Academic Editors: Giuseppe Nasso and Giuseppe Santarpino
Surgical ablation is a well-established therapy for patients with atrial fibrillation (AF) undergoing cardiac surgery. However, it is not clear if this translates to an improvement in patient important outcomes such as mortality, stroke, and quality of life (QoL). Electronic searches were performed of Ovid Medline and PubMed from their inception to October 2021. Eligible literature included comparative studies with patient undergoing surgical ablative treatment for AF concomitant to any cardiac surgery procedure and patients without specific AF treatment. For this paper, the studies listed are presented descriptively without statistical processing or collection of a meta-analysis. Freedom from AF at 1 year was consistently shown to be improved by surgical ablation. No differences in 30-day mortality or in safety outcomes were observed between the group who received ablation and the control group. A significant increase in pacemaker implantation in the ablation group was generally detected among studies, especially if the lesions were biatrial. Amongst the studies that reported on health-related quality of life (HRQoL) a statistically significant improvement was seen in the ablation group over the control, especially in the physical domains. Surgical ablation is the most effective procedure to treat AF during cardiac surgery, and it is a unique opportunity to return to sinus rhythm with no added mortality risk and a potential improvement in quality of life. There is however an increased risk of pacemaker implantation and complications such as renal failure which must be weighed with tailored treatment and patient selection. It is also not clear how long-term outcomes are affected due to underpowered randomized controlled trials. This review summarized short term outcomes of concomitant AF treatment during cardiac surgery and highlight the importance of reporting long-term outcomes to confirm the benefits.
Atrial fibrillation (AF) affects about 3% of the population, making it the most common arrythmia encountered by healthcare professionals [1]. Among patients undergoing cardiac surgery, preoperative AF reaches an incidence of about 10% [2], with a higher incidence among patients presenting for mitral valve replacement (30–50%) and a lower incidence in patients undergoing coronary artery bypass graft (CABG) (5%) [3, 4].
AF is characterized by loss of atrial contraction and rapid, irregular ventricular contraction, leading to progressive cardiac dysfunction. Many patients experience significant symptoms such as palpitations, dyspnea, and weakness, leading to reduced quality of life (QoL) [5]. Beyond symptoms, AF is an independent risk factor all-cause mortality, stroke, heart failure and hospitalization compared to normal sinus rhythm [6, 7], and if present at the time of surgery, it negatively affects 30 day outcome and survival [8].
Surgical ablation (SA) is a well-established treatment option for patients with AF undergoing cardiac surgery. Current ESC/EACTS guidelines recommend concomitant AF ablation for all patients with a history of AF if the heart team believes added rate control may be beneficial [9, 10]. The aim of SA is to eliminate AF by using surgical lesions to block aberrant electrical conduction which therefore inhibits the generation and propagation of macro re-entrant circuits in the atria [11, 12].
Technical aspects include a wide range of energy including “cut and sew”, radio-frequency ablation (RFA) and cryoablation (CA). Similarly, current procedures include simple pulmonary vein isolation (PVI), left atrial (LA) ablation and the biatrial Cox-Maze IV procedure (CM-IV) [13, 14, 15].
On follow-up SA has repeatedly been shown to promote a return to NSR [16]. However, it is not clear if there is a global benefit of imposing NSR, and whether this translates to an improvement in patient important outcomes such as mortality, stroke and QoL.
The aim of this study is to review randomized controlled trials, propensity score matched studies, cohort studies and metanalysis to assess the benefits and risks associated with concomitant SA in patients undergoing cardiac surgery.
Electronic searches were performed of Ovid Medline and PubMed from their inception to October 2021. Randomized controlled trials, propensity matched studies, cohort studies and metanalysis were considered as eligible study design for this paper. To improve sensitivity, the terms ‘concomitant cardiac surgery’ AND ‘atrial fibrillation’ AND ‘ablation’ were used either as key words or medical subject heading (MeSH) terms. Eligible literature for the present review included those in which relevant patient cohorts with any type of AF (Fig. 1) underwent any cardiac surgery concomitantly with a surgical ablative treatment. When duplicate studies were identified with increased follow-up lengths or accumulating numbers of patients, the most complete reports only were selected for assessment at each time interval. All publications were limited to those involving human subjects. Case reports, abstracts, editorials, and reviews were excluded. The reference lists of all retrieved articles were then reviewed using inclusion/exclusion criteria. Search strategies including exploded MeSH terms have been used. English language restriction was imposed. Additional articles by manually searching the reference lists from recent reviews and the extracted papers have been looked for. Attempts has been made at collecting unpublished data from the Authors of potentially pertinent papers. Outcomes of interest included freedom from AF at 12 month follow up, 30-day mortality, permanent pacemaker implantation in 12 month follow up and safety outcomes at 12 month follow up. The primary safety outcomes were incidence of any major adverse cardiovascular event (MACE) (death, myocardial infarction, heart failure), renal failure requiring dialysis or cerebrovascular events such as transient ischemic attack (TIA) or stroke. Health Related Quality of Life (HRQoL) was also examined where possible. Fig. 2 summarizes the search strategy. Initial search retrieved 792 papers from databases and 12 papers from registries; after duplicate removal and ineligible records, 483 papers were screened. After removing papers not describing outcomes of interest and inappropriate study design (i.e., case report, abstracts from meeting, editorials, opinion articles…), a total of 26 papers were integrated in this review. In details, 19 randomized trials, 7 high quality non-randomized trials (cohort studies and propensity matched studies) and 5 metanalysis were included in this review.
