Academic Editor: Jane A. Leopold
Background: Available nomograms to predict aortic root (AoR) diameter
for body surface area have limitations. The purpose of this study was to evaluate
the use of a new multivariate predictive model to identify AoR dilatation in
hypertensive patients with left ventricular hypertrophy. Methods: 943 of
961 patients in the Losartan Intervention For Endpoint reduction in hypertension
(LIFE) echocardiographic sub-study had the necessary baseline characteristics and
echocardiographic 2D measurements of AoR size to be included. Results:
Predicted AoR (Sinus of Valsalva) diameter was 1.519 + (age [years]
Being the immediate receiver of the total cardiac output the aortic root (AoR) is important when studying pathological changes in the heart and equally important is the heart when evaluating pathology in the proximal aorta. AoR dilatation is an important pathophysiological mechanism behind aortic regurgitation (AR) in patients with Marfan syndrome [1, 2], bicuspid aortic valve [3, 4] and in severe, pure AR in patients with no valvular abnormality [5, 6]. With increasing AoR diameter the degree of cusp overlap is reduced leading to AR. AoR dilatation is also associated with serious conditions such as aortic dissection [7]. AoR diameter is frequently evaluated by echocardiography in patients with valvular heart disease, aortic aneurisms or manifest heart failure.
Early necropsy [8] and cross sectional studies [9] showed AoR dimensions to be related to body size, gender and age, and hence nomograms based on body surface area (BSA) and age intervals have been widely used and adopted in guidelines [10, 11, 12]. These nomograms have several limitations including no consideration of gender differences, broad age-intervals and indexation by BSA to adjust for differences in body size. This latter is compromised by overweight (increasing BSA makes larger diameters fall into the normal range) and has previously been described as mathematically incorrect [13, 14]. In addition, reference values indexed to height are warranted by various researchers, e.g., in a recent recommendation paper from the European Association of Echocardiography [11]. To counter the limitations of existing nomograms Devereux et al. [15] developed a multivariate predictive model based on measurements of AoR size in 1207 healthy individuals. Patients with hypertension and left ventricular hypertrophy are of particular interest in this context. The present study was a sub-study of the LIFE study [16, 17], an investigation of 9193 hypertensive patients with left ventricular hypertrophy. This study had an echocardiographic sub-study comprising 961 patients with echocardiographic variables to access the aortic root diameter. The aim of this study was to validate further this new multivariable model by determining the prevalence and predictors of AoR dilatation in hypertensive patients with left ventricular hypertrophy (LVH). Furthermore, we compared the results obtained using this new model to those obtained using the existing nomograms in our study population.
N = 961 patients with essential stage II–III hypertension and ECG-LVH were
enrolled from the Losartan Intervention for Endpoint Reduction in Hypertension
(LIFE) echocardiographic sub-study (representing 11% of the total LIFE study
population). The LIFE Study was a randomized, prospective, double-blinded,
parallel group study designed to compare the effects of losartan and atenolol
regarding cardiovascular morbidity and mortality. Eligible patients were men and
women age 55 to 80 with baseline blood pressure 160–200/95–115 mmHg and LVH by
sex-adjusted Cornell voltage-duration criteria (
Ethical committees for all participating centers in the LIFE echocardiographic
sub-study approved the protocol for the sub-study and all participating patients
in the echo sub-study signed written informed consent. Echocardiograms were
obtained at baseline and annually thereafter during the 5-year study period.
Echocardiographic procedures for this study were performed using commercially
available echocardiographs with M-mode, 2-dimensional and color-flow Doppler
capabilities. All techniques were based on procedures employed in previous
studies [19, 20, 21, 22] and have been described in detail elsewhere [23, 24].
