Academic Editor: Antonio Mangieri
Objectives: To assess the outcomes of transcatheter mitral
valve repair (TMVr) for failed previous surgical mitral valve repair (MVr).
Methods: We searched Pubmed, Embase, and Cochrane Library
databases for studies that reported the outcomes of TMVr for failed initial
surgical MVr. Data were extracted by 2 independent investigators and subjected to
meta-analysis. The 95% confidence interval (CI) was calculated for preoperative
demographics, peri-operative outcomes, and follow-up outcomes using binary and
continuous data from single-arm studies. Results: Eight single-arm
studies were included, with a total of 212 patients, and mean follow-up ranged
from 1.0 to 15.9 months. The pooled rate of residual procedural mitral
regurgitation
Mitral valve repair (MVr) is the treatment of choice for severe symptomatic mitral regurgitation (MR) recommended by current guidelines, especially for degenerative mitral valve (MV) disease [1, 2]. However, MVr carries a potential risk for reoperation, reducing late survival [3]. Redo surgery including MVr and mitral valve replacement (MVR) has been the gold standard for failed surgical MVr, defined by the recurrence of moderate or severe MR, mitral valve re-operation for any reason, such as mitral regurgitation, stenosis, hemolysis, or infective endocarditis [4]. However, it is associated with increased technical difficulty inherent to reoperations and greater frailty of the patients [5, 6].
Transcatheter procedures provide a minimally invasive alternative to redo surgery in high-risk patients. For this challenging scenario, transcatheter mitral valve replacement (TMVR) using valve-in-valve (ViV) and valve-in-ring (ViR) techniques have been focused on in the past few years [7, 8, 9, 10]. However, since first reported by Lim et al. [11] in 2010, there have been very few studies reporting transcatheter mitral valve repair (TMVr) for failed MVr. The safety and effectiveness of TMVr for failed surgical MVr have not been fully established. Also, there have been no clinical trials comparing TMVr, TMVR, or redo surgery for these patients. Whether the advantages of re-repair compared with Redo MVR can be applied in transcatheter procedures is not clear yet.
Thus, we conducted the present systematic review and meta-analysis to assess the outcomes of TMVr for failed previous surgical MVr.
The study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. On May 29, 2022, a comprehensive literature search was conducted of the Pubmed, Embase, and Cochrane Library databases, for relevant studies reporting the outcomes of TMVr for failed surgical MVr. The search strategy is the following words in full text: ((failed) OR (recurrent)) AND ((mitral valve repair) OR (mitral regurgitation) OR (mitral annuloplasty) OR (ring)) AND ((transcatheter) OR (percutaneous) OR (Mitraclip) OR (Neochord)).
The study protocol was registered with PROSPERO, ID: CRD42022336807.
The studies were considered for inclusion if they met the following criteria: (1) the population consisted of patients with previous surgical MVr; (2) re-intervention due to mitral repair failure; (3) previous surgical MVr either with or without an annuloplasty ring; (4) techniques of TMVr were not restricted: transcatheter mitral annuloplasty, edge-to-edge repair, and chordal implantation were acceptable.
The exclusion criteria were: (1) the initial surgery included transcatheter
procedures or surgical MVR; (2) re-intervention due to causes other than repair
failure; (3) TMVR procedures; (4) open cardiac reoperation; (5) studies with
Three authors (HX, SL, ZZ) screened and assessed the studies independently for inclusion. Disagreements regarding inclusion were resolved via a group consensus.
Two authors (WS, ZZ) reviewed and extracted the reported data from the studies, which included: details of the study (study design, inclusion criteria, study period, follow-up duration); baseline demographics; procedural details (echocardiographic evaluation of MR and stenosis); perioperative details (major morbidities, mortality, hospital stay); follow-up outcomes (follow-up duration, regurgitation recurrence, mortality, functional status).
The study quality and risk of bias were assessed using the methodological index for non-randomized studies (MINORS) [13]. Disagreements were resolved by consensus.
