†These authors contributed equally.
Academic Editor: Vincenzo Lionetti
Objectives: Although injury of myocardium after percutaneous coronary
intervention (PCI) has been reported, the mechanism and effect of exogenous
phosphocreatine (PCr) supplementation on the injury are yet to be elucidated.
Biomarkers, such as interleukin-6 (IL-6) and variations in white blood cells for
inflammation, and serum cardiac troponin I (cTnI) for myocardial injury are
examined. Methods: A total of 105 patients undergoing PCI were included
and randomly divided into two groups: control (treated with routine hydration
therapy) and PCr (treated with additional intravenous infusion of exogenous PCr).
The serum levels of biomarkers were detected at administration and 4, 12, 24, and
48 h after PCI, with natural logarithmic (log
Cardiovascular disease (CVD) is the main cause of disease burden worldwide, with high morbidity and mortality, and is continuously increasing in most countries [1]. Since Gruentzig successfully performed the first percutaneous transluminal coronary angioplasty (PTCA) in 1977, percutaneous coronary intervention (PCI) has rapidly evolved into the first-line therapy in most patients with acute coronary syndrome [2]. However, PCI causes myocardial injury, inflammatory reaction, and ischemia-reperfusion injury in ischemic and necrotic areas, making some patients suffer from major adverse cardiovascular events (MACEs) after the operation and affecting the therapeutic effect [3]. Therefore, identifying the mechanism and intervention measures of myocardial injury after PCI is essential. Reportedly, stent implantation triggered the acute phase response and systemic inflammation, which was associated with increased plaque burden and pathological features [4].
Phosphocreatine (PCr) is the main form of chemical energy reserve of myocardial cells with a critical role in energy metabolism during myocardial contraction. A declined level of PCr may have adverse effects on myocardial contraction and recovery of cardiac function [5]. Sodium PCr is an efficient energy supplement, which can effectively supply exogenous myocardial energy and play a critical role in reducing myocardial injury and maintaining normal cardiac function [6]. A recent study found that additional sodium PCr treatment improves early cardiac dysfunction and 28-day survival outcomes in patients with septic shock [7]. Thus, PCr is recommended to be involved in inflammation. However, the mechanism and effect of exogenous PCr on myocardial injury after PCI lacks clinical evidence.
In the present study, we hypothesized that exogenous PCr supplementation before operation alleviates systemic inflammation triggered by PCI to protect the myocardium. A series of experiments were designed and performed to test this hypothesis. Interleukin-6 (IL-6) and blood cell count were used to reflect the inflammation. Cardiac troponin I (cTnI) was adopted as a sensitive marker of myocardial injury [8].
A total of 105 patients with coronary heart disease, admitted to the Department of Geriatric Medicine, Qilu Hospital of Shandong University (Jinan, China) from August 2019 to June 2021, undergoing PCI and meeting the enrollment criteria, were recruited in this study. The patients were randomly assigned to the control group (n = 51) or PCr group (n = 54) according to a random number table.
We included patients who had a non-ST-segment elevation of acute coronary syndromes (NSTE-ACS), identified by non-invasive examination or cardiac catheterization with at least 75% stenosis of one major coronary artery suitable for elective PCI, without extreme risk indicators.
The patients who fulfilled the following criteria were excluded from the study:
severe heart valve (mitral or aortic valve) stenosis or hypertrophic/restrictive
cardiomyopathy; allergic to contrast agent or exogenous PCr; chronic kidney
disease or estimated glomerular filtration rate (eGFR)
The minimum number of samples was calculated as 41 in each group, based on
Software PASS 11 (NCSS, LLC, Kaysville, Utah). Herein,
The patients received routine pharmacological treatment for coronary artery disease (CAD) as recommended by guidelines in both groups: anti-platelets, nitrates, statins, and beta receptor blockers. Patients with diabetes mellitus ceased metformin administration 24 h before the operation and were injected insulin subcutaneously to control the plasma glucose level. The patients in the control group were infused 100 mL saline intravenously, and those in the PCr group were treated with 4 g of sodium PCr (Harbin Lebotong Pharmaceutical Co., Ltd, Harbin, China) within 30 min before PCI.