Standardized examinations included two-dimensional guided M-mode echocardiograms
as well as selected two-dimensional and Doppler recordings. AoR diameter was
evaluated at the level of the Sinus of Valsalva using two-dimensional
measurements of the maximal distance between the leading edges of the anterior
and posterior aortic root walls in end-diastole. The Sinus of Valsalva was used
because it fits with other similar studies, and it is also recommended to use by
the American Society of Echocardiography
(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3462295/). Aortic regurgitation was
assessed using color flow Doppler recordings from parasternal and apical windows
[25]. Measures of LV internal dimension and wall thickness were performed at
end-diastole and end-systole following recommendations of American Society of
Echocardiography [26]. All echocardiographic recordings were sent to the
Echocardiographic Reading Center at New York Hospital - Cornell Medical Center
(New York, NY, USA) for standardized and blinded interpretation by experienced technician
and physician readers. LVH was defined as left ventricular mass index
The predicted AoR diameter at the Sinus of Valsalva for age, sex and height was
calculated using a multi-variate regression model recently developed by Devereux
et al. [15] based on measurements of 1207 healthy adults: AoRM [cm] =
1.519 + (age [years]
Data management and analysis were performed using SPSS 20.0 software (SPSS Inc.,
Chicago, IL, USA). The study population was divided into 2 groups by the presence
or absence of AoR dilatation as described above. In the following, continuous
variables are expressed as mean
Descriptive data of the entire LIFE Study population have been reported
elsewhere [16, 17]. Of the 961 patients included in the LIFE echocardiographic
sub-study, 945 patients had the necessary baseline 2D measurements of the AoR to
be included in the present study. Baseline anthropometric measures for two
patients were missing, and they were therefore excluded. N = 943 patients met the
inclusion criteria (58.7% men) and their characteristics are presented in Table 1. Mean age was 65.9
| N = 943 | |
| Male sex (%) | 58.7 |
