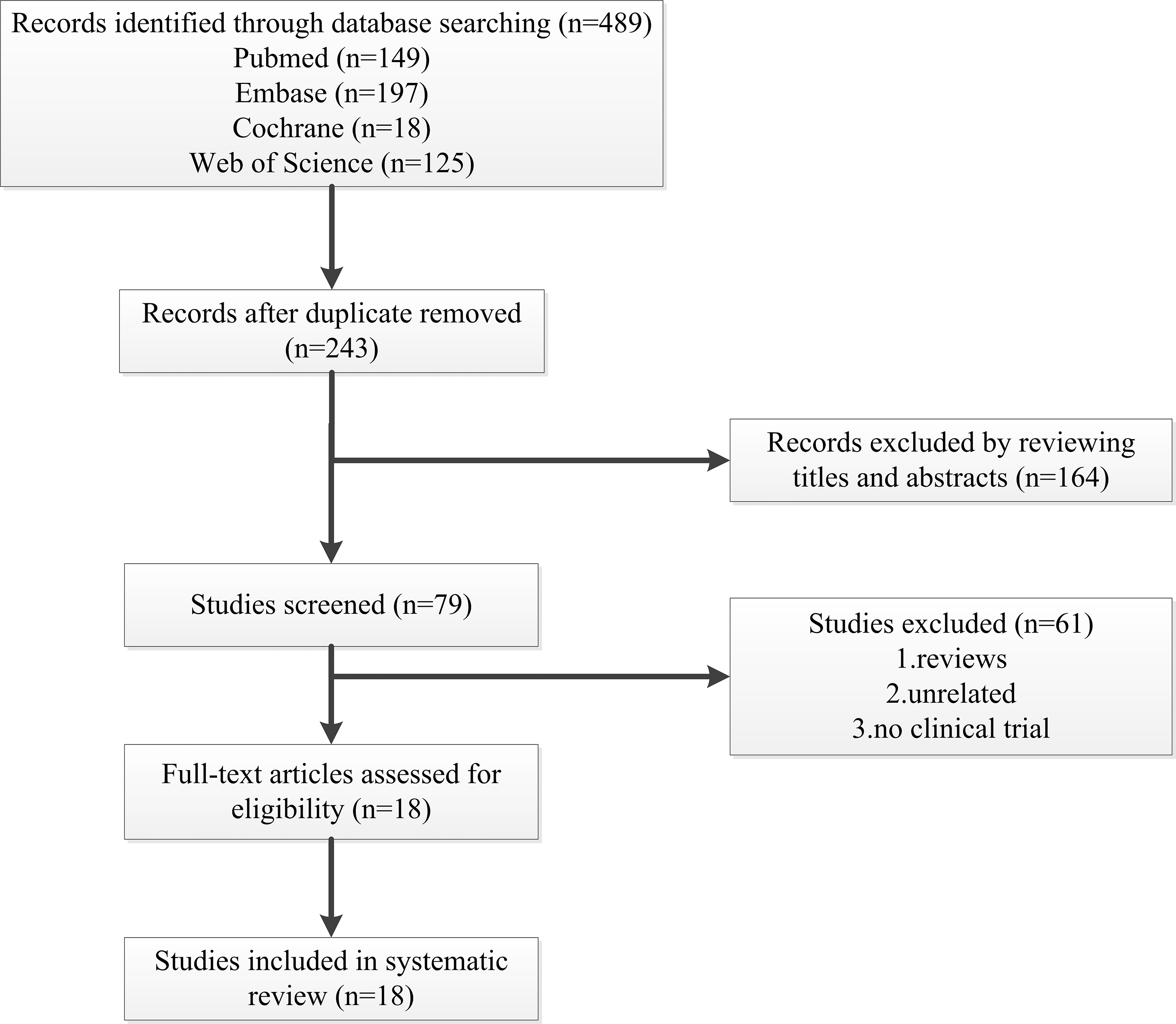 Fig. 1.
Fig. 1.Flow diagram of studies selected in the present systematic review.
| Study and study type | Total patients (N) | Patient number | Gender | Age (yr) | Diagnosis | Xanthomas | CVD events | |
| Single-arm studies (2) | ||||||||
| Harada-Shiba et al. [26] | 9 | 1 | Female | 40 | HoFH (4); HeFH (5) | Nm | Nm | |
| 2 | Male | 46 | + | Nm | ||||
| 3 | Male | 33 | Nm | Nm | ||||
| 4 | Female | 52 | Nm | Nm | ||||
| 5 | Male | 43 | + | Nm | ||||
| 6 | Female | 35 | Nm | Nm | ||||
| 7 | Male | 75 | Nm | Nm | ||||
| 8 | Female | 63 | Nm | Nm | ||||
| 9 | Male | 66 | Nm | Nm | ||||
| Cuchel, et al. [21] | 29 | Nm | HoFH | Nm | Nm | |||
| Retrospective case series (2) | ||||||||
| Aljenedil et al. [28] | 12 | 1 | Male | 36 | HoFH | Nm | Unstable angina; PTCA; PTCA stent | |
| 2 | Male | 57 | HoFH | Nm | Peripheral vascular disease; left CEA; right CEA; CABG; Aortic valve replacement and aortic root replacement; STEMI aorto-coronary bypass; CABG | |||
| 3 | Male | 22 | HeFH | Nm | Aortic valve replacement; Aortic valve prothesis | |||
| 4 | Female | 30 | HeFH | Nm | Aortic valve replacement; Aortic valve prothesis | |||
| 5 | Female | 34 | HeFH | Nm | Nm | |||
| 6 | Female | 49 | HoFH | Nm | CABG | |||
| 7 | Male | 48 | HeFH | Nm | CABG; portacaval shunt | |||
| 8 | Male | 36 | HoFH | Nm | CAD | |||
| 9 | Male | 39 | HoFH | Nm | None | |||
| 10 | Female | 83 | HoFH | Nm | None | |||
| 11 | Female | 70 | HoFH | Nm | None | |||
| 12 | Male | 29 | HeFH | Nm | None | |||
| D’Erasmo et al. [27] | 15 | 1 | Female | 43 | ARH | 13/15 (+) | CHD (5); aortic valve stenosis (6) | |
| 2 | Male | 47 | ARH | |||||
| 3 | Female | 38 | ARH | |||||
| 4 | Female | 48 | ARH | |||||
| 5 | Female | 19 | HoFH | |||||
| 6 | Female | 23 | HoFH | |||||
| 7 | Female | 34 | ARH | |||||
| 8 | Male | 29 | HoFH | |||||
| 9 | Female | 25 | HoFH | |||||
| 10 | Male | 38 | HoFH | |||||
| 11 | Female | 67 | HoFH | |||||
| 12 | Male | 49 | HoFH | |||||
| 13 | Male | 52 | HoFH | |||||
| 14 | Male | 32 | HoFH | |||||
| 15 | Female | 20 | HoFH | |||||
| Case reports (14) | ||||||||
| Ben-Omran et al. [37] | 11 | 1 | Female | 13 | HoFH | Nm | Aortic root plaque | |
| 2 | Male | 12 | HoFH | Nm | Left ventricle dilatation; Mild aortic regurgitation and atherosclerotic plaques in both carotid bulbs, and in the common and internal carotid arteries | |||
| 3 | Male | 16 | HoFH | + | Nm | |||
| 4 | Male | 7 | HeFH | Nm | Aortic plaque | |||
| 5 | Female | 11 | HoFH | Nm | Non-critical aortic stenosis/supra-aortic stenosis, and non-obstructive plaques in the carotid arteries | |||
| 6 | Male | 16 | HeFH | + | Carotid plaques occluding 25–30% of the carotid lumina | |||
| 7 | Female | 3 | HoFH | Nm | Mild aortic thickening; Mild aortic valve regurgitation | |||
| 8 | Male | 14 | HeFH | Nm | Mild aortic regurgitation; Bentall procedure | |||
| 9 | Male | 15 | HoFH | + | None | |||
| 10 | Female | 8 | HoFH | + | Supra-aortic stenosis; Mild tricuspid regurgitation | |||
| 11 | Male | 8 | HoFH | Nm | Aortic insufficiency; Focal intimal thickening; thickened tricuspid aortic valve leaflets | |||
| Yahya et al. [30] | 2 | 1 | Male | 25 | HeFH | + | Stable moderate aortic valve stenosis; Mild to moderate insufficiency | |