The patients in both groups were administered lidocaine for local anesthesia, heparin, nitroglycerin, and non-ionic contrast agents during the operation. They also underwent routine electrocardiograph (ECG) examination and bedside ECG monitoring for 24 h after the operation and received secondary prevention of CAD.
Typically, 3–5 mL peripheral venous blood samples were collected from both
groups of patients at administration and 4, 12, 24, and 48 h after PCI. The serum
was separated by centrifugation of the blood samples at 4
Blood cell count was detected by blood routine Sysmex XE2100 hematology analyzer (Sysmex Corp. Kobe, Japan). The parameters were similar to those for the routine blood test. The blood glucose, lipids, and biochemical indicators of the patients were measured in a standard fashion.
The primary endpoint was the evaluation of IL-6 at 48 h after PCI to investigate the mechanism of PCr supplementation. The secondary endpoint was the evaluation of the myocardial injury based on serum cTnI after PCI, as well as the ratio of neutrophils (NEUR) to white blood cells (WBCs) to define the effect of PCr.
All statistical analyses were performed using SPSS 20.0 software (IBM, Armonk,
NY, USA). The quantitative data were assessed for normality and homogeneity of
variance. For non-normally distributed continuous variables, the natural
logarithmic transformation was carried out, and the normal or approximately
normal distribution was confirmed using the quantile-quantile (Q-Q) plot, while
the normally or approximately normally distributed data were represented as mean
A total of 105 patients, aged 64.14
 Fig. 1.
Fig. 1.Consort flow diagram. Patients were randomly assigned to the control and PCr groups. No patient withdrew from the study.
The proportion of patients with the previous diagnosis of hypertension,
diabetes, old myocardial infarction, heart failure with New York Heart
Association (NYHA) classification of cardiac function worse than grade II, and
coronary intervention was 44.76%, 24.76%, 15.00%, 12.38%, and 11.43%,