| Age (years) | 65.9 |
| Body surface area (m |
1.89 |
| Body mass index (kg/m |
27.3 |
| Systolic blood pressure (mmHg) | 174 |
| Diastolic blood pressure (mmHg) | 95 |
| Pulse pressure (mmHg) | 78 |
| Mean blood pressure (mmHg) | 122 |
| Pulse rate (beats/min) | 72 |
| Serum cholesterol (mmoL/L) | 6.0 |
| Serum creatinine ( |
90 |
| Serum glucose (mmoL/L) | 6.0 |
| Diabetes mellitus (%) | 11.2 |
| Smoking (%) | 20.2 |
| Previous stroke (%) | 7.8 |
| Previous myocardial infarction (%) | 5.5 |
The distributions of measured AoR dimensions in the study population of 943
patients are presented in Fig. 1. The mean AoR diameter (at the level of
Sinus of Valsalva) was 3.60
 Fig. 1.
Fig. 1.Aortic root diameter at the Sinus of Valsalva measured by 2D
echocardiography. The distributions of measured AoR dimensions in the study
population of 943 patients are presented in men and women. Men had larger AoR
diameters than women (3.75
Using the new multivariate predictive model including height, age and gender,
the mean predicted AoR diameter was 3.52
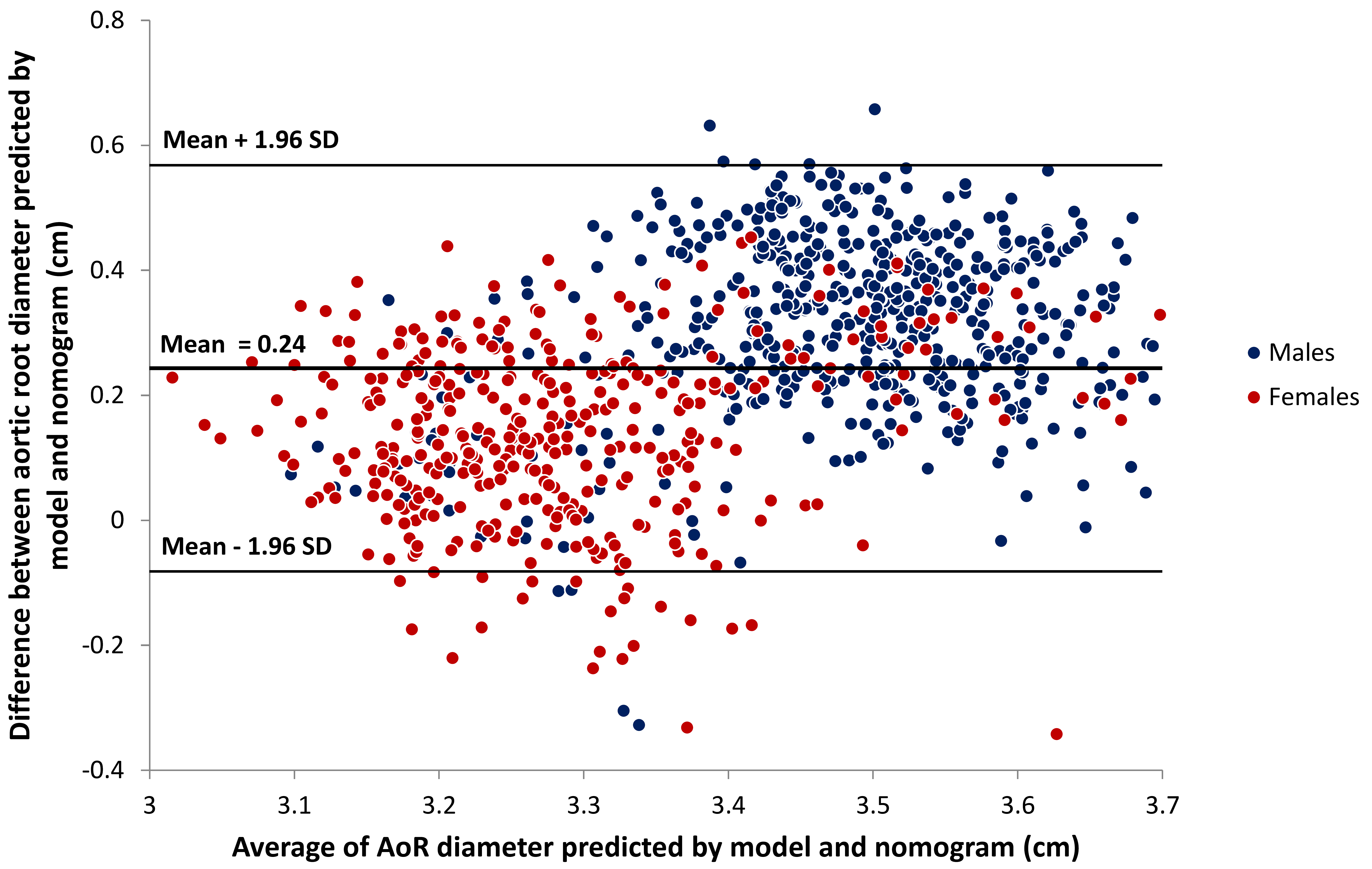 Fig. 2.
Fig. 2.Bland-Altman plot comparing AoR diameter predicted by model and
by nomogram. Using the new multivariate predictive model including height, age
and gender, the mean predicted AoR diameter was 3.52
Using the new multivariate predictive model to identify patients with AoR
dilatation the overall prevalence was 13.1% (124 of 943 patients) and there was
no difference between genders (13.7% in men, 12.3% in women, p =
0.537). Using the existing nomograms the prevalence of AoR dilatation was 11.7%
(110 of 943 patients) with a significantly higher prevalence among men (15.9%
vs. 5.7%, p
Overall there was a consensus of AoR phenotype between nomogram and model in 875 of the 943 patients (92.8%, Table 2). Regarding gender there was disagreement concerning AoR dilatation in 28 women (7.2%) of which 27 women had AoR dilatation only by model definition and one woman had AoR root dilatation only by nomogram definition. In men, disagreement existed for 40 patients (7.2%) of which 14 had AoR dilatation only by model definition and 26 had AoR dilatation only by nomogram definition.
