Academic Editor: Peter A. McCullough
Heart Failure (HF) is characterized by an elevated readmission rate, with almost 50% of events occurring after the first episode over the first 6 months of the post-discharge period. In this context, the vulnerable phase represents the period when patients elapse from a sub-acute to a more stabilized chronic phase. The lack of an accurate approach for each HF subtype is probably the main cause of the inconclusive data in reducing the trend of recurrent hospitalizations. Most care programs are based on the main diagnosis and the HF stages, but a model focused on the specific HF etiology is lacking. The HF clinic route based on the HF etiology and the underlying diseases responsible for HF could become an interesting approach, compared with the traditional programs, mainly based on non-specific HF subtypes and New York Heart Association class, rather than on detailed etiologic and epidemiological data. This type of care may reduce the 30-day readmission rates for HF, increase the use of evidence-based therapies, prevent the exacerbation of each comorbidity, improve patient compliance, and decrease the use of resources. For all these reasons, we propose a dedicated outpatient HF program with a daily practice scenario that could improve the early identification of symptom progression and the quality-of-life evaluation, facilitate the access to diagnostic and laboratory tools and improve the utilization of financial resources, together with optimal medical titration and management.
Heart Failure (HF) is the leading cause of outpatient visits in the Medicare system [1]; the increased prevalence of HF reflects a major health burden with respect to age-adjusted rates of first hospitalization, poor overall survival, and premature mortality when compared to the most common forms of cancer [2, 3]. Several items remain poorly explored; they include: (1) readmissions for worsening HF most often occur during the early months post-discharge (30 to 50% within the first 30–90 days) or in the last months before death, with similar trends among patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) [4]. (2) The EVEREST Trial clearly shows that one-third of all hospitalizations are due to non-HF-related causes, another third are due to ischemic or arrhythmic reasons, and the remaining are related to incomplete decongestion during hospitalization [5]. (3) Despite the re-hospitalization rates for HFpEF (rates for HFrEF are similar), the mechanisms leading to destabilization and the risk profile are quite different [6]. In HFpEF, more than half of hospitalizations are not related to any specific cardiac causes, due to the exacerbation of the underlying comorbidities; conversely, hospitalizations for cardiovascular reasons are much more prevalent in HFrEF [7]. Therefore, the vulnerable phase represents the period when patients go from a sub-acute to a stabilized chronic phase; transitional care programs reduce 30-day readmission, optimize the use of evidence-based therapies, improve the patient’s necessary knowledge of the disease and save financial resources [8, 9, 10, 11]. The structure and organization of HF clinics need a multidisciplinary team, physician- and nurse-directed, with easy access to available slots for laboratory and imaging exams and to other specialists with expertise in treating patients with HF [12]. Most care programs are based on the main diagnosis and the HF stages, but a model focused on specific HF etiology is lacking. For all these reasons, we propose a dedicated outpatient HF care that could improve the early identification of symptom progression and the quality-of-life evaluation, facilitate the access to diagnostic and laboratory tools and improve the utilization of evidence-based medications, with the aim of reducing HF hospitalizations. We also suggest a specific model to organize an optimal network between hospital clinics, outpatient visits, peripheral medical support and patient care.
Dilated cardiomyopathy (DCM) is one of the most frequent causes of HFrEF
worldwide, and HF is a common clinical presentation [13]. Compared to other HF
etiologies, DCM patients are younger and with lower left ventricle ejection
fraction (LVEF) at diagnosis; women show better survival rates than men in
relation to a better left ventricle (LV) systolic function. Outpatient visits
should be scheduled every 3 months—or monthly—in subjects recently diagnosed
or hospitalized who need therapy uptitration and patients with left bundle brunch
block, high arrhythmic burden potentially candidates for cardiac
resynchronization therapy (CRT), or with implantable cardioverter defibrillator
(ICD). Cardiac magnetic resonance (CMR) is indicated in all patients at the first
diagnosis of DCM, with a diagnostic and prognostic implication. In 30 to 40% of
non-ischemic DCM, LV fibrosis is localized in the mid-myocardial septum; however,
the characteristics of fibrosis are variable and can involve other locations,
such as the LV free wall [14]. The presence and extension of LV fibrosis are both
related to adverse cardiovascular outcomes [15]. CMR is recommended to assess the
exact LV systolic dysfunction prior to CRT/ICD implantation in patients who are
candidates for a LV assistant device or Heart Transplantation (HT). Together with
ischemic heart disease (ICM), DCM is the most common indication for HT in younger
subjects (less than 60 years old). A dedicated pathway addressing the advanced HF
management and a HT surgery center may be recommended [16]. In this context,
cardiopulmonary exercise testing (CPET) provides an objective evaluation of the
functional capacity and represents the key to defining the clinical severity and
to stratifying the outcomes. CPET is essential for the anaerobic threshold
measured by V-slope analysis of VO
A dedicated nursing staff with HF skills may measure and record weight and blood pressure (BP), and provide patients with an education regarding their daily diuresis and body weight. The physician organizes the follow-up based on disease stability and progression (including booking the next visit, laboratory exams and second-level imaging tools).
The clinical course of HF in DCM may be variable; however, around 40%
of DCM patients show a significant LV reverse remodeling. Complete functional LV
systolic recovery and reverse LV remodeling can be achieved if a correct etiology
has been performed, especially following a guideline-directed medical therapy
[20]. Female sex, a higher LVEF at baseline, a reduced LV dimension, and a
limited LGE area are all associated with positive LV reverse remodeling [21].