| 2 | Female | 23 | HeFH | – | Stable moderate aortic valve stenosis; Mild insufficiency | |||
| Yahya et al. [29] | 4 | 1 | Female | 29 | HoFH | + | The details are not clear | |
| 2 | Female | 20 | HoFH | + | None | |||
| 3 | Male | 36 | HoFH | + | The details are not clear | |||
| 4 | Female | 62 | HoFH | + | The details are not clear | |||
| Sperlongano et al. [31] | 2 | 1 | Male | 62 | HoFH | + | Premature CHD; CABG | |
| 2 | Female | 52 | HoFH | – | AF and atherosclerosis; CHD | |||
| Roeters van Lennep et al. [32] | 4 | 1 | Female | 20 | HoFH | Nm | Nm | |
| 2 | Female | 62 | HoFH | Nm | PCI; 4 stents implanted; | |||
| 3 | Male | 42 | HeFH | Nm | PCI; Aortic valve replacement | |||
| 4 | Female | 36 | HoFH | Nm | 2 CABG and mechanical; Aortic valve replacement | |||
| Raper et al. [19] | 1 | 1 | Female | 49 | HoFH | + | Premature CAD; AF | |
| Mahzari and Zarif et al. [33] | 2 | 1 | Male | 17 | HoFH | + | CAD | |
| 2 | Female | 26 | HoFH | + | Severe aortic stenosis | |||
| Littmann et al. [24] | 1 | 1 | Male | 26 | HoFH | + | Mild to moderate central aortic insufficiency | |
| Kolovou et al. [38] | 1 | 1 | Female | 8 | HoFH | + | Stenotic aortic valve | |
| Suppressa et al. [36] | 1 | 1 | Female | 28 | HoFH | + | ACS; Moderate valvular insufficiency; Intimal thickening and calcified plaques in both carotid arteries | |
| Cuchel et al. [35] | 6 | 1 | Female | 18 | HoFH | Nm | Absent (4); Present (2) | |
| 2 | Male | 18 | HoFH | Nm | ||||
| 3 | Female | 35 | HoFH | Nm | ||||
| 4 | Male | 40 | HoFH | Nm | ||||
| 5 | Male | 22 | HoFH | Nm | ||||
| 6 | 8M/4F | 21 | HoFH | Nm | ||||
| Kolovou et al. [25] | 12 | Male | 8–62 | HoFH | + | ASCVD | ||
| Stefanutti et al. [34] | 7 | 1 | Female | 32 | HoFH | + | Slight aortic valve disease | |
| 2 | Female | 24 | HoFH | + | CAD+ aortic valve disease; bypass 2009; Aortic and mitral valves replaced 2009 | |||
| 3 | Male | 24 | HoFH | + | Slight aortic valve disease | |||
| 4 | Female | 25 | HoFH | + | Slight aortic valve disease | |||
| 5 | Female | 26 | HoFH | + | Moderate aortic valve disease | |||
| 6 | Female | 30 | HeFH | + | Slight aortic valve disease | |||
| 7 | Female | 28 | HeFH | + | Moderate aortic valve disease | |||
| Chacra et al. [39] | 1 | 1 | Female | HoFH | + | Atherosclerotic carotid; Aortic valve disease | ||
| Nm, Not mentioned; HoFH, Homozygous familial hypercholesterolaemia; HeFH, Heterozygous familial hypercholesterolaemia; ARH, Autosomal recessive hypercholesterolemia; PTCA, Percutaneous transluminal coronary angioplasty; CEA, Carotid endarterectomy; CABG, Coronary artery bypass graft surgery; STEMI, ST-elevation myocardial infarction; CAD, Coronary artery disease; CHD, Coronary heart disease; PCI, Percutaneous coronary intervention; ACS, acute coronary syndrome; AF, atrial fibrillation; ASCVD, Atherosclerotic cardiovascular disease. | ||||||||
Quality assessment of the 2 single-arm studies revealed that the first 7 cases were reported and comprehensive in both studies (Table 2, Ref. [21, 26]). Quality assessment of the 2 retrospective case series showed that 7 cases were highly suitable for all the 10 questions (Figs. 2,3).
