Academic Editor: Takatoshi Kasai
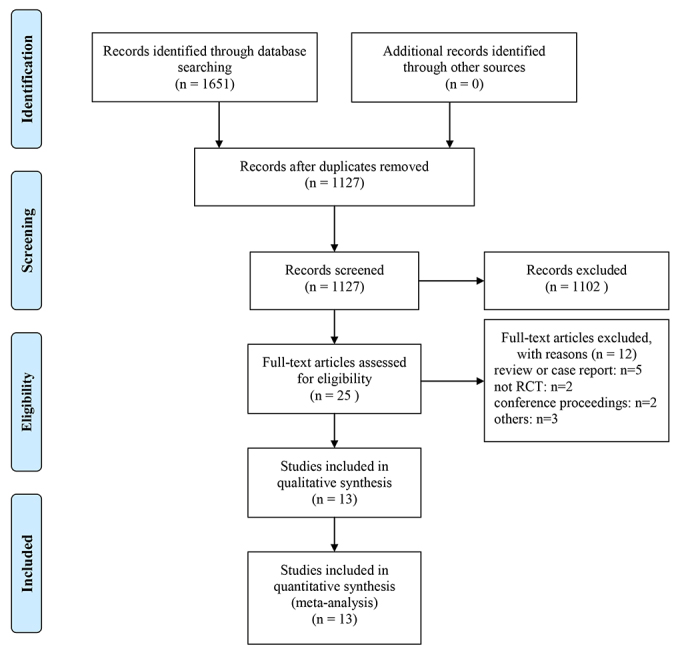
Evaluation of the effects of alirocumab on cardiovascular (CV) events, CV mortality and all-cause mortality. Data search was carried out using the Cochrane Library, PubMed, Web of Science and Embase. The search time is up to November 18, 2020. All randomized clinical trials (AEs) comparing alirocumab with placebo were searched. Meta-analysis was performed by Review Manager version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark), and the heterogeneity between studies was tested by Cochrane’s Q test and measured with I2 statistics. A total of 13 randomized controlled trials with 24,815 participants were included. Alirocumab usage can considerably lower the incidence of CV events when compared to the control group (risk ratio(RR) 0.89, 95% confidence interval(CI) 0.83–0.95). No significant difference in CV mortality between the two groups was observed (RR 0.87, 95% CI 0.74–1.04). Treatment with alirocumab has been associated with a major decrease in all-cause mortality compared to placebo (RR 0.80, 95% CI 0.66–0.96). The incidence of serious adverse events (AEs) was similar in the two groups (RR 0.94, 95% CI 0.90–0.99). Alirocumab can reduce CV events and all-cause mortality. The AEs were mild and tolerable.