 Fig. 1.
Fig. 1.Classification of atrial fibrillation by duration and ability to cardiovert. Recurrent atrial fibrillation can be divided into paroxysmal, persistent, long-standing and permanent based on duration and ability to cardiovert.
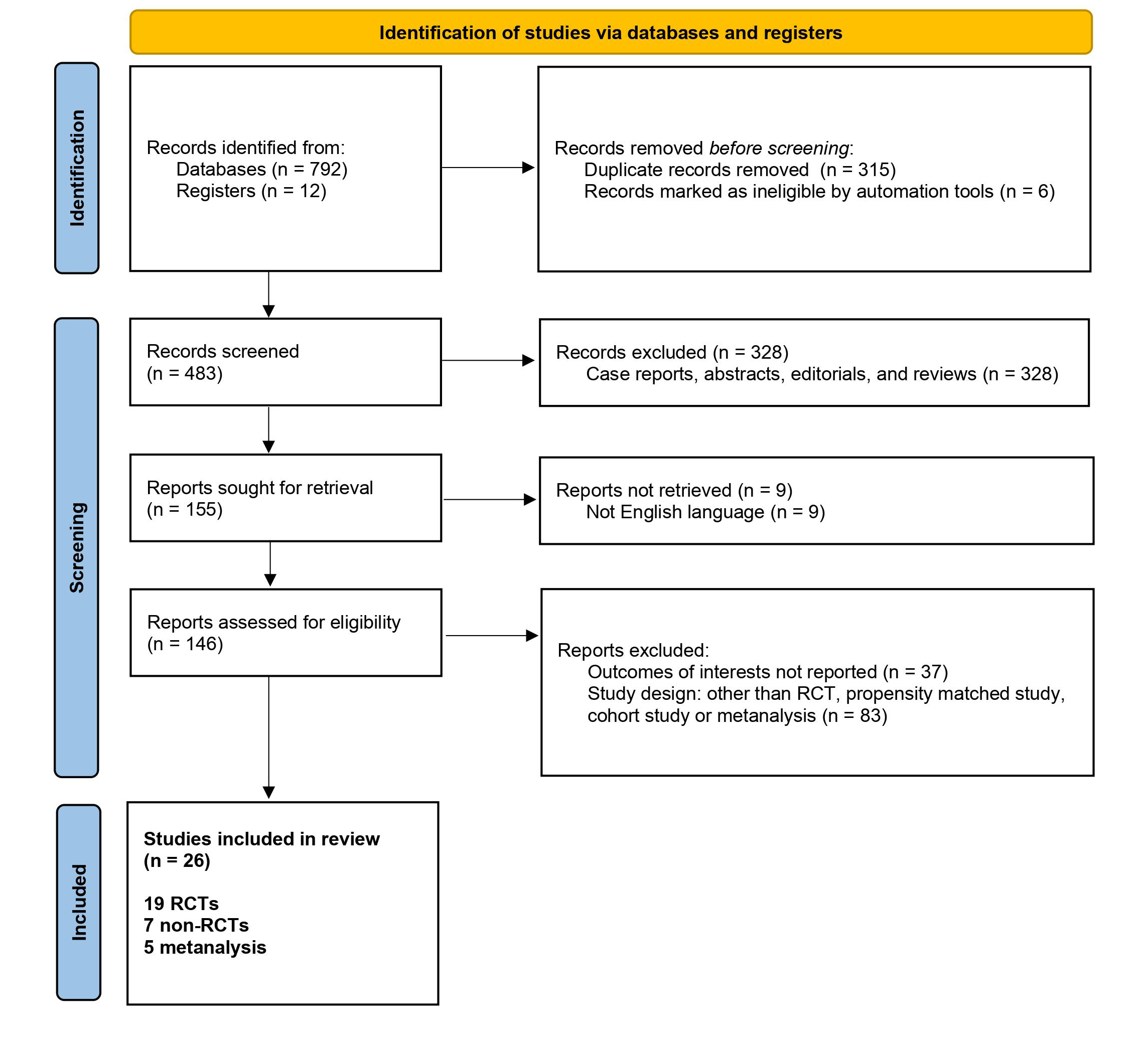 Fig. 2.