The analyses were performed utilizing R software version 4.2.0 (The R Foundation for Statistical Computing) with the open-source package Meta version 5.2-0, Metamedian version 0.1.5, and Metafor version 3.4-0. Both R and the packages were available as free software released under GNU General Public Licenses. The R software was developed by the R Foundation, downloaded from “https://www.r-project.org/”, the packages were downloaded from the Comprehensive R Archive Network (CRAN) within R.
Statistical heterogeneity was assessed using I
A total of 1266 studies were identified utilizing the search criteria. Based on title and abstract, 44 studies were retrieved for full-text review. TMVR was reported in 12 studies and open cardiac redo surgery in one study. In 2 studies, the previous surgery included MVR or non-mitral cardiac surgery. There were 6 review articles and 15 case reports with less than 5 cases. The remaining 8 studies [15, 16, 17, 18, 19, 20, 21, 22] comprised the pooled data (Fig. 1).
 Fig. 1.
Fig. 1.PRISMA Flow chart. The selection of studies included in the meta-analysis.
Two studies were single-arm prospective studies and 6 were single-arm retrospective studies. Two studies included only degenerative MR, two studies included only functional (Carpentier IIIb) MR and the other 4 did not specify pathological types. A total of 212 patients were included, with 197 patients undergoing MitraClip and 15 undergoing NeoChord. There was no study reporting percutaneous direct annuloplasty for failed surgical MVr. All studies reported short-term follow-up results; mean follow-up ranged from 1 to 15.9 months. The basic characteristics of the studies were listed in Table 1 (Ref. [15, 16, 17, 18, 19, 20, 21, 22]).
| Study Period | Center | Country | Pathology | Patients | Technique | Mean Follow-up (m) | Conclusion | |
| Rahhab 2021 [22] | 2009–2017 | Multi-center International | International | Not specified | 104 | MitraClip | N/A | MitraClip is safe and less invasive |
| Gerosa 2021 [21] | 2014–2018 | Multi-center European | European | Degenerative | 15 | Neochord | 1.5 |
Selected patients can be treated successfully with Neochord |
| Niikura 2019 [20] | N/A | Abbott Northwestern Hospital | U.S. | Degenerative | 12 | MitraClip | 18.5 |
TMVr with MitraClip is effective, in properly selected patients without mitral stenosis |
| Pleger 2019 [19] | 2013–2018 | University Hospital Heidelberg | Germany | Not specified | 7 | MitraClip | 1 |
MitraClip-in-ring is feasible and safe |
| Braun 2017 [18] | 2010–2016 | University of Munich | Germany | Not specified | 57 | MitraClip | 15.9 |
MitraClip is an alternative for high-risk patients, especially when valve-in-ring is not possible |
| Saji 2016 [17] | 2007–2013 | University of Virginia | U. S. | Degenerative and functional | 5 | MitraClip | 7.1 |
MitraClip assisted by intracardiac echocardiography is feasible in patients with prior surgical rings |
| Estévez-Loureiro 2016 [16] | 2010–2015 | Complejo Asistencial Universitario de León | Spain | Degenerative and functional | 6 | MitraClip | 11.1 |
MitraClip is safe and effective following surgical annuloplasty |
| Grasso 2014 [15] | 2008–2013 | Ferrarotto Hospital | Italy | Funcional | 6 | MitraClip | 12.8 |
MitraClip is safe and effective in patients with an annuloplasty ring |
The studies scored 7–11 out of 24 on the MINORS index, losing points mainly for lack of prospective data collection, unbiased endpoint assessment, and study size calculation. Quality assessment of included studies was listed in Table 2 (Ref. [15, 16, 17, 18, 19, 20, 21, 22]). Funnel plot analysis (Supplementary Figs. 1,2) did not suggest potential publication bias.