| AoR dilatation by nomogram [BSA] | No | Yes | No | Yes | Total | Consensus |
| AoR dilatation by model [age, sex, height] | No | No | Yes | Yes | ||
| Women | 340 (87.4) | 1 (0.3) | 27 (6.9) | 21 (5.4) | 389 (100) | 361 (92.8) |
| Men | 452 (81.6) | 26 (4.7) | 14 (2.5) | 62 (11.2) | 554 (100) | 514 (92.8) |
| All patients | 792 (84.0) | 27 (2.9) | 41 (4.3) | 83 (8.8) | 943 (100) | 875 (92.8) |
| Legend: BSA, body surface area; AoR, aortic root diameter. | ||||||
The two predicted variables (predicted ad modum Devereux and predicted ad modum
Roman) were moderately correlated (R = 0.60, p
Patients with AoR dilatation identified by the new multivariate predictive model
were, in univariate analyses, younger (64.2
| MODEL (height, sex, age) | p | NOMOGRAM (BSA) | p | |||
| Normal | Dilatation | Normal | Dilatation | |||
| N = 819 (86.9%) | N = 124 (13.1%) | N = 833 (88.3%) | N = 110 (11.7%) | |||
| Clinical characteristics: | ||||||
| Men (%) | 58 | 61 | 0.537 | 56 | 80 | |
| Age (years) | 66.2 |
64.2 |
0.004 | 65.8 |
66.3 |
0.480 |
| Height (cm) | 170 |
170 |
0.945 | 169 |
171 |
0.072 |
| Weight (kg) | 78 |
81 |
0.026 | 79 |
76 |
0.032 |
| Body surface area (m |
1.89 |
1.92 |
0.107 | 1.89 |
1.88 |
0.409 |
| Body mass index (kg/m |
27.1 |
28.2 |
0.010 | 27.5 |