respectively. The clinical characteristics of the patients are summarized in
Table 1. The basic fasting blood glucose level and low-density lipoprotein
cholesterol (LDL-C) of all patients measured at the time of admission were 5.81
| Clinical factors | PCr group | Control group | t/x |
p value |
| (n = 54) | (n = 51) | |||
| Age (year, |
62.87 |
65.49 |
1.232 | 0.221 |
| Gender (male) (n, %) | 33 (61.11%) | 36 (70.59%) | 1.046 | 0.307 |
| BMI (Kg/m |
26.30 |
25.72 |
–1.028 | 0.306 |
| Hypertension (n, %) | 24 (44.44%) | 23 (45.10%) | 0.005 | 0.946 |
| Diabetes mellitus (n, %) | 13 (24.07%) | 13 (25.49%) | 0.028 | 0.867 |
| Old myocardial infarction (n, %) | 8 (14.81%) | 5 (9.80%) | 0.607 | 0.436 |
| Cardiac dysfunction (NYHA |
5 (9.26%) | 7 (13.73%) | 0.517 | 0.472 |
| Previous PCI (n, %) | 12 (22.22%) | 8 (15.69%) | 0.727 | 0.394 |
| Statins (pitavastatin) (n, %) | 29 (53.70%) | 26 (50.98%) | 0.078 | 0.780 |
| Diseased vessels (n, |
2.46 |
2.63 |
0.998 | 0.320 |
| Implanted stents (n, |
1.50 |
1.73 |
1.517 | 0.132 |
| Procedural time (minutes, |
52.54 |
52.84 |
0.089 | 0.930 |
| volume of contrast medium (mL, |
90.46 |
101.67 |
1.771 | 0.081 |
| trans-arterial approach (radial artery) (n, %) | 49 (90.74%) | 46 (90.20%) | 0.000 | 1.000 |
| Gensini scores (per case, |
48.94 |
40.49 |
–1.420 | 0.159 |
| NYHA, cardiac function grading of New York Heart Academy; BMI, body mass index,
calculated by dividing weight (Kg) by the square of height (m | ||||
The usage of statins, average amounts of diseased vessels, implanted stents,
procedural time, the volume of contrast medium, transarterial approach, and
Gensini scores in the major branches of coronary circulation (left main coronary
artery, left anterior descending branch, left circumflex branch, and right
coronary artery) did not show significant intergroup gaps (all p
The natural logarithm of IL-6 (log
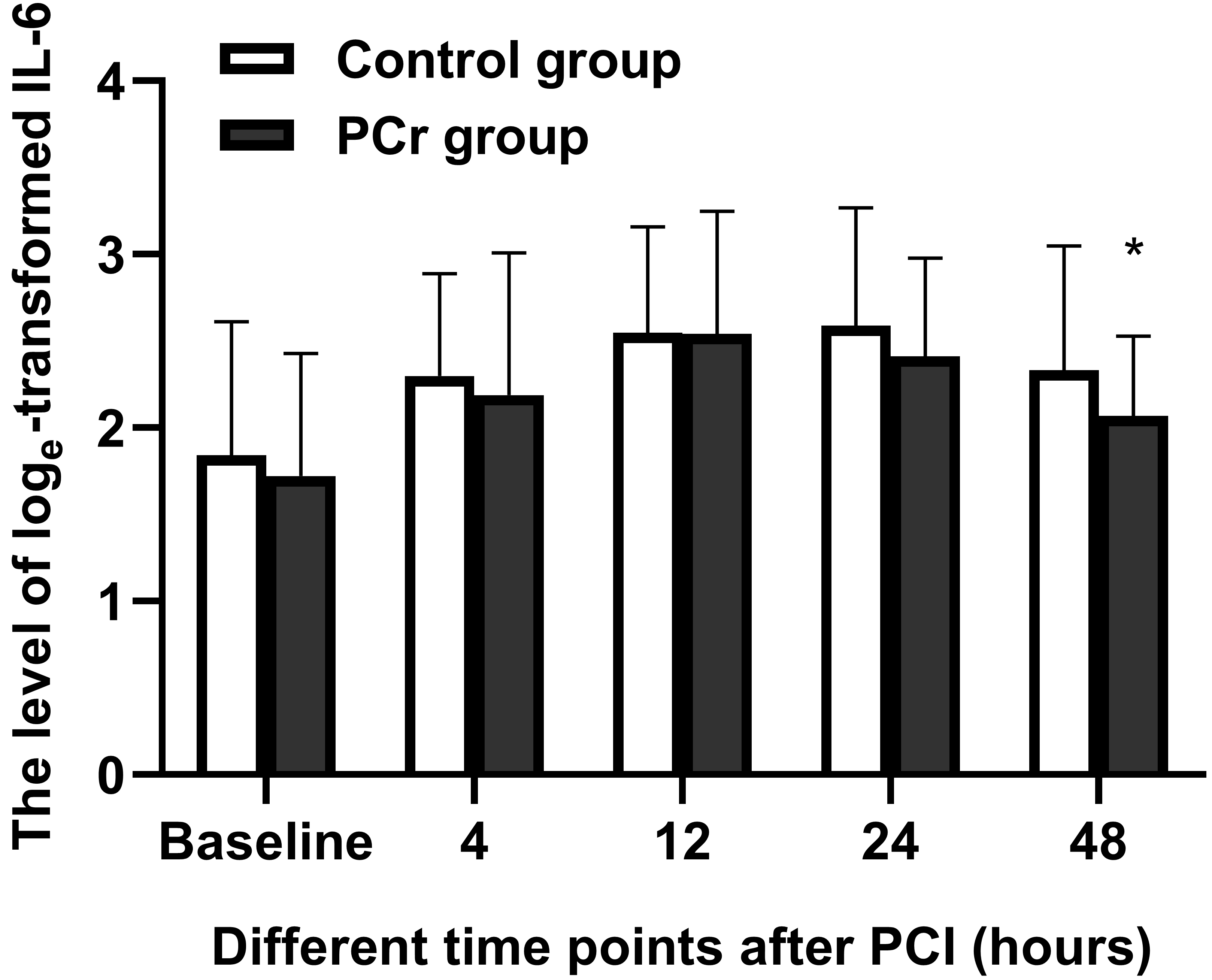 Fig. 2.
Fig. 2.Effects of PCr on IL-6. The number of patients enrolled was 51
in the control group and 54 in the PCr group. Values are log
No statistical difference was detected in log
 Fig. 3.