| A clearly stated aim | Inclusion of consecutive patients | Prospective data collection | Endpoints appropriate to the aim of the study | Unbiased assessment of study endpoint | Follow-up period appropriate to the study aim | Loss to follow up less than 5% | Prospective calculation of the study size | |
| Harada-Shiba et al. [26] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Cuchel et al. [21] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Items are scored 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate). The global ideal score non-comparative studies is 16. | ||||||||
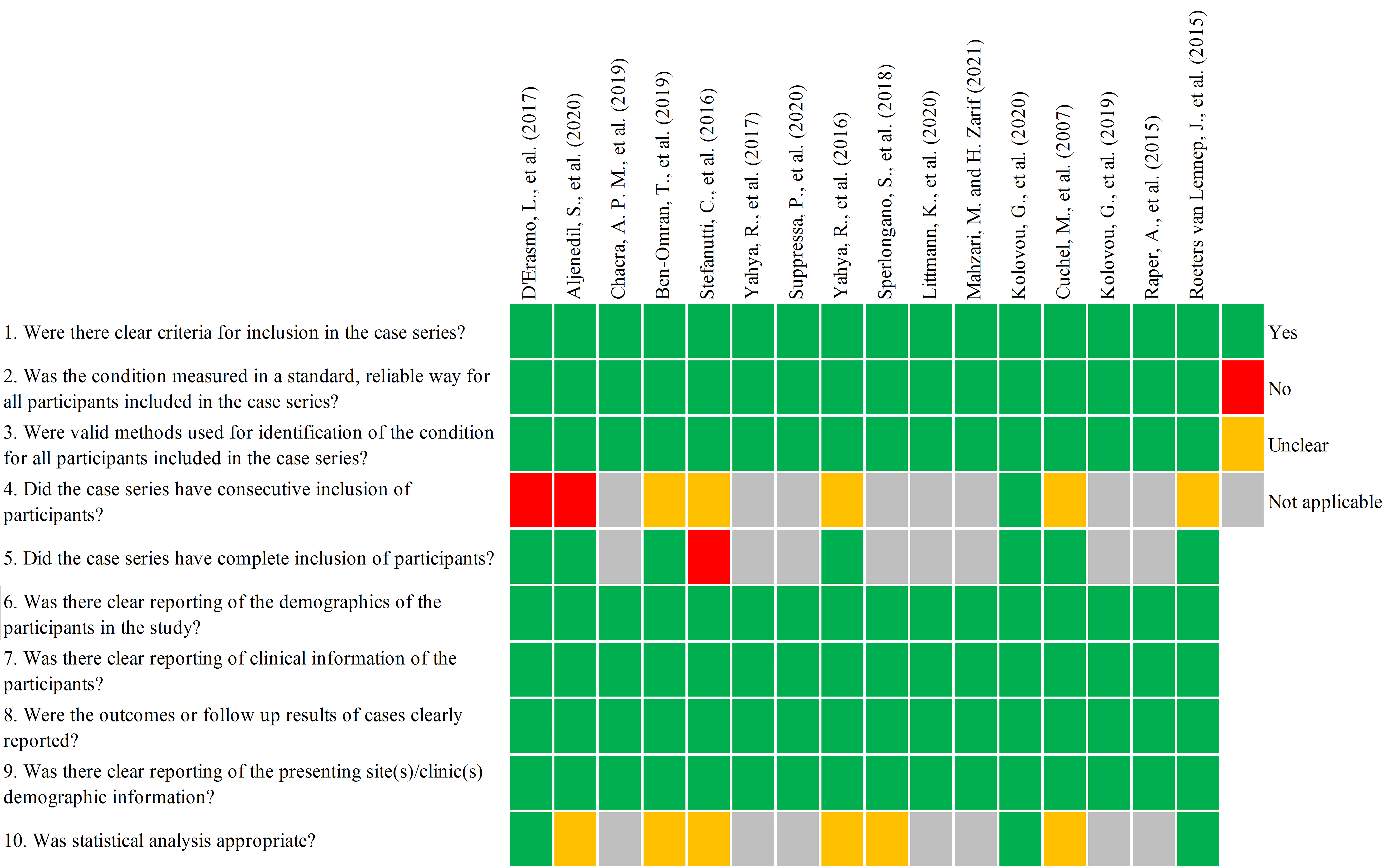 Fig. 2.
Fig. 2.Individual quality assessment of case series according to the JBI Checklist. Green, yes; red, no; orange, unclear; grey, not applicable.