| Rahhab 2021 [22] | Gerosa 2021 [21] | Niikura 2019 [20] | Pleger 2019 [19] | Braun 2017 [18] | Estévez-Loureiro 2016 [17] | Saji 2016 [16] | Grasso 2014 [15] | |
| A clearly stated aim | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 1 |
| Inclusion of consecutive patients | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 2 |
| Prospective collection of data | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Endpoints appropriate to the aim of the study | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Unbiased assessment of the study endpoint | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Follow-up period appropriate to the aim of the study | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Loss to follow up less than 5% | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Prospective calculation of the study size | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Additional criteria in the case of comparative study | ||||||||
| An adequate control group | ||||||||
| Contemporary groups | ||||||||
| Baseline equivalence of groups | ||||||||
| Adequate statistical analyses | ||||||||
| Total | 10 | 11 | 7 | 8 | 8 | 7 | 9 | 11 |
| †The items are scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The global ideal score being 16 for non-comparative studies and 24 for comparative studies. MINORS: methodological index for non-randomized studies. | ||||||||
The pooled mean age was 73.5 (95% CI: 70.15%~76.77%; I
Mean preoperative left ventricular ejection fraction (LVEF) was 42.6% (95% CI:
33.6%~52.0%; I
The operative risk was estimated using the Society of Thoracic Surgeons (STS)
score in 6 studies and the EuroSCORE in 3 studies. The pooled STS score was 6.7%
(95% CI: 5.1%~8.4%; I
The pooled rate of procedural MR reduction
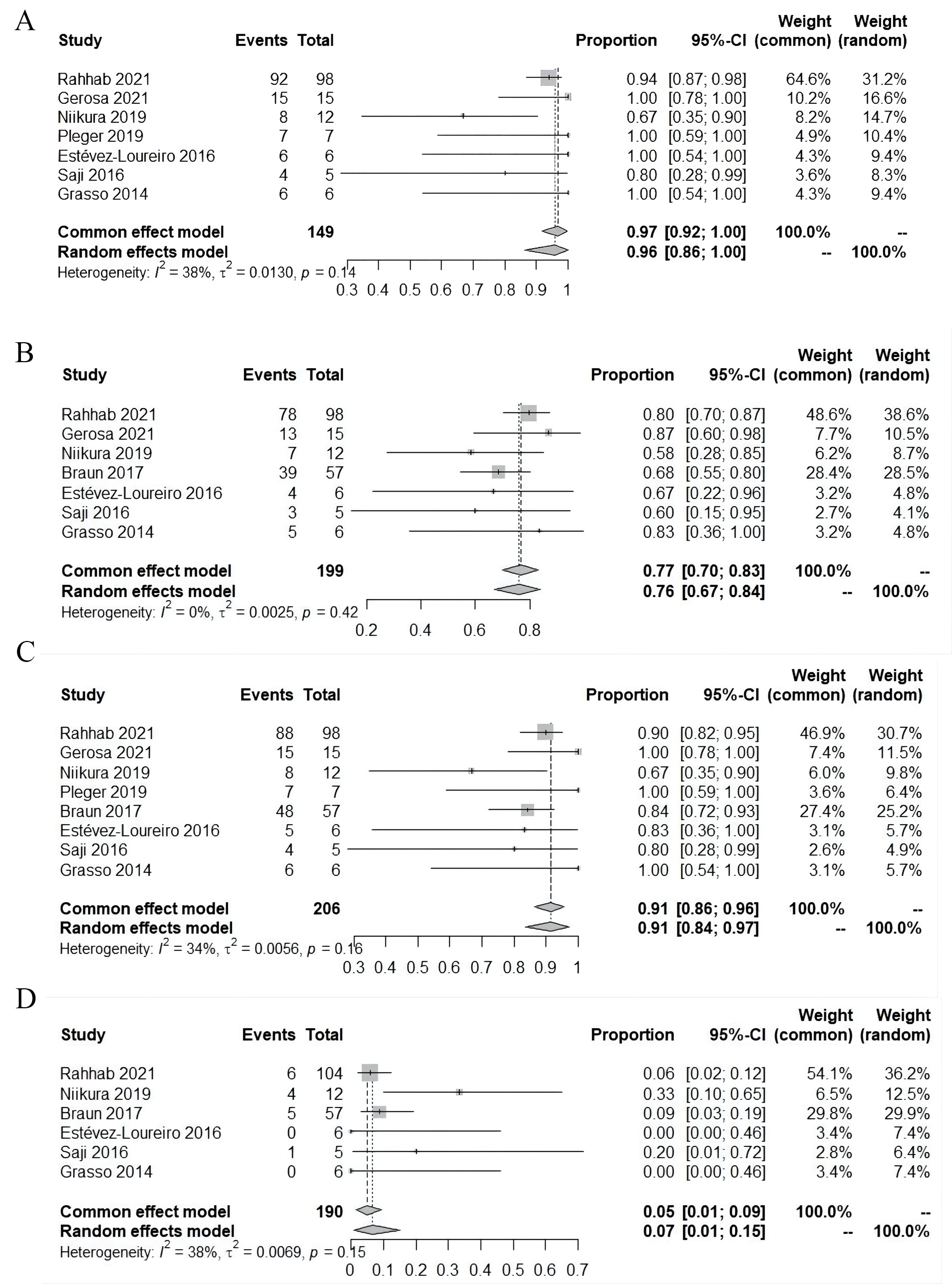 Fig. 2.