25.9 |
|
| Blood tests: | ||||||
| Serum cholesterol (mmoL/L) | 6.0 |
5.8 |
0.083 | 6.0 |
5.7 |
0.032 |
| Serum creatinine ( |
91 |
90 |
0.713 | 90 |
92 |
0.324 |
| Serum glucose (mmoL/L) | 6.0 |
5.9 |
0.586 | 6.0 |
6.0 |
0.572 |
| Cardiovascular disease risk factors: | ||||||
| Diabetes mellitus (%) | 11.7 | 8.1 | 0.230 | 11.3 | 10.9 | 0.907 |
| Smoking (%) | 19.2 | 26.6 | 0.055 | 19.1 | 28.2 | 0.026 |
| Previous stroke (%) | 7.9 | 7.3 | 0.792 | 7.6 | 10.0 | 0.372 |
| Previous myocardial infarction (%) | 6.1 | 1.6 | 0.041 | 5.8 | 3.6 | 0.359 |
| Blood pressures and pulse: | ||||||
| Systolic blood pressure (mmHg) | 174 |
169 |
0.017 | 174 |
172 |
0.304 |
| Diastolic blood pressure (mmHg) | 95 |
97 |
0.037 | 95 |
96 |
0.438 |
| Pulse pressure (mmHg) | 79 |
72 |
79 |
75 |
0.100 | |
| Mean blood pressure (mmHg) | 121 |
121 |
0.997 | 121 |
121 |
0.938 |
| Pulse rate (beats/min) | 72 |
73 |
0.488 | 72 |
72 |
0.939 |
| Legend: BSA, body surface area. | ||||||
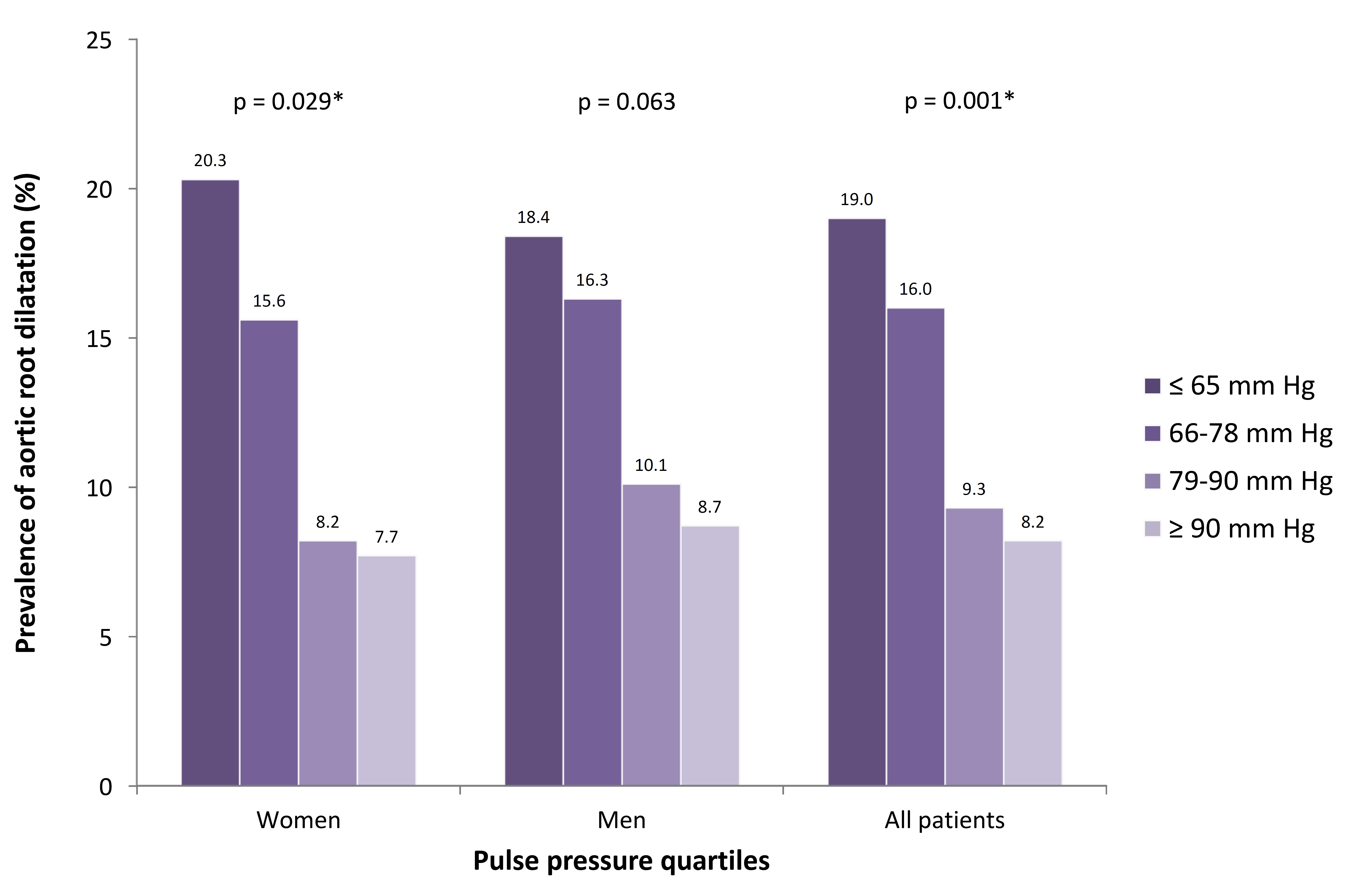 Fig. 3.
Fig. 3.Prevalence of aortic root dilatation for pulse pressure quartiles. When dividing pulse pressure into quartiles,there was a significant (*) higher prevalence of AoR dilation for lower quartiles of pulse pressure in both women and in all patients.