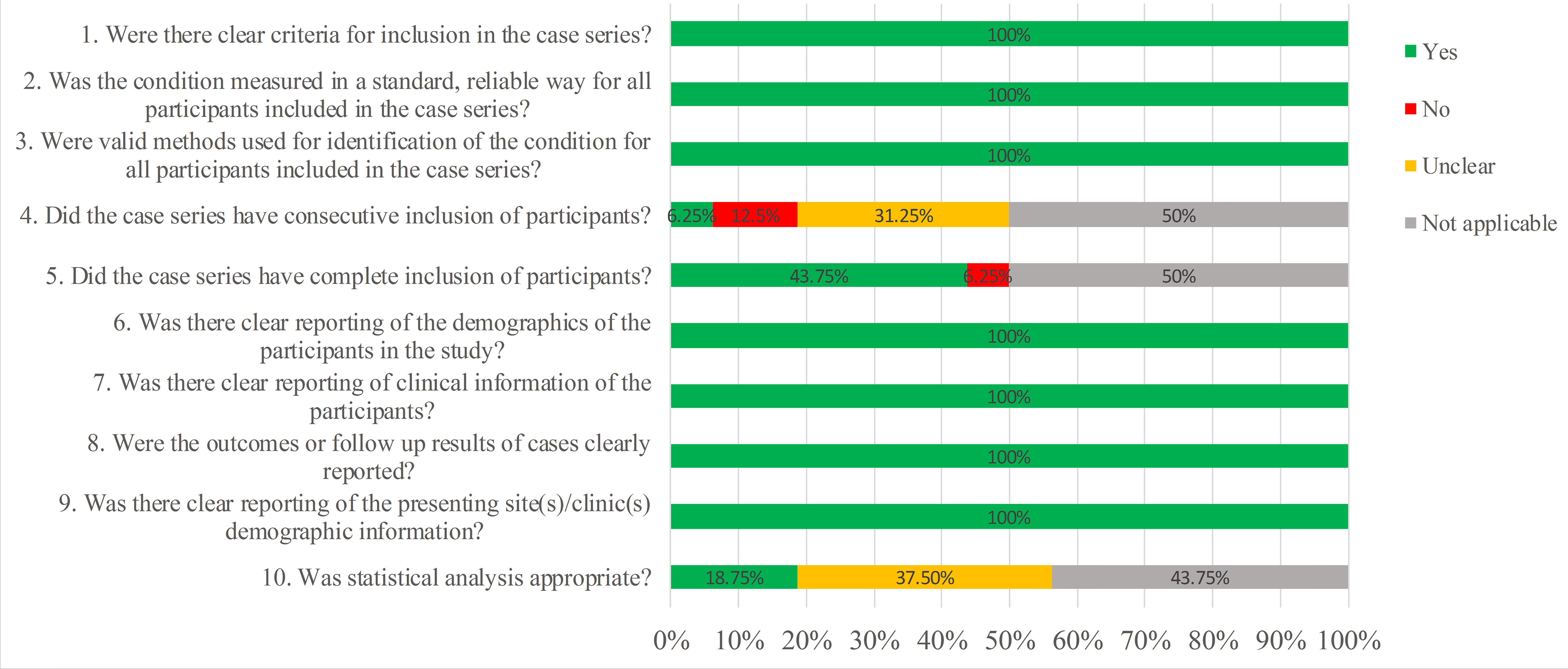 Fig. 3.
Fig. 3.Overall quality assessment of case series according to the JBI Checklist.
Results on the analysis of LDL-C, current LLT regimens, duration of lomitapide treatment, and whether lomitapide treatment was discontinued are presented in Table 3 (Ref. [19, 21, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39]). Lipid-lowering effects of lomitapide on HoFH were explored. CVD complications, lomitapide-related adverse events (AEs) were evaluated as safety outcomes (Table 4, Ref. [19, 21, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39]).
| Study and study type | Patient number | Baseline LDL-C (mmol/L) | Current LLT | Duration of lomitapide treatment | Discountinuation lomitapide treatment (Yes/No) | LDL-C prior to lonitapide | LDL-C at nadir (mmol/L) | LDL-C decrease (%) | |
| Single-arm studies (2) | |||||||||
| Harada-Shiba et al. [26] | 1 | 5.15 | rosuvastatin + ezetimibe + LA | 56 w | No | 5.15 | 2.98 | 42% | |
| 2 | 4.74 | rosuvastatin + ezetimibe + colestilan | 56 w | No | 4.74 | 4.92 | –4% | ||
| 3 | 8.57 | ethyl eicosapentaenoic acid + LA | 22 w | Yes | 8.57 | 3.57 | 58% | ||
| 4 | 6.71 | ezetimibe + ethyl eicosapentaenoic acid + LA + lomitapide | 56 w | No | 6.71 | 0.31 | 95% | ||
| 5 | 5.18 | atorvastatin + ezetimibe + LA + lomitapide | 56 w | No | 5.18 | 1.40 | 73% | ||
| 6 | 5.72 | atorvastatin + ezetimibe + probucol + LA + lomitapide | 56 w | No | 5.72 | 2.15 | 62% | ||
| 7 | 3.13 | atorvastatin + lomitapide | 56 w | No | 3.13 | 1.04 | 67% | ||
| 8 | 3.47 | atorvastatin + ezetimibe + lomitapide | 56 w | No | 3.47 | 1.37 | 61% | ||
| 9 | 3.81 | rosuvastatin + ezetimibe + colestilan + LA | 56 w | No | 3.81 | 3.39 | 11% | ||
| Cuchel et al. [21] | 8.70 | statins (27) + ezetimibe (22) + niacin (3) + fibrate (1) + bile acid sequestrant (1) + LA (18) + lompitade (23) | 26 w; 56 w; 78 w | 7/29 discontinued | 8.70 | Nm | Nm | ||
| Retrospective case series (2) | |||||||||
| Aljenedil et al. [28] | 1 | 9.20 | atorvastatin + ezetimibe + LA | 37.5 m | Yes | 4.90 | 3.90 | 58% | |
| 2 | 18.40 | rosuvastatin + ezetimibe + evolocumab + LA | 20 m | Yes | 8.10 | 7.30 | 60% | ||
| 3 | 20.00 | rosuvastatin + ezetimibe + LA + lomitapide | 25 m | No | 5.70 | 3.80 | 81% | ||
| 4 | 19.00 | atorvastatin + ezetimibe + evolocumab + LA | 4 m | Yes | 7.30 | 6.30 | 67% | ||
| 5 | 10.90 | atorvastatin + LA | 11.5 m | Yes | 11.60 | 10.70 | 2% | ||
| 6 | 21.30 | atorvastatin + ezetimibe + lomitapide | 117 m | No | 15.00 | 4.60 | 78% | ||
| 7 | 10.60 | atorvastatin + ezetimibe + evolocumab + lomitapide | 124 m | No | 7.10 | 2.30 | 78% | ||
| 8 | 10.40 | rosuvastatin + ezetimibe + lomitapide | 41 m | No | 12.30 | 3.00 | 71% | ||
| 9 | 13.90 | rosuvastatin + ezetimibe + lomitapide | 38 m | No | 10.20 | 4.80 | 65% | ||
| 10 | 18.80 | rosuvastatin + ezetimibe + alirocumab + lomitapide | 15 m | No | 11.40 | 7.50 | 60% | ||
| 11 | 11.10 | rosuvastatin + ezetimibe + alirocumab + lomitapide | 29 m | No | 7.50 | 4.60 | 59% | ||
| 12 | 10.20 | rosuvastatin + ezetimibe + fenofibrate + evolocumab + lomitapide | 8 m | No | 7.80 | 5.60 | 45% | ||
| D’Erasmo et al., (2017) [27] | 1 | 12.76* | background therapies + lompitade* | No | 7.99 | 3.42* | 73%* | ||
| 2 | No | 6.06 | |||||||
| 3 | No | 16.06 | |||||||
| 4 | No | 13.16 | |||||||
| 5 | No | 12.17 | |||||||
| 6 | No | 4.35 | |||||||
| 7 | No | 6.92 | |||||||
| 8 | No | 21.83 | |||||||
| 9 | No | 14.27 | |||||||
| 10 | No | 18.80 | |||||||
| 11 | No | 6.27 | |||||||
| 12 | No | 6.89 | |||||||
| 13 | No | 5.49 | |||||||
| 14 | No | 11.89 | |||||||
| 15 | No | 13.36 | |||||||
| Case reports (14) | |||||||||
| Ben-Omran et al. [37] | 1 | 10.85* | atorvastatin + lomitapide | 16 m | No | 7.74 | 1.45 | Nm | |
| 2 | rosuvastatin + ezetimibe + lomitapide + LA | 15 m | No | 8.44 | 2.41 | Nm | |||