Fig. 2.Perioperative outcomes. (A) Procedural mitral regurgitation
reduction
Perioperative mortality was reported in all studies, including 6 studies with no
hospital death. The pooled mortality rate was 0% (95% CI:
0%~1%; I
Median hospital stay was 4.1 days (95% CI: 2.9%~6.1%; I
Follow-up MR was reported in 6 studies. MR
Follow-up survival was reported in 7 studies, and pooled survival was 94% (95%
CI: 88%~98%; I
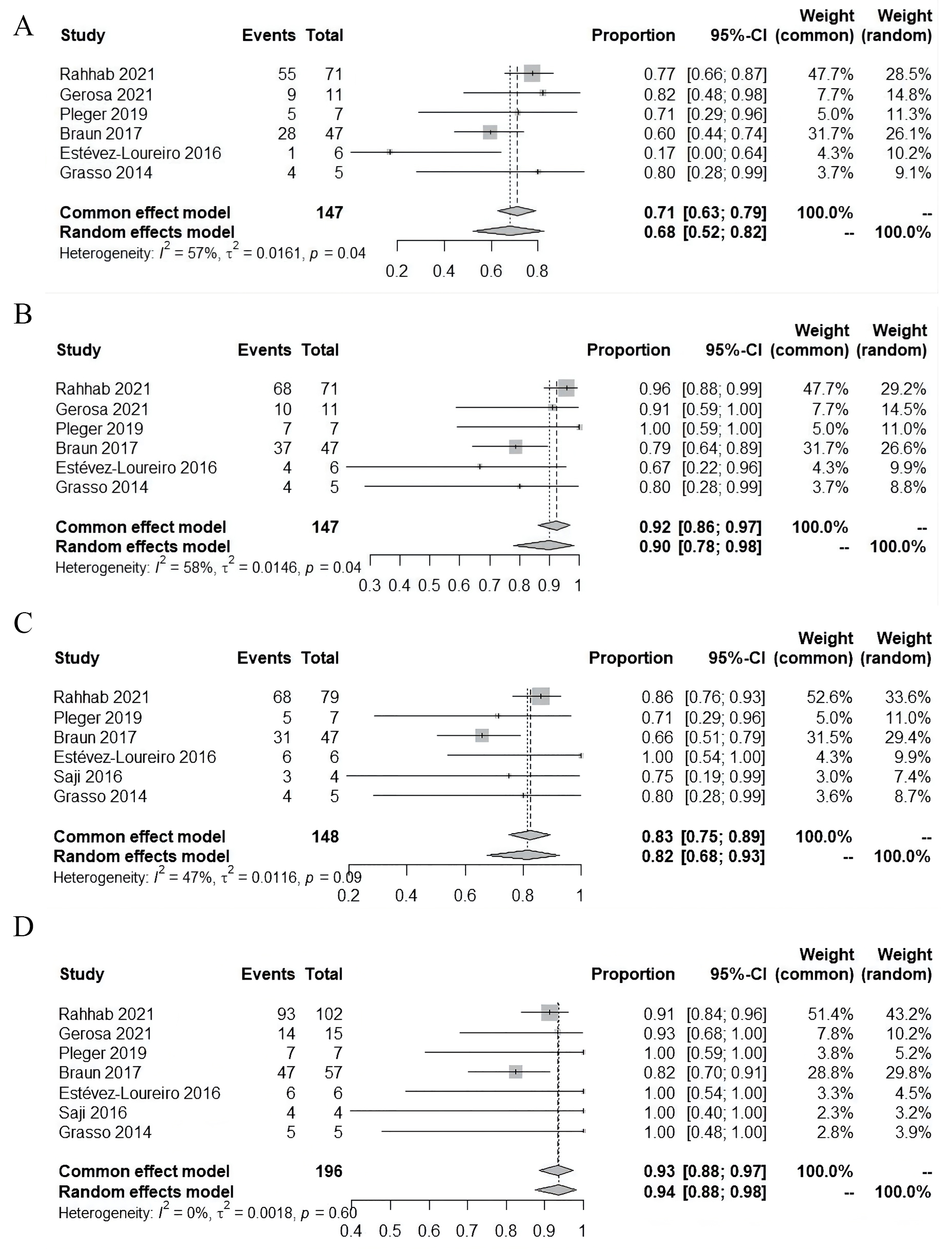 Fig. 3.
Fig. 3.Follow-up outcomes. (A) Residual mitral regurgitation
In the present systematic review and meta-analysis, the major finding was that TMVr was safe and effective for failed surgical MVr. For patients who were not a candidate or at high risk for reoperation, TMVr reduced MR and improved functional status less invasively. This was the first systematic review and meta-analysis that focused on the transcatheter repair of failed previous surgical MVr.
Redo mitral valve surgery has been the golden standard for failed MVr before the era of percutaneous interventions [4]. However, it was associated with higher perioperative risk. Kwedar et al. [5] analyzed early mitral reoperation data from Medicare. The hospital mortality was 9.8% for re-repair, 12.7% for MVR with bioprosthesis, and 12.2% for mechanical prosthesis [5]. Ejiofor et al. [6] reported a group of mitral reoperative patients who were eligible for TMVR (ViR or ViV), and the operative mortality was 5% for previous MVr versus 9% for the previous MVR. In retrospective studies focusing on reoperation for failed MVr, the hospital mortality was even lower, especially for re-repair groups. In our study, the pooled hospital mortality for TMVr was less than 1%, lower than predicted by the STS score (6.7%) and EuroSCORE (13.2%). It was relatively low for the group of high-risk patients, some of which with prohibitive medical conditions for redo surgery.