Fig. 2.Study flow chart of search strategy. After literature search and evaluation, a total of 26 articles have been included in this review (19 randomized studies, 7 non randomized studies, 5 metanalysis).
Letters, editorial, reviews, animal studies and reports with duplication data have been excluded. PICOS approach (Patients, Intervention, Comparator, Outcomes, Study design) was used for inclusion/exclusion criteria (Supplementary Table 1). To identify eligible studies, a two-step selection process has been applied. Authors checked eligibility criteria and selected the studies for inclusion in the present systematic review. As a second round, authors independently screened records for inclusion. They were blinded to each others’ decisions. Disagreements between individual judgements have been resolved by consensus. Studies were excluded if they did not meet the criteria. Authors independently extracted data from all eligible studies using a standardized Excel file, focusing on study design, study size, type of intervention and outcomes. Any disagreement was solved by consensus.
The presented results are based only on descriptive data from randomized, propensity matched studies and metanalysis; conclusions are drawn directly from these data and not from a formal meta-analysis.
Literature presents many randomized control trials (RCTs) [5, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33], propensity score matched studies [34, 35] and cohort studies [3, 36, 37, 38, 39]. Tables 1,2 Ref. [16, 17, 18, 19, 20, 21, 22, 23, 34, 35, 36] show a summary of the RCTs and propensity matched studies included in the review. A large variety of lesion sets and energy sources were used to perform the ablation and there were also significant differences in the type of AF that was allowed according to specific inclusion criteria. Most studies included persistent AF (PerAF), long standing persistent AF (LsPerAF) or permanent AF.
| Study name/Author | Patients | Study type | Type of concomitant procedure | Intervention groups | Type of energy | Type of AFib population |
| PRAGUE-12, Budera et al. 2012 [17] | 218 | multicenter RCT | non valvular: 54 (25%) | (Group A) left atrial ablation: 116 (53%) | CA: 113 (96.6%) | Group A: PAF 26 (22.2%), PerAF 30 (25.6%), LSPerAF 61 (52.1%) |
| valvular: 164 (75%) | (Group B) no ablation: 102 (47%) | RFA: 4 (3.4%) | Group B: PAF 33 (30.8%), PerAF 25 (23.4%), LSPerAF 49 (45.8%) | |||
| Gillinov et al. 2015 [16] | 260 | multicenter RCT | valvular: 260 (100%) | (Group 1) PVI: 67 (26%) | CA: 133 (100%) | Group 1+2: LSPerAF 70 (52.6%), PerAF 63 (47.4%) |
| valvular + CABG: 52 (20%) | (Group 2) modified biatrial Maze: 66 (26%) | |||||
| (Group 3) no ablation: 127 (48%) | Group 3: LSPerAF 71 (55.9%), PerAF 56 (44.1%) | |||||
| CURE-AF, Damiano et al. 2014 [36] | 150 | multicenter RCT | Valvular |
Biatrial Cox Maze IV: 150 (100%) | RFA: 150 (100%) | PAF: 4 (3%) |
| CABG alone: 22 (15%) | PerAF: 33 (22%) | |||||
| LSPerAF: 113 (75%) | ||||||
| Von Oppell et al. 2009 [18] | 49 | single center RCT | Valvular: 49 (100%) | Control group) no ablation: 24 (49%) | RFA: 25 (100%) | Control group: permanent AF 22 (92%), PerAF 2 (8%) |
| Valvular + CABG: 26 (53%) | Intervention group) Cox Maze IV: 25 (51%) | Intervention group. permanent AF 22 (88%), PerAF 3 (12%) | ||||
| Cherniavsky et al. 2014 [19] | 95 | single center RCT | CABG: 95 (100%) | (Group 1) CABG + PVI, 31 (32%) | RFA: 61 (100%) | PerAF 95 (100%) |
| (Group 2) CABG + mini Maze (PVI + line to mitral annulus), 30 (32%) | ||||||
| (Group 3) no ablation, 34 (36%) | ||||||
| McCarthy et al. 2013 [34] | 4349 | retrospective propensity matched cohort | Patients with PAF: MVS 296 (54%), CABG 198 (36%), AVS 228 (41%), TVS 102 (18%). | (Group 1) no history of AF: 3797 (87%) | RFA or CA depending on surgeon’s choice, not specified. | No history of AF: 3797 (87%) |