Fig. 3.Effects of PCr on myocardial injury. The number of patients
enrolled was 51 in the control group and 54 in the PCr group. Values are natural
logarithmic transformed highly sensitive cardiac troponin I plus 2
(log
The WBC count, including neutrophils (NEU), lymphocytes (LYM), eosinophils
(EOS), basophils (BAS), and monocytes (MON), and their ratios to WBCs, including
NEUR, LYMR, EOSR, BASR, and MONR, did not show any significant difference between
the PCr and control groups before PCI (all p
Compared to the baseline at admission, the WBC count in both groups was
increased significantly at 4 h and 12 h after PCI, and that in the control group
was still higher at 24 h (p
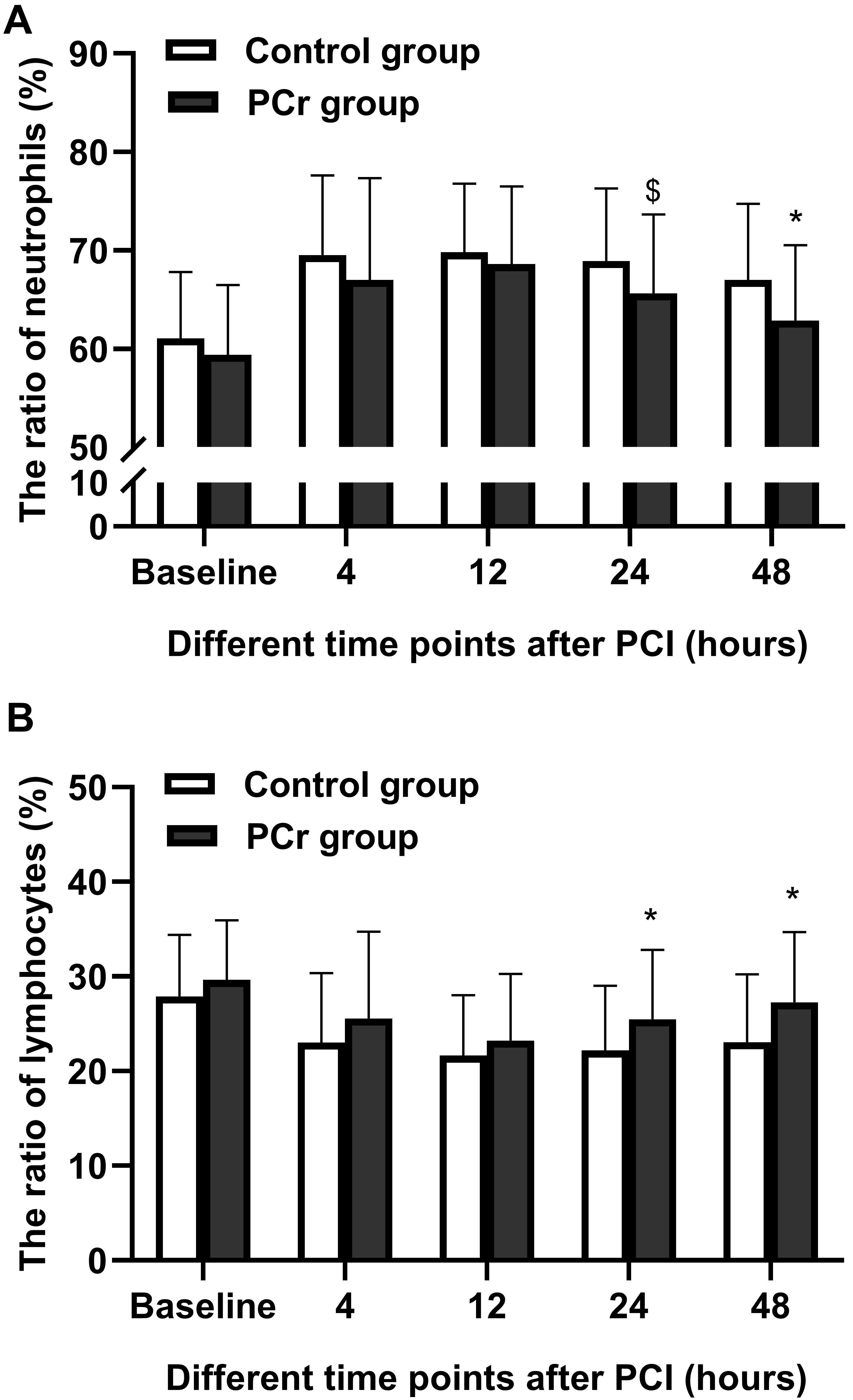 Fig. 4.