While “optimal” correction of MR was achieved by open cardiac surgery, TMVr
sometimes provided only “acceptable” results [23]. Pooled data in our
meta-analysis suggested that 96% of patients had
The long-term outcome of TMVr for failed MVr has yet to be studied. In the
present study, 83% of patients were in NYHA class I or II at follow-up, 90% of
patients had
TMVr for failed MVr can only be performed in a subset of patients. For example, it was contraindicated in the setting of endocarditis and seldomly used in patients with mitral stenosis. For patients with a small rigid annuloplasty ring or stiff leaflets, elevated transmitral pressure gradient might be a concern [26]. In most cases, the re-repair rate for open surgery was less than half, but Anyanwu et al. [27] reported a re-repair rate of 85% (90% for degenerative disease) in an experienced heart center. The feasibility of TMVr is based on an individualized analysis of the MV pathology.
Thus, redo surgery provides definite immediate and long-term results with acceptable perioperative risk for most patients. Re-repair should be preferred to TMVr for appropriately selected patients. For high-risk patients, TMVr is an alternative which reduces MR and improves functional status less invasively.
For primary MR, the result MVr was superior to MVR, even for complex repair, for elderly patients, and other causes such as papillary muscle rupture and infective endocarditis [1]. For secondary MR, the proof of surgical correction was limited, but different techniques of subvalvular repair were being evaluated. For failed MVr, re-repair was also associated with significantly lower peri-operative mortality and improved late survival compared with MVR [4].