| Patients with no AF:MVS 949 (25%), CABG 1913 (50%), AVS 1517 (40%), TVS 185 (5%) | (Group 2) PAF treated with ablation: 423 (77%). Procedural details: LA-only Maze 199 (47%), PVI 119 (28%), biatrial Maze 85 (20%), “cut and sew” Cox Maze 20 (5%) | PAF: 552 (13%) | ||||
| (Group 3) Untreated PAF: 129 (23%) | ||||||
| Badhwar et al. 2017 [35] | 86941 | retrospective propensity matched cohort | Isolated CABG: 33%, with lowest rate of surgical AF ablation (33.1%) | (Group 1) surgery with no concomitant AF ablation, 44875 (52%) | RFA: 30516 (49.2%) | Group 1: PAF 26207 (58%), PerAF 18848 (42%) |
| MVR |
(Group 2) surgery with concomitant AF ablation, 42066 (48%). Procedural details: left atrium only 52.9%, biatrial 35.8%, right atrium 1.7%, unspecified 9.6% | CA: 16933 (27.3%) | Group 2: PAF 19098 (45%), PerAF 22968 (55%) | |||
| cut-and-sew: 8125 (13.1%) | ||||||
| other: 2.5% | ||||||
| unspecified: 7.9% | ||||||
| Doukas et al. 2005 [20] | 97 | single center RCT | All patients underwent MVS. | (Control group) no ablation: 48 (49%) | RFA: 49 (100%) | History of 6 months of continuous AF (LSPerAF 100%). |
| Associated procedures in control group: CABG 6 (12.5%), TVR 7 (14.6%). | ||||||
| Associated procedures in intervention group: CABG 5 (10.2%), TVR 9 (18.4%) | (Intervention group) LA ablation 49 (51%) | |||||
| SAFIR, Chavelier et al. 2009 [21] | 43 | multicenter RCT | MVS 100% | (Control group) no ablation: 22 (51%) | RFA: 21 (100%) | LSPerAF 100% |
| (Intervention group) concomitant LA ablation 21 (49%) | ||||||
| Deneke et al. 2002 [22] | 30 | single-center RCT | MVS 100% | (Group A) concomitant Maze 15 (50%) | RFA: 15 (100%) | Permanent AF with previous failed attempts at cardioversion |
| (Group B) no ablation 15 (50%) | ||||||
| Van Breugel et al. 2010 [23] | 132 | multicenter RCT | CABG 41 (31%) | (Control group) no ablation, 67 (51%) | not reported | PAF 57 (43%) |
| valve replacement 53 (40%) | (Intervention group) concomitant PVI 65 (49%) | PerAF 30 (22.7) | ||||
| valve surgery + CABG 30 (23%) | Permanent AF 43 (32.6%) | |||||
| other 8 (6%) | ||||||
| Legend: PAF, Paroxysmal AF; PerAF, Persistent AF; LSPerAF, Long-standing Persistent AF; MVRR, Mitral Valve Repair/Replacement; MVS, Mitral Valve Surgery; PVI, Pulmonary Vein Isolation; TVR, Tricuspid Valve Replacement; LA, Left Atrium; CA, Cryoablation; RFA, Radiofrequency Ablation; MACE, Major Adverse Cardiac Event; TrPAF, Treated PAF; UnTrPAF, Untreated PAF; ILR, Implantable Loop Recorder. | ||||||
| Study name/Author | Freedom from AFib at 1 year, type of monitoring | 30-days mortality | Safety primary outcomes at 1 year | Permanent pacemaker implantation |
| PRAGUE-12, Budera [17] | PAF: Group A 61.9% vs Group B 58.3% | Group A: 9 (7.8%) | Group A: 45 (40.5%) | Group A: 11 (9.5%) |
| PerAF: Group A 72% vs Group B 50% | Group B: 9 (8.8%) | Group B: 37 (40.2%) | Group B: 12 (11.8%) | |
| LSPerAF: Group A 53.2% vs Group B 13.9% | p = 0.809 | p = 0.785 | p = 0.654 | |
| ECG ad 24 h Holter | ||||
| Gillinov et al. 2015 [16] | Group 1: 36 (61%) | Group 1+2: 3 (2.3%) | Group 1+2: 31 (23.3%) | Group 1+2: 26 (19.5%) |
| Group 2: 31 (66%) | ||||
| Group 3: 30 (29.4%) | Group 3: 5 (3.9%) | Group 3: 26 (20.5%) | Group 3: 9 (7.1%) | |
| 72 h Holter | p = 0.49 | p = 0.58 | p = 0.01 | |
| CURE-AF, Damiano et al. 2014 [36] | follow up only for 9 months | 6 (4%) | 10 (7%) at 9 months | 1 (0.7%) at 9 months |
| 24 h Holter | ||||
| Von Oppell et al. 2009 [18] | Control: 9 (39%) | 0 | no significant differences reported, p = NR | Control: 1 (4%) |
| Intervention: 18 (75%) | Intervention: 1 (4%) | |||
| ECG | p = NR | |||