| 3 | lomitapide + LA | 20 m | No | 4.84 | 1.89 | Nm | |||
| 4 | rosuvastatin + lomitapide | 15 m | No | 21.58 | 12.07 | Nm | |||
| 5 | atorvastatin + ezetimibe + lomitapide | 48 m | No | 11.47 | 5.98 | Nm | |||
| 6 | rosuvastatin + ezetimibe + lompitade | 15 m | No | 6.29 | 0.60 | Nm | |||
| 7 | atorvastatin + ezetimibe + lomitapide | 12 m | No | 16.81 | 6.11 | Nm | |||
| 8 | atorvastatin + ezetimibe + lomitapide | 22 m | No | 5.78 | 1.94 | Nm | |||
| 9 | atorvastatin + ezetimibe + lomitapide + LA | 18 m | No | 2.1 | 1.61 | Nm | |||
| 10 | atorvastatin + ezetimibe + lomitapide | 19 m | No | 16.32 | 11.42 | Nm | |||
| 11 | atorvastatin + ezetimibe + lomitapide | 19 m | No | 18.26 | 11.91 | Nm | |||
| Yahya et al., (2017) [30] | 1 | 19.60 | atorvastatin + ezetimibe + lomitapide | 5 y | No | 9.00 | 1.71 | 91% | |
| 2 | 17.80 | atorvastatin + lomitapide | 3 y | No | 8.80 | 0.75 | 96% | ||
| Yahya et al., (2016) [29] | 1 | Nm | atorvastatin + ezetimibe + lomitapide | 9.5 w | Yes | 14.50 | 2.40 | Nm | |
| 2 | Nm | atorvastatin + cholestagel + lomitapide | 36.5 w | No | 14.10 | 0.77 | Nm | ||
| 3 | Nm | simvastatin + ezetimibe + lomitapide | 9 w | No | 3.90 | 4.50 | Nm | ||
| 4 | Nm | questran + modalim +lomitapide | 9 w | Yes | 12.90 | 2.00 | Nm | ||
| Sperlongano et al. [31] | 1 | 7.64 | background therapies + lompitade | 52 w | No | 7.64 | Nm | Nm | |
| 2 | 5.49 | rosuvastatin + ezetimibe + LA + lomitapide | 55 w | No | 2.77 | Nm | Nm | ||
| Roeters van Lennep et al. [32] | 1 | 14.11 | atorvastatin + lomitapide | 50 w | No | 14.11 | 2.40 | 83% | |
| 2 | 10.35 | lomitapide (stopped permanently) | 44 w | Yes | 10.35 | 0.77 | 93% | ||
| 3 | 7.16 | lomitapide + rosuvastatin + ezetimibe + colesevelam | 20 w | No | 7.16 | 4.50 | 37% | ||
| 4 | 1.30 | LA + simvastatin + ezetimibe + Lomitapide | 24 w | No | 7.30 | 2.00 | –54% | ||
| Raper et al. [19] | 1 | 5.78 | lomitapide + rosuvastatin + ezetimibe | No | 16.50 | 0.73 | 87% | ||
| Mahzari. And Zarif et al. [33] | 1 | 16.50 | Lomitapide (stopped) + rosuvastatin + ezetimibe + evolocumab | Yes | 13.3 | 2.20 | 87% | ||
| 2 | 15.30 | rosuvastatin + ezetimibe + lomitapide (the patient died) | 3 m–1 y | No | 15.30 | 6.90 | 55% | ||
| Littmann et al. [24] | 1 | 18.50 | lomitapide + LA | No | 3–4 | Nm | Nm | ||
| Kolovou et al. [38] | 1 | 26.00 | lomitapide + rosuvastatin + ezetimibe + colesevelam | 2 y | No | 26.00 | 10.00 | 62% | |
| Suppressa et al. [36] | 1 | 7.77 | rosuvastatin + ezetimibe + lomitapide | 2 y | No | 14.04 | 1.17 | 85% | |
| Cuchel et al. [35] | 1 | 12.43 | Nm | 16 w | No | Nm | 5.80 | 53% | |
| 2 | 20.44 | Nm | 16 w | No | Nm | 9.92 | 51% | ||
| 3 | 15.77 | Nm | 16 w | No | Nm | 10.44 | 34% | ||
| 4 | 16.50 | Nm | 16 w | No | Nm | 7.80 | 53% | ||
| 5 | 13.83 | Nm | 16 w | No | Nm | 5.21 | 62% | ||
| 6 | 16.47 | Nm | 16 w | No | Nm | 7.93 | 52% | ||
| Kolovou et al. [25] | 23.31* | LL drugs + lomitapide + LA (9/12) | 3–24 m* | 2/12 stopped* | 7.46* | 1.81* | 92% | ||
| Stefanutti et al. [34] | 1 | Nm | LA + lomitapide | Nm | No | Nm | 1.27 | Nm | |
| 2 | Nm | LA + lomitapide | Nm | No | Nm | 1.92 | Nm | ||
| 3 | Nm | Lomitapide + LA | Nm | No | Nm | 3.89 | Nm | ||
| 4 | Nm | LA + lomitapide | Nm | No | Nm | 3.89 | Nm | ||
| 5 | Nm | LA + atorvastatin + ezetimibe + lomitapide | Nm | No | Nm | 2.62 | Nm | ||
| 6 | Nm | lomitapide + LA | Nm | No | Nm | 1.61 | Nm | ||
| 7 | Nm | lomitapide + LA | Nm | No | Nm | 3.26 | Nm | ||
| Chacra et al. [39] | 1 | 26.13 | atorvastatin + ezetimibe + lomitapide | 49 m | No | 11.09 | 5.98 | 77% | |
| Nm, Not mentioned; *, Represents the level or protocol of the study; LLT, Lipid
lowering therapy; LDL-C, Low-Density Lipoprotein Cholesterol; LA, Lipoprotein
apheresis; w, Week; m, Month; y, Year; LDL-C decrease (%) = (Baseline LDL-C –
LDL-C at nadir)/Baseline LDL-C | |||||||||