Using nomogram definition, AoR dilatation was associated with male gender (80
vs. 56%, p
| MODEL (height, gender, age) | p | NOMOGRAM (BSA) | p | |||
| Normal | Dilatation | Normal | Dilatation | |||
| N = 819 (86.9%) | N = 124 (13.1%) | N = 833 (88.3%) | N = 110 (11.7%) | |||
| Aortic root diameter (cm) | 3.52 |
4.14 |
3.52 |
4.20 |
||
| Left ventricular mass (g) | 231.3 |
252.3 |
232.1 |
248.8 |
0.005 | |
| Left ventricular mass/BSA (g/m |
122.3 |
131.6 |
122.3 |
132.7 |
0.000 | |
| Left ventricular mass/height |
55.6 |
60.6 |
56.0 |
58.3 |
0.076 | |
| Stroke volume (mL) | 81 |
86 |
0.004 | 81 |
86 |
0.005 |
| Stroke volume/pulse pressure (mL/mmHg) | 1.09 |
1.27 |
1.10 |
1.21 |
0.012 | |
| Cardiac output (L/min) | 5.1 |
5.7 |
5.2 |
5.5 |
0.035 | |
| Total peripheral resistance (dyn·sec·cm |
3763 |
3429 |
0.003 | 3743 |
3547 |
0.089 |
| Aortic regurgitation (%) | 13.9 | 29.5 | 14 | 30.6 | ||
| - moderate/severe (%) | 2.4 | 10.7 | 2.6 | 10.2 | ||
| Left ventricular hypertrophy (%) | 68.9 | 79.7 | 0.015 | 69.1 | 79.8 | 0.021 |
| - eccentric (%) | 42.8 | 50.4 | 0.114 | 43.1 | 49.5 | 0.200 |
| - concentric (%) | 26.1 | 29.3 | 0.460 | 26.0 | 30.3 | 0.346 |
| Legend: BSA, body surface area. | ||||||
Using either definition of AoR dilatation (multivariate predictive model or
nomogram), patients with dilated AoR’s had higher LV mass, LV mass index, stroke
volume, stroke volume/pulse pressure ratio and cardiac output (Table 4). Using
the multivariate model the difference was greater between patients with dilated
and normal AoR and the p-values were lower than using nomogram
definition. With the new model patients with AoR dilatation had higher left
ventricular mass indexed by height
The prevalence of aortic regurgitation (AR) in the present study population was
16.0% with no difference between genders (15.6 in men vs. 16.4% in women,
p = 0.774). The prevalence of mild (1+) AR was 12.5% and
moderate/severe (
Factors associated with AoR dilatation in our population (by multivariate
predictive model definition) were used as predictor variables (Table 5). In a
multivariate logistic regression model with age, weight, history of myocardial
infarction, pulse pressure, left ventricular mass index, stroke volume and aortic
regurgitation, AoR dilatation was predicted by age (OR 0.75 per SD increase,
p = 0.023), pulse pressure (OR 0.64 per SD increase, p
| Univariate | Multivariate | |||||
| Odds ratio | 95% CI | p | Odds ratio | 95% CI | p | |
| Age (per SD increase) | 0.75 | 0.62–0.92 | 0.004 | 0.75 | 0.59–0.96 | 0.023 |
| Weight (per SD increase) | 1.23 | 1.02–1.47 | 0.027 | 0.94 | 0.74–1.2 | 0.616 |
| History of myocardial infarction | 0.25 | 0.06–1.05 | 0.058 | 0.30 | 0.07–1.32 | 0.110 |
| Pulse pressure (per SD increase) | 0.66 | 0.54–0.81 | 0.64 | 0.51–0.82 | ||
| Left ventricular mass index (per SD increase) | 1.38 | 1.16–1.64 | 1.36 | 1.08–1.7 | 0.008 | |
| Stroke volume (per SD increase) | 1.54 | 1.27–1.87 | 1.45 | 1.15–1.84 | 0.002 | |
| Aortic regurgitation | 2.59 | 1.64–4.1 | 2.67 | 1.58–4.52 | ||
| Legend: SD, standard deviation; CI, confidence interval. | ||||||
This is the first study to evaluate the use of a multivariate model to predict normal aortic root size and identify aortic root dilatation in patients with hypertension and LVH. Our study shows three new observations using the proposed multivariate predictive model for gender, age and height. First, the overall prevalence of AoR dilatation in this specific population of hypertensive patients with LVH was 13%, which is higher than seen in previous reports. Second, in contrast to previous studies [28, 29, 30, 31], we report AoR dilatation to be equally prevalent in men and women. Finally, our study complements previous studies by the multivariate logistic regression analysis showing left ventricular mass index, stroke volume, pulse pressure and presence of aortic regurgitation to be predictors of AoR dilatation.