Fig. 4.Levels of NEUR and LYMR of the patients at different time
points. The number of enrolled patients was 51 in the control group and 54 in
the PCr group. Values are mean
The indicators of RBCs, including the RBC count, hemoglobin (HGB), hematocrit
(HCT), erythrocyte mean corpuscular volume (MCV), mean corpuscular hemoglobin
(MCH), mean corpuscular hemoglobin concentration (MCHC), and red cell
distribution width (RDW), did not differ significantly between the PCr and
control groups before PCI (all p
Compared to the baseline at admission within groups, the RBC count, HGB, HCT,
MCH, MCHC, and RDW, PLT count, MPV, and PCT, decreased after PCI in both groups.
On the other hand, the MCV and PDW were predisposed to increase after PCI.
However, no obvious differences were detected between the PCr and control group
in these indicators (all p
At administration, no statistical alteration was detected in biochemical
biomarkers for hepatic function, including alanine transaminase (ALT),
| Variables | PCr group (n = 54) | Control group (n = 51) | t | p value |
| ( |
( | |||
| ALT (U/L) | 18.98 |
24.80 |
1.783 | 0.079 |
| GGT (U/L) | 26.30 |
27.82 |
0.445 | 0.657 |
| AKP (U/L) | 72.41 |
74.04 |
0.420 | 0.676 |
| TBIL ( |
10.59 |
10.38 |
–0.245 | 0.807 |
| DBIL ( |
3.62 |
3.69 |
0.263 | 0.793 |
| IBIL ( |
6.97 |
6.69 |
–0.460 | 0.646 |
| TBA ( |
5.99 |
6.99 |
0.988 | 0.325 |
| TC (mmol/L) | 3.71 |
3.99 |
1.349 | 0.180 |
| HDLC (mmol/L) | 1.02 |
1.07 |
1.153 | 0.252 |
| LDLC (mmol/L) | 2.15 |
2.36 |
1.309 | 0.194 |
| TG (mmol/L) | 1.44 |
1.34 |
–0.812 | 0.418 |
| Glu (mmol/L) | 5.81 |
5.80 |
2.072 | 0.041 |
| BUN (mmol/L) | 5.24 |
5.58 |
–0.021 | 0.983 |
| Cys-c (mg/L) | 0.95 |
1.02 |
1.127 | 0.262 |
| UA ( |
326.15 |
329.47 |
1.601 | 0.112 |
| C1q (mg/L) | 171.94 |
167.62 |
0.207 | 0.837 |
| NT-proBNP (pg/mL) | 211.36 |
300.93 |
–0.777 | 0.439 |
| eGFR (mL/min/1.73 m |
99.31 |
92.89 |
–1.481 | 0.142 |
| ALT, alanine transaminase; GGT, | ||||
Recurrent angina pectoris comprised the main cardiovascular adverse events occurring within 48 h after PCI, and no significant difference was observed between the two groups (Fisher’s exact test, p = 1.000) (Table 3).
| Variables | Pcr group (n = 54) | Control group (n = 51) | ||
| n | % | n | % | |
| Recurrent angina pectoris | 1 | 1.85 | 1 | 1.96 |
| Malignant arrhythmia | 0 | 0.00 | 0 | 0.00 |
| Hypotension | 0 | 0.00 | 0 | 0.00 |
| Aggravation of ST-T segment change | 0 | 0.00 | 0 | 0.00 |
| Cardiovascular death | 0 | 0.00 | 0 | 0.00 |
| Deterioration of cardiac function | 1 | 1.85 | 0 | 0.00 |
| Stroke | 0 | 0.00 | 0 | 0.00 |
| Sum | 2 | 3.70 | 1 | 1.96 |
| There was no significant difference in the incidence of adverse cardiovascular
events between the PCr group and the control group (Fisher’s exact test,
p = 1.000 | ||||
CVD is mainly caused by stenosis or occlusion of the lumen due to atherosclerosis, which leads to myocardial ischemia, hypoxia, or infarction. PCI is one of the major methods to treat CVD by restoring coronary circulation and myocardial reperfusion. Some studies have shown that the myocardium is damaged during PCI, which is one of the main factors of cardiovascular adverse events in postoperative patients [9]. The myocardium is a tissue with high oxygen demand and consumption. When coronary artery stenosis occurs, the blood flow decreases, which destroys the balance of oxygen supply and demand. The primary changes caused by ischemia are the destruction of myocardial cells and vascular endothelial cells [10].