| Study and study type | Total patients (N) | Patient number | AEs | Notes on AEs management | |
| Single-arm studies (2) | |||||
| Harada-Shiba et al. [26] | 9 | GIs (8); Increased hepatic enzymes (3) | Reducing the dose or discontinuation of lomitapide treatment | ||
| Cuchel et al. [21] | 29 | GIs (27); Increased hepatic enzymes (4); ACS and AP and LRTI (1); Elective hysterectomy for menorrhagia (1); Chest pain (1) | Reducing the dose or temporary interruption of treatment | ||
| Retrospective case series (2) | |||||
| Aljenedil et al. [28] | 12 | 1 | Increased hepatic enzymes | Reducing the dose then discontinuation | |
| 2 | Noncompliance; GIs | Discontinuation | |||
| 3 | Diarrhea | Dose adjustment | |||
| 4 | Moderate diarrhea | Discontinuation | |||
| 5 | Noncompliance; Moderate diarrhea | Discontinuation | |||
| 6 | Increased hepatic enzymes; Moderate nausea and diarrhea | Dose adjustment | |||
| 7 | Moderate diarrhea and nausea | Dose adjustment | |||
| 8 | None | None | |||
| 9 | Moderate diarrhea only upon early | No drug adjustment | |||
| 10 | Moderate vomiting and diarrhea | Dose adjustment then discontinuation | |||
| 11 | Moderate nausea and diarrhea | None | |||
| 12 | Tired 3 days after starting lomitapide; normalized after; Rare abdominal discomfort | None | |||
| D’Erasmo et al. [27] | 15 | GIs | Dietary modifications; Dose adjustment; Antidiarrheic medications | ||
| Case reports (14) | |||||
| Ben-Omran et al. [37] | 11 | 1 | Mild GIs | Can tolerate | |
| 2 | Nm | Nm | |||
| 3 | Mild GIs | Can tolerate | |||
| 4 | None | None | |||
| 5 | Mild GIs | Dietary modifications; Adjusted dosage | |||
| 6 | Mild GIs; Increased hepatic enzymes | Adjusted dosage | |||
| 7 | None | None | |||
| 8 | Increased hepatic enzymes | Adjusted dosage | |||
| 9 | None | None | |||
| 10 | None | None | |||
| 11 | None | None | |||
| Yahya et al. [30] | 2 | 1 | None | None | |
| 2 | None | None | |||
| Yahya et al. [29] | 4 | 1 | GIs | Dietary modifications | |
| 2 | GIs | Dietary modifications | |||
| 3 | GIs | Dietary modifications | |||
| 4 | GIs; Increased hepatic enzymes | Dietary modifications; Stopped | |||
| Sperlongano et al. [31] | 2 | 1 | Mild GIs | Dietary modifications; Antidiarrheic medications | |
| 2 | Mild GIs | Dietary modifications; Adjust the dosage | |||
| Roeters van Lennep et al. [32] | 4 | 1 | Mild GIs | Dietary modifications | |
| 2 | Mild GIs; Increased hepatic enzymes | Antidiarrheic medications; Stopped permanently | |||
| 3 | None | None | |||
| 4 | None | None | |||
| Raper et al. [19] | 1 | 1 | Mild GIs; Increased hepatic enzymes | Adjusted dosage | |
| Mahzari. and Zarif et al. [33] | 2 | 1 | Nm | Nm | |
| 2 | Nm | Nm | |||
| Littmann et al. [24] | 1 | 1 | GIs | Adjusted dosage | |
| Kolovou et al. [38] | 1 | 1 | None | None | |
| Suppressa et al. [36] | 1 | 1 | GIs | Adjusted dosage | |
| Cuchel et al. [35] | 6 | GIs; Increased hepatic enzymes | Dietary modifications; Adjusted dosage | ||
| Kolovou et al. [25] | 12 | GIs; Increased hepatic enzymes | Nm | ||
| Stefanutti et al. [34] | 7 | 1 | Mild GIs | Dietary modifications | |
| 2 | Increased hepatic enzymes | Temporary interruption; Diet modification | |||
| 3 | None of note | None of note | |||
| 4 | None of note | None of note | |||
| 5 | None of note | None | |||
| 6 | None of note | None | |||
| 7 | None of note | Diet modification | |||
| Chacra et al. [39] | 1 | 1 | GIs | Diet modification; Adjusted dosage | |
| Nm, Not mentioned; AEs, Adverse events; GIs, Gastrointestinal symptoms; ACS, Acute coronary syndrome; AP, Atherosclerotic plaque; LRTI, lower respiratory tract infection. | |||||
Lipid-lowering efficacies of lomitapide combined with other LLTs in adult HoFH
patients were investigated. Cuchel et al. [21] assessed the efficacy and
safety of lomitapide in a single-arm study comprising 29 adult HoFH patients.