| Cherniavsky et al. 2014 [19] | Group 1: 24 (80%) | 0 | no significant differences reported, p = NR | 0 |
| Group 2: 25 (86%) | ||||
| Group 3: 11 (44%) | ||||
| ILR monitoring | ||||
| McCarthy et al. 2013 [34] | TrPAF vs UnTrPAF: 81% vs 60% | TrPAF vs UnTrPAF: 2% vs 5%, p = 0.28 | not reported | TrPAF: 4% |
| TrPAF vs no AF: 84% vs 93% | TrPAF vs no AF: 3% vs 3%, p = 0.97 | UnTrPAF: 4% | ||
| different monitoring techniques | UnTrPAF vs no AF: 6% vs 4%, p = 0.51 | No AF: 5% | ||
| Increased need for pacemaker in the biatrial group vs LA-only group (16.5% vs 7.5%. p = 0.02) | ||||
| Badhwar et al. 2017 [35] | not reported | Group 1: 1292 (4.5%) | Group 2 had lower risk-adjusted mortality (p |
Group 1: 1693 (5.9%) |
| Group 2: 1118 (4.1%) | Group 2: 2253 (7.8%) | |||
| p = 0.449 | p | |||
| Doukas et al. 2005 [20] | Control group: 2 (4.5%) | Control group: 4 (8.3%) | no significant differences in morbidity, p = NR | not reported |
| Intervention group: 20 (44.4%) | Intervention group: 3 (6.1%) | |||
| ECG and 48 h Holter | p = NR | |||
| SAFIR, Chavelier et al. 2009 [21] | Control group: 7 (33%) | Control group: 0 (0%) | stroke: control 4.5% vs intervention 14%, p = NR | Control group: 2 (9%) |
| Intervention group: 20 (95%) | Intervention group: 1 (4.8%) | other results not reported. | Intervention group:3 (14%), p = NR | |
| 24 h Holter | p = NR | |||
| Deneke et al. 2002 [22] | Group A: 9 (60%) | 0 | Overall survival at 12 months was 83% (73% in Group A, 93% in Group B, p = 0.131). No thromboembolic events occurred. | Group A: 1 (6.7%) |
| Group B: 3 (20%) | Group B: 1 (6.7%) | |||
| ECG and Holter | p = NR | |||
| Van Breugel et al. 2010 [23] | Control group: 26 (42%) | Control group: 5 (7.5%) | no significant differences | Control group: 1 (0.8%) |
| Intervention group: 36 (58%) | Intervention group: 2 (3.1%) | Intervention group: 1 (0.8%) | ||
| p = NR | p = NR | |||
| Legend: PAF, Paroxysmal AF; PerAF, Persistent AF; LSPerAF, Long-standing Persistent AF; MVRR, Mitral Valve Repair/Replacement; MVS, Mitral Valve Surgery; PVI, Pulmonary Vein Isolation; TVR, Tricuspid Valve Replacement; LA, Left Atrium; CA, Cryoablation; RFA, Radiofrequency Ablation; MACE, Major Adverse Cardiac Event; TrPAF, Treated PAF; UnTrPAF, Untreated PAF; ILR, Implantable Loop Recorder; p = NR, p value not reported in the original publication. | ||||
Freedom from AF at 1 year was consistently shown to be improved by surgical ablation. All studies reported statistically significant increases in the number of patients that returned to NSR in the ablation arm compared to the control, except Van Breugel et al. [23] in which the increase was not sufficient to reach significance (p = 0.28). Monitoring tools to investigate freedom from AF, however, were rather heterogenous across articles.
All studies reported on 30-day mortality, without any significant difference between the group who received ablation and the control group.
No significant differences were found in the context of MACE or stroke between the group who received concomitant ablation and the group who did not. The only statistically significant result observed was an increased risk of renal failure requiring hemodialysis described by Badhwar et al. [35] among patients undergoing AF ablation.
Most studies included data on the number of pacemaker implantations at latest follow up. 14 described no statistically significant difference between the ablation and the control group in the number of pacemakers implanted. However, in the study by Badhwar et al. [35] the ablation group had a significantly higher number of pacemakers implanted compared to the control group. Also in the study by Gillinov et al. [16] ablation was associated with increased risk of requiring a permanent pacemaker than no ablation (p = 0.01).