AoR dilatation is associated with aortic regurgitation [1, 2, 3, 4, 5, 6] and risk for
serious events such as aortic dissection [7]. Available nomograms to predict
normal AoR diameter for body size are based on modest-sized reference populations
and lack consideration of gender effects and direct continuous relationship with
age. The new multivariate predictive model was derived from echocardiographic
assessment of 1207 normal individuals
Evidently the prevalence of AoR dilatation in a studied population reflects the definition used and the purpose of this study was to evaluate the use of a more dynamic definition than the available nomograms and cutoff values. Interestingly, in our population the mean AoR diameter predicted by the multivariate model was higher than that predicted by the nomogram and this was true overall, in men and in women. Because the standard error of the estimate was lower with the multivariate model (0.22 cm vs. 0.37 cm), the average upper normal limit of normal AoR diameter was lower with this model, leading to an overall higher prevalence of AoR dilatation (13.1 vs. 11.7%). Consensus between the new multivariate model and the existing nomogram concerning the presence or absence of AoR dilatation was 92.8%.
The prevalence of AoR dilatation observed in our population is slightly higher than that found by Cipolli et al. [30] (10.5%) in comparable population of 438 patients with hypertension and LVH and by Cuspidi et al. [28] (11.8%) in a population of 2229 hypertensive patients referred to echocardiographic assessment of hypertension-related cardiac damage. The prevalence in our study is approximately twice that previously observed by Cuspidi et al. [28] (6.1%) in a population of 3366 patients with uncomplicated hypertension [31] and by Palmieri et al. [29] (4.6%) in 2096 hypertensive and 361 normotensive patients. The higher prevalence in our study may reflect that our patients are older and have more hypertension-related target organ damage and that hypertension mediated target organ damage may also be found in the central vasculature.
The multivariate model identified more women and fewer men with dilated aortic roots, leading to an equal prevalence of AoR dilatation in men (13.7%) and women (12.3%). It is a matter of discussion whether AoR dilatation should be as much as 2–4 times more common in men than women as observed in previous studies using various definitions of normal AoR size [28, 29, 30, 31]. However, gender is a prime determinant of AoR diameter independent of age and body size, emphasizing the need of differentiated normal values. In our population the difference in mean AoR diameter was 0.37 cm with men having larger diameters compared to women and this difference remained significant after controlling for age and height (data not shown). From our data, the multivariate model seems to address the issue of significant gender differences in AoR size by identification of equal prevalence among men and women.