The destruction of intima also decreases nitric oxide production, which disrupts
the endothelium-dependent vasodilation function. The myocardial cells also press
the microvessels due to cell swelling and ischemia-induced injury, which is due
to the decrease in adenosine triphosphate (ATP) caused by long-term ischemia and
hypoxia, the inactivation of sodium-potassium-exchanging ATPase on the cell
membrane, the decrease in calcium ion outflow, and the limitation of endoplasmic
reticulum’s absorption of calcium ions, resulting in the overload of calcium ions
in myocardial cells. These changes in myocardial cells are accompanied by the
activation of intracellular protease, damaging the myofibrils. The reperfusion
injury potentiates the ischemic injury. Previous studies have shown that the
increase in serum cTnI level is closely related to the occurrence of MACEs after
PCI, which guides the therapeutic strategy [11]. The current results showed that
the log
The highly sensitive cardiac troponin I (cTnI) describes the damage of tiny
myocardium, and its molecular weight is smaller than that of myocardial
enzymology index, with high sensitivity and specificity. When the myocardium is
damaged, the intracellular adenosine triphosphate (ATP) level decreases, leading
to acidosis and increased permeability of the cell membrane; also, cTnI is easily
released into the blood [12]. The supply of energy is vital to relieve myocardial
ischemia and PCI-induced injury. PCr is a high-energy phosphate compound found in
the brain cells, myocardium and skeletal muscle. It participates in ATP
synthesis, forms actin through the hydrolysis effect, and supplies energy for the
contraction of the myocardium. The decline in the PCr level in the myocardium
affects the oxidative metabolism of myocytes, causes myocardial injury, and slows
down the repair process [13]. In addition, as an endogenous substance, PCr can
directly enter cardiac myocytes, providing energy for calcium-transporting
ATPases and sodium-potassium-exchanging ATPase, facilitating the transport of
intracellular Ca
Recent studies have reported that inflammation is involved in the development of
atherosclerotic plaque, and the release of inflammatory mediators activates the
complements, promotes leukocyte aggregation, damages the intima of blood vessels,
and induces the formation of plaque and the progression of nontarget lesions [4].
IL-6 is an acute-phase protein of inflammation. Typically, the level of IL-6 is
low in the body. Damaged or infected tissue results in a sharp increase in the
synthesis of IL-6 and its release into the blood, which in turn promotes the
monocytes to release tissue factors, thus increasing the risk of local thrombosis
[17]. IL-6 is also involved in the formation of atherosclerosis, causing an
intimal reaction in arteries, aggravating inflammation, and possibly causing
vasospasm [18]. The higher the level of IL-6, the more serious the inflammation
in patients with CVD, which further increases the risk of cardiovascular adverse
events that are detrimental to the prognosis of patients [19]. The current
results showed that log
Some debris (foam-like macrophages, aggregated platelets, cholesterol crystals, intimal debris, and thrombus fragments) fell to the distal end of blood vessels due to the rupture of unstable plaques and physical injuries, such as the entry of guidewires, balloon dilatation, and stent implantation during PCI [20]. Although a small amount of debris does not affect the forward blood flow, it induces the aggregation of inflammatory mediators and releases vasoconstrictors, such as thromboxane A2, serotonin, and tumor necrosis factor. Some studies have shown that shed debris can induce NEU aggregation, activate PLTs, and promote thrombosis. The primary mechanism of myocardial damage lies in the aggregation of NEUs and PLTs and the release of reactive oxygen species (ROS). Activated NEUs and PLTs release a large number of vasoconstrictors, inflammatory factors, ROS, and proteases, which further aggregate the NEUs and PLTs, aggravate the inflammatory reaction, form a vicious circle, cause the destruction and edema of the myocardium, and eventually lead to reperfusion injury [21].