Only 3 studies [5, 18, 23] reported data on health related quality of life (HRQoL)
using the RAND 36-item Health Survey assessment tool (SF-36). The SF-36
questionnaire evaluates eight health domains: physical functioning, role-physical
functioning, general health, bodily pain, social functioning, vitality,
role-emotional functioning, and mental health [40]. Van Breugel et al.
[23] and Von Oppel et al. [18] both found a significant increase in the
physical functioning domain between preoperative and post-operative assessment at
one year, however the difference in the increase between the ablation and control
groups was not statistically significant. Van Breugel et al. [23] also
reported using the EuroQoL 5D assessment tool and found a statistically
significant deterioration in the Pain/Discomfort sub-scale for both groups
(p
No studies reported a significant difference in mortality or safety outcomes in relation to the lesion sets or energies used. However, in the study by McCarthy et al. [34] there was a statistically significant increase in need for pacemaker implantation in the group who underwent bi-atrial ablation rather than left atrial only (p = 0.02).
A total of 5 metanalysis have been published in literature about concomitant treatment of AF during cardiac surgery (Table 3, [41, 42, 43, 44, 45]). The milestone Cochrane systematic review published by Huffman et al. [41] in 2016 summarized all the significant literature, concluding that concomitant AF surgery significantly halves the risk of recurrent arrhythmias (RR 2.04, 95% CI 1.63–2.55), while increasing the risk of permanent pacemaker implantation (RR 1.69, 95% CI 1.12–2.54), but with no differences in all-cause mortality (RR 1.14, 95% CI 0.81–1.59) and early mortality (RR 1.25, 95% CI 0.71–2.20). Starting from the late 1980’s when first RCTs were reported, this paper included 34 reports of 22 RCTs with about 1900 patients. Notably, authors reported a high risk of bias across at least one domain, and although they included only RCTs by study design, the risk of detection bias, small-study bias and reporting bias negatively affected the quality of the evidence, which were considered to be qualitatively poor. Most trials (77%) had less than 200 patients and were performed in a single center. Surgical technique also was very variable, with cut-and-sew technique, microwave ablation, cryoablation and radiofrequency ablation equally distributed among studies, thus increasing the inter-study variability and confounding the interpretation of results. At the publication of this review, 8 studies were still under recruitment and therefore results were not included but most studies were halted prematurely. Authors concluded that adequately powered RCTs and future secondary analysis would overcome those limitations, but this remains the most comprehensive metanalysis of RCTs on this topic.
| Metanalysis | Included studies | Patients included, number of studies | Early mortality | postoperative adverse events | Stroke and neurologic events | Late mortality | Freedom from recurrent arrhythmias | permanent pacemaker | Results |
| Huffman et al. 2016 [41] | randomized controlled trials, any AF procedure vs control (no AF treatment) | 22 studies, 1899 patients | 2.3% vs 3.1%, RR 1.25 with 95% CI 0.71–2.20, I |
all complications 24.8% vs 23.6%, RR 1.07 with 95% CI 0.85–1.34, I |
- | 7.0% vs 6.6%, RR 1.14 with 95% CI 0.81–1.59, I |
51.0% vs 24.1%, RR 2.04, with 95% CI 1.63–2.55, I |
6.0% vs 4.1%, RR 1.69 with 95% CI 1.12–2.54, I |
concomitant AF treatment halved risk of recurrent arrhythmias, increased risk of permanent pacemaker |
| Wang et al. 2018 [42] | randomized controlled trials, left atrial treatment vs control (no AF treatment) | 11 studies, 666 patients | 2.7% vs 2.3%; OR 1.06; 95% CI, 0.43–2.60; p = 0.90; I |
reoperation for bleeding 5.3% vs 5.1%; OR 1.05; 95% CI 0.31–3.55; p = 0.94; I |
3.2% vs 3.2%; OR 1.05; 95% CI 0.41–2.67; p = 0.92; I |
1.7% vs 2.4%; OR 1.25; 95% CI 0.30–5.29; p = 0.76; I |
31.6% vs 67.5%; OR 0.41; 95% CI 0.37–0.46, p |
5.5% vs 5.1%; OR 1.08; 95% CI 0.48–2.40; p = 0.85; I |
concomitant AF treatment reduced risk of recurrent arrhythmias; no other significant differences between groups |