Among the 29 patients, 23 completed the efficacy period (26 weeks) and the full
study (78 weeks). The main treatment involved increasing lomitapide dose based on
efficacy of the original LLT. Gradually, the lomitapide dose was increased from 5
mg/d to 60 mg/d. Mean LDL-C levels ranged from 8.7 mmol/L at baseline to 4.3
mmol/L at week 26. Eight patients had LDL-C levels below 2.6 mmol/L. At weeks 56
and 78, the decrease in LDL-C levels were 44% and 38% respectively.
Gastrointestinal symptoms, which were effectively alleviated by dietary
adjustments or dose reductions. Were the most common AEs. ALT activities
D’Erasmo et al. [27] conducted two retrospective studies and obtained
clinical as well as biochemical data from 15 HoFH patients treated with LLT and
lomitapide. During treatment, average LDL-C levels were 426
Littmann et al. [24] reported a case of a female patient diagnosed with
HoFH at the age of 6 and treated with lomitapide. The patient completely lacked
normal LDLR activities and did not exhibit any responses to statin therapy. At 7
years of age, the patient was treated with LA. When lomitapide was combined with
LA treatment, LDL-C levels significantly improved. Then, the LA dose was reduced
from 2 times a week to once every 2 weeks, after which the quality of life of the
patient improved. The patient did not present with any AEs. Raper et al.
[19] conducted a case study of a 49-year-old woman with HoFH and a complex
cardiovascular history who was treated with lomitapide for 5 years in combination
with other LLTs. Long-term lomitapide administration significantly suppressed
LDL-C levels to
Sperlongano et al. [31] reported findings on two lomitapide-treated HoFH patients. Compared to baseline levels, after lomitapide administration, there was a 78% reduction in LDL-C levels in patient 1 and an 86% reduction in patient 2. LA therapy was stopped in patient 2. During lomitapide administration, two patients presented with mild gastrointestinal symptoms. Side effects were alleviated by a low-fat diet and antidiarrheal medications. Patients did not show any elevations in ALT and liver fat levels. These findings indicate that lomitapide administration to patients in middle and early stages can reduce LDL-C levels and the risk of CVD. Roeters et al. [32] performed a study involving 4 adult HoFH patients. Each patient was administered with lomitapide and subjected to routine follow-up. In all 4 patients, LDL-C levels were reduced by 35 to 73%, with 3 of the 4 patients presenting with gastrointestinal AEs that were alleviated via appropriate dieting. During the whole study period, three patients were administered with lomitapide, while lomitapide administration was stopped in one patient due to elevated ALT levels, which were restored to normal levels after treatment withdrawal. Mahzari et al. [33] conducted a case study involving two HoFH patients treated with lomitapide in Saudi Arabia. After lomitapide treatment, LDL-C levels of patient 2 decreased from 15.3 mmol/L to 6.9 mmol/L. After one year of lomitapide administration, patient 2 died, which was attributed to cardiovascular surgery associated complications. However, the two patients did not show severe lomitapide-associated side effects. Suppressa et al. [36] reported a case of a 28-year-old female HoFH patient who had been diagnosed with xanthoma at age 2. The patient rejected LA therapy, therefore, LLT treatment was initiated using statins, ezetimibe and evolocumab, however, this therapy did not significantly decrease LDL-C levels. Treatment with increasing lomitapide doses (up to 30 mg/d) was initiated at month 24 of follow-up, resulting in decreased LDL-C level to 45 mg/dL. During lompitade treatment, the patient did not present CVD complications.
Cuchel et al. [35] conducted study in which six HoFH patients aged 18–40 years were treated with increasing lomitapide doses (0.03, 0.1, 0.3, 1.0 mg/kg/d). Four weeks prior to lompitade treatment, LLT therapy was suspended for 4 weeks in each group. After a 4-week drug elution period, patients returned for a final follow-up. All patients tolerated lomitapide treatment to a maximum dose of 1.0 mg/kg/d, which reduced LDL-C levels by 50.9% and Apo B levels by 55.6%, compared to baseline levels. The most severe AEs included elevated ALT levels and hepatic fat accumulation. Stefanutti et al. [34] reported on the effects of administration of lomitapide in addition to LA in 7 adult HoFH patients. In most (5/7) patients, the dose range of lomitapide was 10–30 mg/d. One patient received 60 mg/d lomitapide whereas another patient received 5 mg/d lomitapide. LDL-C levels reduced by more than 50% in 3 patients. Six patients receiving LA in this trial showed a reduction in dosing frequency, with three patients permanently discontinuing LA intake. Notably, patients who received the lowest lomitapide dose of did not achieve significant benefits from treatment. Gastrointestinal AEs were managed by a low-fat diet.
One study investigated the efficacy and safety of lomitapide in paediatric HoFH
patients. Ben-omran et al. [37] reported on lomitapide outcomes in
paediatric HoFH patients for the first time. The mean age for patients in the
study was 11.6
Eighteen clinical trials involving 120 lomitapide-treated HoFH patients were included in this study (2 single-arm studies, 2 retrospective case series and 14 case reports). Lomitapide significantly reduced LDL-C levels in HoFH paediatrics and adults, but also increased the risk for gastrointestinal reactions, ALT elevations, and liver fat accumulation. However, the adverse effects were controllable.
Lomitapide can significantly reduce LDL-C levels and the risk of CVD during HoFH
treatment [40]. Modeling data in adult patients revealed that early interventions
with lomitapide has the potential to increase the life expectancy and delay the
onset of the first major adverse cardiovascular events [41]. The potential of
lomitapide in long-term HoFH management has been evaluated. Among the included
studies in this systematic review, Raper et al. [19] reported
on lomitapide administration for
Compared to previous systematic reviews, this study informs on the efficacy and safety of lomitapide in paediatrics with HoFH. Early identification of CVD children and their timely referral to specialists are crucial active LLT measures for reducing CVD risks [3]. Currently, lomitapide is not permitted for use in children, however, clinical studies have been conducted through expanded access programs or on a designated patient basis. Clinical trials involving HoFH patients treated with lomitapide included in this study showed that lomitapide significantly reduces LDL-C levels in HoFH patients, reduces the frequency of LA treatment, reduces the risk of early CVD, and improves the quality of life for patients. There were few lomitapide-associated adverse reactions, with gastrointestinal disorders being the most important. However, adverse reactions could be controlled using low fat diets or through treatment dose adjustment.