NEUs are the main source of ROS during reperfusion and the key inflammatory cells of myocardial injury. In the event of severe inflammation, apoptosis decreases the LYM count while increasing the NEU count [22]. This variation trend was also observed in the present study. The current results showed that after PCI, the counts of WBC, NEU, and NEUR are higher than those before PCI, while those of LYM, EOS, BAS, and MON and the ratios are lower than the baseline. Moreover, the NEUR and LYMR altered at 12 h after PCI. This phenomenon was consistent with the mechanism of reperfusion injury. Therefore, preventing NEU-triggered inflammation during PCI perioperative period may be a key to treating myocardial injury. In this study, NEUR and LYMR in the PCr group were significantly improved compared to those in the control group at 48 h after the operation. This indicated that the intervention of exogenous PCr alleviates the inflammation related to NEUs after PCI, thus protecting the myocardium.
PDW and MPV are crucial features of PLT activation [23]. The current results described that PDW is increased after PCI, while MPV, PLT count, and PCT are decreased. PCr intervention had no effect on the PLT features. These phenomena may be essential to detect sensitive indicators of PLT reactivity [24, 25].
HGB is a specific protein that transports oxygen in RBCs. Anemia is related to serious cardiovascular diseases (such as thromboembolism and hemorrhage), which in turn increases the mortality of anemia patients during hospitalization [26]. The disease is also related to inflammation, which increases the release of erythropoietin and cytokines, leading to vascular endothelial damage, accelerating atherosclerosis, activating platelets, putting the body in a hypercoagulation state, and increasing the risk of thrombosis [27]. The current results showed that RBC, HGB, HCT, MCH, and MCHC were lower after PCI than those before PCI, which was similar to the previous studies. Compared to the control group, these indicators in the PCr group improved slightly, albeit not significantly. Thus, additional studies with larger sample sizes may be needed. Interestingly, the plasma concentration of free hemoglobin (fHGB) was significantly higher in patients with acute myocardial infarction (AMI) between 12 h and 24–48 h time points after stent implantation [28], suggesting that the decrease in HGB may be related to perioperative sterile inflammation and hemolysis.
Nevertheless, the present study has the following limitations. Firstly, this is a single-center, unblinded study, and no confounding factors have been identified to influence the results. Secondly, measuring troponin post-intervention is not clear, and indirect variables for myocardial injury and inflammation are used. Finally, no long-term follow-up was carried out in this study and can be continued in future studies to further evaluate the prediction of the risk of adverse cardiovascular events.
In conclusion, a series of operations during PCI in coronary arteries leads to vascular endothelial damage and vasospasm, increases the risk of thrombosis, affects the blood perfusion of distal vessels, aggravates myocardial ischemia and inflammation, and cause myocardial injury and adverse cardiovascular events. Exogenous PCr regulates the levels of serum cTnI, IL-6, NEUR, and LYMR in patients undergoing PCI, which is crucial for improving myocardial perfusion, alleviating inflammation, and regulating the functions of granulocytes and mononuclear cells. Additional studies are warranted to clarify the detailed mechanism and the long-term prognosis.
MYL performed the analysis, processed the experimental data, and wrote the article. YQX designed and directed the project, performed the statistical analysis, and edited the manuscript. YPS and CL were involved in planning and supervising of the experiments. YPS, ZHW and YW aided in interpreting the results and edited the manuscript. XHL, ZZ and RXZ performed the measurements. LYQ and ML were involved in planning the project and contributed to sample preparation. YLX and HQC aided in interpreting the results. All authors approved the final version to be submitted.
This study complied with the Declaration of Helsinki, was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University and registered at the Chinese Clinical Trial Registry Center (registration number: ChiCTR-IOQ-15007475). All participants provided written informed consents.
We thank all study coordinators and patients for their invaluable contribution.
This study was supported by the National Natural Science of China (grant numbers 81570356, 81700725, 82100472), the Key Research and Development Program of Shandong Province (grant number 2017GSF218101), the Natural Science Foundation of Shandong Province (grant number ZR2017BH003), the Science and Technology Foundation of Jinan City (grant number 201805057) and the Medical and Health Science and Technology Development Foundation of Shandong Province (grant number 2016WS0366).
The authors declare no conflict of interest.