ViR procedures for patients with a prior annuloplasty ring/band have been
introduced since the invention of TMVR. Mortality with ViR-TMVR at 30 days is
0%~18%, and 0%~34% at 1 year [8]. Recently,
the midterm results of the VIVID registry were evaluated, including 222 ViR
patients. Residual stenosis (26.9% severe patient-prosthesis mismatch), residual
regurgitation (16.6%
The outcomes of the two procedures were influenced by anatomic and technical factors. For example, the discontinuous portions of prior incomplete rings might result in a paravalvular leak. Large septal bulge and small aorto-mitral angle increased the risk of LVOT obstruction [8, 9]. Some of these factors were the intrinsic characteristics of the ViR procedures and were difficult to avoid. For patients with these unfavorable factors, TMVr should be considered as an alternative therapy to ViR-TMVR. The feasibility of TMVr should be analyzed individually, aiming to optimize hemodynamics and device durability.
Thus, TMVr was associated with better immediate and sustained outcomes and should be attempted if technically feasible, especially for those with unfavorable anatomical factors for ViR-TMVR.
Transcatheter edge-to-edge repair has been the most frequently used TMVr procedure. Besides edge-to-edge repair, there was also growing evidence for transcatheter procedures targeting the annulus and the chordae [28]. In open MV re-repair surgery, the frequently used techniques included leaflet resection, ring removal/annuloplasty, edge-to-edge repair, and neochordae [4, 27].
For the choice of TMVr techniques for failed surgical MVr, current literature was limited by a small number of patients and short follow-up. No comparison could be made in this challenging scenario. The use of Neochord procedures might offer several advantages. It was not limited by the presence of an annuloplasty ring and minimizes the risk of mitral stenosis [29]. Compared with MitraClip, it offered more physiological hemodynamics [26] and an annular shape [30]. However, whether these advantages could be translated into improved clinical prognosis has not been determined. For a degenerative disease with the precise site of recurrent prolapse, chordal implantation might be more effective, while for leaflet tethering or annular dilation, edge-to-edge repair might provide a simple but effective choice.
To our knowledge, there has been no study reporting percutaneous mitral annuloplasty for failed surgical MVr, although it has been used for failed MitraClip [31]. Combined transcatheter procedures of concomitant annuloplasty and chordal implantation, have already been performed to treat complex MR [32, 33], but not failed surgical MVr.
In conclusion, there has been a paucity of data on the long-term outcomes comparing transcatheter edge-to-edge and chordal repair. NeoChord might be an alternative in anatomically suitable cases.
There were several limitations of our meta-analysis. Firstly, the studies included were all single-arm studies with small sample sizes. Of the studies included, only 2 had decent numbers, and these two appear to sway the results. Meanwhile, no comparison was performed between TMVr and surgical MVr in failed surgical MVr due to lack of research addressing the issue. Secondly, in spite of increased interest, there are limited studies on TMVr after failed surgical MVr. Accordingly, most recent studies are observational and retrospective, with small sample size, a variety of follow-up periods, and different ethnicities of participants. Due to this, studies were scored poorly on the MINORS index, heterogeneous, and biased. Thirdly, there was no uniform standard for selecting redo surgery vs. transcatheter, and TMVr vs. TMVR. The choice was made based on the surgeons or institutional experience. Fourthly, most studies included focused on procedural and in-hospital outcomes, with relatively short follow-up.
TMVr for failed surgical MVr had encouraging short-term outcomes and should be recommended in selected high-risk and anatomically suitable patients if technically feasible.
HX and ZZ contributed to the study design, data analysis, manuscript drafting, and revision. WS, SL acquired data, critically reviewed, and revised the manuscript. HX, WS performed a literature search. SL and ZZ made substantial contributions to revising the article critically for important intellectual content. All authors contributed to and discussed the results and critically reviewed the manuscript. All authors read and approve the final version to be published.
Not applicable.
Not applicable.
This study was funded by Capital Science and Technology Program, Beijing, Grant number: Z201100005520005.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