In both men and women we detected a higher brachial pulse pressure in patients with AoR dilatation compared to patients with normal aortic roots on basis of both lower systolic blood pressure and a higher diastolic blood pressure. This finding confirms what has been found in the Framingham cohort [34, 35]. The relationship between AoR dimensions and systemic blood pressure has been investigated in numerous studies yielding conflicting results. Some studies find AoR size to be associated with both higher systolic and diastolic blood pressure [36], while others report association only to higher diastolic blood pressure [28, 29, 37]. The apparent relationship between AoR size and blood pressure has led to the notion that smaller AoR size predisposes to hypertension, but longitudinal studies on AoR dilatation and incidence of new onset hypertension have failed to show a causal relationship [38]. Intuitively it seems reasonable that a high distending pressure (both in systole and diastole) leads to dilatation of the AoR by pure mechanical force. On the other hand, a stiff and calcified arterial system with a non-dilated AoR leads to a higher systolic blood pressure, lower diastolic blood pressure and consequently a higher pulse pressure. Hypertension is known to increase wall stress and promote atherosclerotic changes in the vessel wall and the explanation to the chicken-and-egg dilemma of AoR dilatation and hypertension [14] might be that patients susceptible to atherosclerotic changes have a diminished age-dependent dilatation and compliance of the AoR whereas less susceptible patients dilate and retain a low pulse pressure. The idea of AoR dilatation being a non-atherosclerotic process is in line with previous studies showing risk factors of atherosclerosis to account for less than 2% of the variability in aortic dimensions [39] and this study finding no association with diabetes or smoking. Furthermore, pulse pressure was associated with cardiovascular morbidity and mortality and in our population we detected a lower prevalence of previous myocardial infarction in patients with AoR dilatation.
There are some limitations to the present study. The existing nomograms, the proposed multivariate model and our echocardiographic measurements all concentrate on the diameter at a single level of the AoR (Sinus of Valsalva), which prevents us from correlating our findings to the entire AoR. Furthermore, this study investigated patients enrolled in a randomized clinical trial, which might have excluded patients with more severe cardiovascular disease. The external validity of our findings might not extend beyond comparable patients with hypertension and LV hypertrophy. Finally, this is a descriptive study in nature and to address the question of prognostic value of AoR dilatation a prospective trial with a long observation period is needed using the proposed model to define AoR dilatation.
AoR dilatation was associated with higher LV mass, LV hypertrophy (in particular eccentric hypertrophy) and stroke volume as well as aortic regurgitation. In our hypertensive population patients with aortic root dilatation might constitute a subgroup of patients with increased volume load in addition to the pressure load of hypertension.
The clinical implication of this study is that we now have a more precise tool to predict AoR size and thereby identify patients with AoR dilatation. This is important for the clinician in echocardiographic assessment of a patient and for researchers designing future studies to investigate the implications of AoR dilatation. Compared to available nomograms for identification of aortic root dilatation, the multivariate predictive model integrating gender and age in addition to body size might identify aortic root dilatation more correctly and seems to identify a more homogenous group of patients. Furthermore, using the proposed multivariate model aortic root dilatation is equally common in men and women with a prevalence of 13% in our population of hypertensive patients with left ventricular hypertrophy.
SEK, SJ and RBD were involved in the study’s conceptualization and methodology. AL, CNB, EG, ACL, SEK, SJ, PMO, KW and RBD were involved in the data collection. AL, CNB, PMO, KW and RBD were in involved in the statistical analysis. AL, KW and RBD were involved in the writing of the first draft. AL, CNB, EG, ACL, SEK, SJ, PMO, KW and RBD were involved in the review and editing of the study. All authors read and approved the final manuscript.
Ethical committees for all participating clinical centers approved the LIFE Study. The study was performed in accordance with the Declaration of Helsinki. The protocol was written and the study was chaired by an academic steering committee, and it was overseen by an independent data and safety monitoring board. Ethical committees for all participating centers in the LIFE echocardiographic sub-study similarly approved the investigator written protocol for the sub-study and all participating patients in the echo sub-study signed written informed consent.
The authors thank the many patients who participated in the study.
The present study was supported by grant no. CDSP-COZ-368 from Merck & Co., Inc., West Point, PA, USA.
Sverre E. Kjeldsen has received lecture honoraria within the past 3 years from Getz Pharma, Merck Healthcare KGaA, Sanofi-Aventis and Vector-Intas. The other authors declare that they have no conflicts of interest.