| Cappabianca et al. 2019 [43] | prospective randomized, prospective observational and retrospective studies, bi-atrial treatment (BA) vs left atrial treatment (LA) | 28 studies, 7065 patients | no differences (OR 1.01, 95% CI 0.59–1.71, p = 0.98) | reopening for bleeding higher in BA group (OR 1.70, 95% CI 1.05–2.75, p = 0.03) | no differences (OR 1.48, 95% CI 0.63–3.45, p = 0.37) | no differences (OR 0.99, 95% CI 0.43–2.29, p = 0.98) | 6- and 12-months prevalence of sinus rhythm were higher in the BA group (OR 1.37, 95% CI 1.09–1.73, p = 0.008 and OR 1.37, 95% CI 0.99–1.88, p = 0.05 respectively) | higher in BA group (OR 1.85, 95% CI 1.38–2.49, p |
BA ablation appears superior to LA ablation in terms of efficacy but is associated with a higher risk of bleeding and of PPM implantation, more frequently due to sinoatrial node dysfunction. LA approach should be preferable in patients with a higher risk of bleeding or with perioperative risk factors for PPM implantation. |
| Guo et al. 2021 [44] | randomized controlled trials, pulmonary vein isolation (PVI), left atrial Maze (LAM), bi-atrial Maze (BAM), or no ablation, Bayesian network meta-analysis | 19 studies, 2031 patients | BAM was associated with an increase in early mortality when compared with no ablation (OR 4.08, 95% CI 1.23–17.30, p |
- | - | - | PVI, LAM, and BAM (OR 5.02, 95% CI 2.72–10.02; OR 7.97, 95% CI 4.93–14.29; OR 8.29, 95% CI 4.90–14.86, p |
- | Bi-atrial ablation is not superior to left atrial ablation strategies in reducing AF recurrence for un-selected surgical patients. BAM has a higher risk of early mortality than no ablation. |
| Maesen et al. 2021 [45] | all studies, focused on quality of life (SF-36) | 9 studies, 545 patients | - | - | - | - | - | - | Quality of life scores improved 1 year after surgical ablation for AF; association between an improved QoL and the procedural effectiveness. |
| Legend: RR, relative risk; OR, odds ratio; AF, atrial fibrillation; QoL, quality of life; PVI, pulmonary vein isolation; LAM, left atrial Maze; BAM, bi-atrial Maze; PPM, permanent pacemaker. | |||||||||
Wang et al. in 2018 [42] confirmed the previous findings, but found a higher risk of mortality and morbidity for AF surgery. Cappabianca et al. in 2019 [43] summarized studies comparing biatrial vs left atrial ablation, concluding that BA is superior at costs of increased risks of bleeding and pacemaker implantation. However, those results were partially confirmed by Guo et al. in 2021 [44] as they found a higher risk of early mortality with BA ablation. Maesen et al. in 2021 [45] published the only metanalysis focused on quality of life, concluding that QoL improves after both stand-alone and concomitant arrhythmia surgery, as this improvement can be attributed to both the cardiac procedure itself and to the arrhythmia surgery, confirming that the main factor determining quality of life is rhythm at long term follow up.
This review suggests that in patients with a history of AF, surgical ablation
concomitant with cardiac surgery significantly increases freedom from AF at 12
months follow up compared to the same surgery without ablation. This appears
crucial among patients presenting for mitral valve repair or replacement, as in
this population preoperative incidence of AF can reach 50%; also, they would
benefit most from SA to improve QoL and reduce AF-associated adverse events [1, 3, 4, 5]. As mentioned previously, AF has repeatedly been identified by multiple
studies as an independent risk factor for morbidity and mortality [46] and in the
AFFIRM study, the mortality risk for patients with AF was shown to be almost
double that of patients in NSR (p
In the present review, ablation appeared to be very effective at converting AF into NSR, however there was significant heterogeneity between studies in the tools used to assess freedom from AF. 18% of the trials used ECG alone at follow up to establish presence of AF, only using Holter monitoring if the patient claimed to be symptomatic. We were concerned that this may have caused over-estimation of freedom from AF. In one study, asymptomatic AF occurred 12 times more frequently than symptomatic AF when followed-up by Holter monitoring [49]. Also, the DISCERN-AF study showed that increased intensity of monitoring resulted in increased AF detection, therefore studies are less likely to achieve freedom from AF if a more intensive method, such as an implantable loop recorder (ILR), is used to assess rhythm [50]. As a result of this, we believe over estimation of freedom from AF to be a limitation of the present review and suggest that future trials contributing to the ablation argument should aim for a more homogenous, intensive monitoring technique to make results more reliable.