Ben-omran et al. [37] reported that lomitapide has a good efficacy in pediatric HoFH patients, with 6 of 11 patients achieving the recommended target of 135 mg/dL. The frequency of LA was decreased in 5 patients, whereas clinical manifestations of the drug were like those in adult patients. Yahya et al. [30] reported that 2 HoFH patients diagnosed at a young age and administered with LLT (including lomitapide) were without AEs and CVD had not yet occurred on the patients. Mahzari et al. [33] documented that one HoFH patient in Saudi Arabia, who had received lomitapide, showed an 87% reduction in LDL-C levels without AEs. Kolovou et al. [38] documented that the xanthoma of an 8-year-old boy with HoFH was improved after lomitapide administration, without any side effects. The first reported long-term (49 months) use of lomitapide in children with HoFH was by Chacra et al. [39]. Lomitapide reduced LDL-C levels by 37%, however, diarrhea occurred during lomitapide use, whereas alterations in liver enzyme levels and hepatic steatosis were controlled through dose reductions and a low-fat diet.
A combination therapy involving statins, ezetimibe and LA is the most effective LLT therapy for HoFH patients [5]. In HoFH patients, statin monotherapy does not significantly reduce LDL-C levels; however, it reduces LDL-C levels by an average of 26% and, significantly reduces CVD events as well as all-cause mortality [12]. Treatment of HoFH with LLTs does not reduce LDL-C levels to required levels, therefore, due to these limitations, LA is the standard treatment option for HoFH. J. Višek et al. [42] analyzed data on FH patients treated with LA for 15 years. They found that long-term LA treatment improved lipid levels and endothelial dysfunction, without cardiovascular complications. However, LA treatment is expensive and requires a long treatment period as well as high patient compliance [43]. Moreover, due to the frequency of treatment (at least two weeks) and the need to maintain vascular access, not all patients are eligible for monotherapy. This technology requires highly specialized facilities and is not available in most countries [44]. LA treatment may also present technical, clinical, and social challenges, especially in children [45, 46]. Although the current lipid-lowering drugs and LA can significantly improve the prognostic outcomes for HoFH patients, they result in LDL-C levels above target levels in most patients [12]. Therefore, new drugs are urgently needed for HoFH treatment. Lomitapide is characterized by a high efficacy and tolerability, therefore, it is an alternative to LA for several patients awaiting liver transplantation. Littmann et al. [24] reported that since it is associated with significantly improved LDL-C levels and it markedly reduces the frequency of LA administration, lomitapide can be used as the drug of choice for HoFH. Stefanutti [34] reported that in addition to LA treatment,7 adult HoFH patients were treated with lomitapide, resulting in reduced LA treatment frequencies in 6 patients and permanent withdrawal of LA in 3 patients. This indicates that lomitapide can be used as an adjunct treatment to LA in HoFH.
Lomitapide-associated adverse reactions may lower patient adherence to treatment and limit the use of the maximum tolerable dose, potentially reducing its efficacy. Adverse reactions included gastrointestinal symptoms, elevated hepatic ALT levels, and accumulation of liver fat, which may lead to steatohepatitis or liver fibrosis [47]. Cuchel et al. reported on AEs in at least 90% of patients treated with lomitapide, with gastrointestinal symptoms (diarrhea, nausea, vomiting, or indigestion) being the most common [16, 21, 35, 48].
Most of the lomitapide-associated adverse reactions can be alleviated through different management approaches. For instance, gastrointestinal symptoms can be minimized through intakes of low-fat diets (20% of energy is obtained from fat). Clinical use of lomitapide can be regulated by gradually increasing the dose under tolerable levels or decreasing the dose when necessary [49]. The effects of lomitapide precursors in healthy volunteers (n = 48) have been investigated. It revealed that gastrointestinal AEs were significantly associated with high-fat diets [50]. Due to associated adverse reactions, lomitapide prescription requires intense patient education and liver function monitoring during treatment [5, 51].
This study is associated with some limitations. First, some unpublished studies were not included in the search, which may result in publication bias. Second, lomitapide is an orphan drug used for the treatment of orphan diseases. Studies on lomitapide as an adjunct to other LLTs are mainly small clinical sample size studies. Currently, due to ethical limitations, there are no long-term large randomized clinical trials on efficacies of lomitapide on hard clinical endpoints in HoFH. Therefore, assessment of the safety of lomitapide is limited. Third, the information in some studies were incomplete. Some studies did not report on differences in exposure time of lomitapide treatment. Some trials did not report the data on dietary fat intake by patients [30]. In addition, no large-scale data are available on lomitapide use in HoFH children. Only 4 case reports documented on the use of lomitapide in infant patients or in HoFH children [33, 37, 38, 39]. Therefore, the efficacy and safety of lomitapide in children and infants with HoFH should be explored further.
Lomitapide is an effective treatment option for significantly reducing LDL-C levels in adult patients with HoFH; however, further data are needed in children. Moreover, lomitapide is suitable for long-term use as an adjunct therapy for patients treated with LA to reduce the frequency of LA dosage. If HoFH patients treated with the maximum tolerable dose of lipid-lowering drugs and LA do not achieve normal LDL-C levels, lomitapide can be administered as an adjunct drug if HoFH patients treated with the maximum tolerable dose of lipid-lowering drugs and LA do not achieve normal LDL-C levels. Lomitapide is associated with adverse reactions, mainly manifested as gastrointestinal reactions, such as diarrhea, nausea, and vomiting. In addition, elevated ALT levels in the liver are associated with high levels of lomitapide administration. However, adverse reactions were alleviated through diet management, regular monitoring, and dosage adjustment.
NW and JS conceived and designed the study. SL and GL performed the database search and extracted the data. NW and NZ analyzed the data and wrote the manuscript. GY and QJ edited the English. HZ and YH revised the manuscript. All authors read and approved the final manuscript.
Not applicable.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research was supported by Sample study on toxicity effect evaluation method of traditional Chinese patent medicine containing toxic components (2020-ZXFZJJ-072).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.rcm2305151.