Although studies have claimed that SA can be performed concomitantly to cardiac surgery with no increased risk to the patient [39] others have shown an almost three fold higher risk of permanent pacemaker placement associated with SA [16]. This phenomenon is believed to be due to underlying sinus node dysfunction, only revealed when the aberrant macro-reentrant circuits associated with AF are terminated following successful ablation [51, 52]. Increased risk of pacemaker implantation has been observed especially with bi-atrial Cox Maze-IV lesions rather than LA or PVI [41]. This evidence was considered robust enough for the ESC/EATCS to downgrade their recommendation for concomitant ablation from I to IIA [10]. Although adequate statistical sub-group analysis has not yet been performed and is needed to confirm our findings, it initially appears that we have corroborated this view. It should however be added that this risk of pacemaker implantation is relatively low, and in our opinion, it should not prevent clinicians from performing ablation on patients if the heart team believe it to be in their best interest.
An important patient-centered outcome that is frequently overlooked in studies is health related quality of life (HRQoL), again due to the inference that return to NSR is directly linked with increased QoL [53]. In the present review, of the 26 studies included, only 3 reported on HRQoL using approved assessment tools such as the SF-36. As shown by the RACE study, AF can commonly cause significant symptoms and reduce QoL [47]. However, in the context of concomitant surgical ablation, the main cardiac disease requiring intervention might have a crucial role in limiting HRQoL preoperatively. Therefore, it can be difficult to ascertain if the improvement in the QoL is due to treatment of AF or the concomitant disease.
Chernyavskiy et al. [5] found that in the immediate post-operative period, HRQoL was significantly improved in all domains in both the control and the ablation groups. However, at 1 year of follow up, the increases in the control group had reduced and had ceased to be significant whereas in the ablation group they remained significant. Furthermore, at this time as recorded by ILR, only 44.1% of the control group where in stable NSR, whereas in the PVI group 80% were in NSR and in the CM-IV group the figure was 86%. Therefore, it is likely that the reduction in QoL in the control group was due to a persistence or relapse into AF in the follow up period which was not observed in the ablation groups.
Our opinion is therefore in agreement with Chernyavskiy et al. [5], and we suggest that improvement in QoL following cardiac surgery with concomitant surgical ablation is attributed to not only resolution of the underlying disease but also return to NSR. Berkowitsch suggested that this may be due to a placebo effect whereby fewer symptoms are reported because the patients believe their treatment has been successful [54]. Also maybe being told that their heart-rate is beating in a “normal” rhythm may increase their psychological well-being by reducing their anxiety [55]. We therefore believe that treatment should be comprehensive and focused on not only the elimination of angina symptoms but also the clinical manifestations of AF. More evidence is needed however in the form of large RCTs investigating HRQoL over long periods to support this argument further.
Surgical ablation is the most effective procedure to treat AF and is a unique opportunity for most patients undergoing cardiac surgery affected by AF to return to sinus rhythm with no added mortality risk and a potential improvement in quality of life. There is however a low risk of potential pacemaker implantation and renal failure which must be weighed against the benefits before selection of treatment. It is also not clear how long-term outcomes are affected due to underpowered RCTs and this review would urge these studies to report on their long-term outcomes in the future to add to this argument.
There is significant heterogeneity between the studies in terms of their inclusion and exclusion criteria. This may question the legitimacy of looking at the results as a collective, however we believe that the comparison is valid since it reflects the heterogeneity in operating procedure observed between surgeons and institutions. Adequate evaluation of SA in specific surgical settings, i.e., patients undergoing isolated mitral valve repair or combined mitral and tricuspid valve repair, is warranted to define the optimal treatment of those patients. Also, homogeneous or comparable technical details (lesion set and energy) are vital to evaluate outcomes. This will add considerable weight to the argument of whether the benefits of surgical ablation extend further than simple maintenance of NSR.
Based on the findings of this review, surgical ablation concomitant to cardiac surgery results in increased freedom from AF at 12 months post-operatively without increasing 30-day mortality. There was no significant effect on safety outcomes such as MACE or stroke in the 12 month follow up, however one study showed a significant increase in the incidence of renal failure requiring hemodialysis in the ablation group. 2 studies also showed a significant increase in the number of patients requiring pacemaker implantation following ablation, especially in those who underwent a biatrial lesion set. Health related quality of life was sparsely reported, however a significant improvement at 12 months follow-up using the SF-36 was seen in patients who underwent ablation compared to the control.
Conceptualization—CD and MC; methodology—CD; writing—original draft preparation—CD; writing—review and editing—CD and MC; supervision—MC. All authors have read and agreed to the published version of the manuscript.
Not applicable.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions. A special thanks to Mohamad Bashir for English language review.
This research received no external funding.
The authors declare no conflict of interest.