Outpatient visits should be scheduled every 6 months in subjects in stable
clinical condition and with recovered LVEF. Holter ECG should be scheduled every
6 months, or more frequently, in the presence of a high arrhythmogenic burden. In
these patients, a CMR should be repeated every 5 years, and should be performed
during follow-up in case of HF progression and relevant worsening of LV/right
ventricle systolic function. Exercise echocardiography (EE) provides a wealth of
additional information during follow-up, such as functional capacity, BP curve,
PH, mitral regurgitation (MR) and arrhythmias, and should be prescribed
especially in case of HF symptoms during the stress test [22]. The 6-minute
walking test (6-MWT) is a simple test capable of identifying variations in
exercise tolerance with prognostic utility. Commonly available and simple, this
test is a valid alternative to the more complex VO
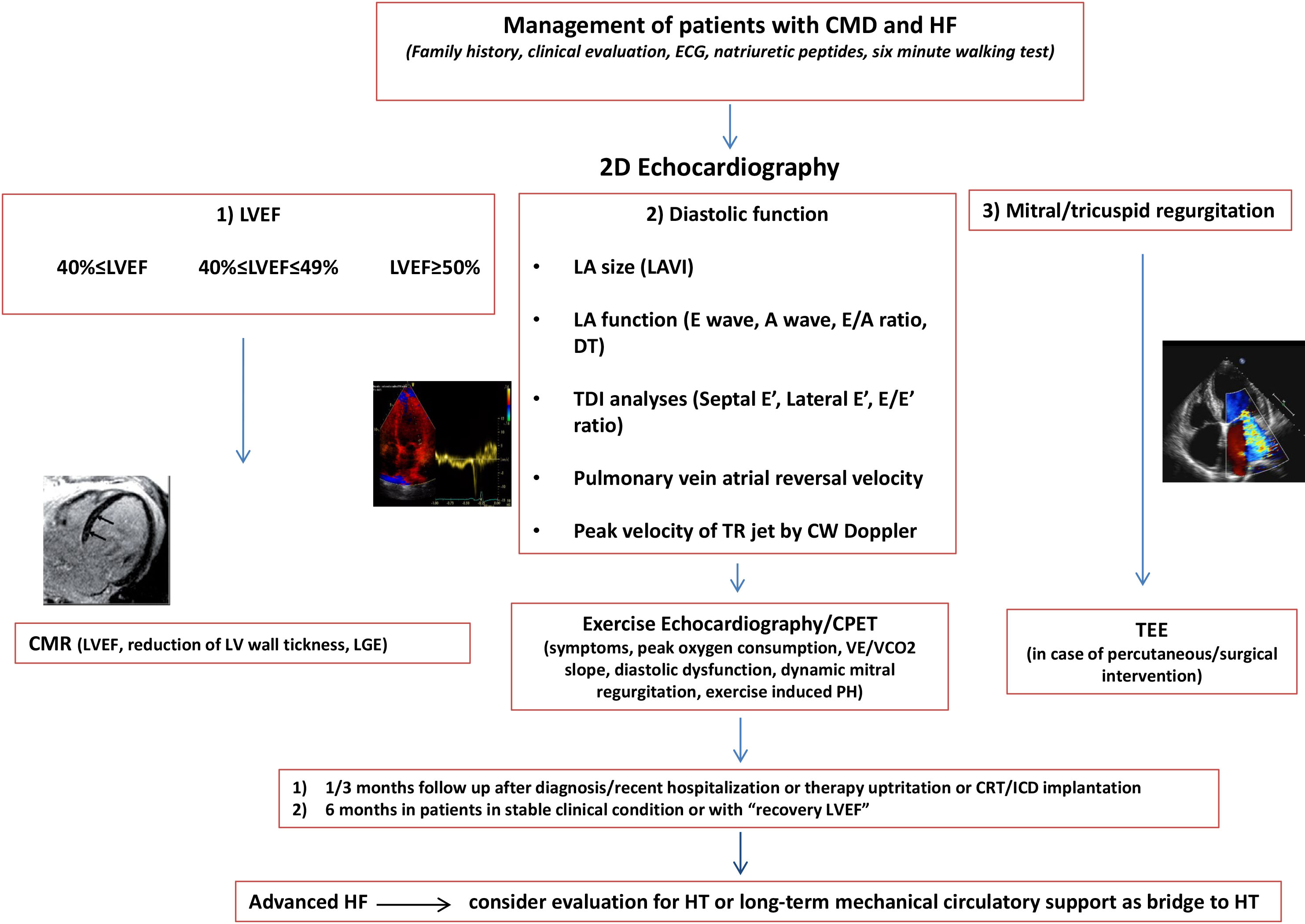 Fig. 1.
Fig. 1.The diagram describes the management of patients with Dilated Cardiomyopathy and Heart Failure: patients may be screened according to baseline LVEF and subsequent changes. Indeed, some patients may experience a systolic function recovery, a consistent percentage remain stable, and others have progressive deterioration with scarce response to the therapy. Outpatient visits should be scheduled every 3 months or monthly in subjects recently diagnosed or hospitalized who need therapy uptitration and patients with left bundle brunch block, high arrhythmic burden potentially candidates for CRT, or with ICD. Outpatient visits should be organized every 6 months in subjects in stable clinical condition and with recovered LVEF. EE/CPET provides functional capacity, anaerobic threshold, BP curve, PH, MR and arrhythmias, and should be prescribed especially in case of HF symptoms during effort and prior to HT. CMR is indicated in all patients at the first diagnosis of DCM. CMR should be repeated every 5 years, and should be performed earlier during follow-up in case of HF progression and relevant worsening of LV/right ventricle systolic function. CMD, Dilated cardiomyopathy; ECG, Electrocardiogram; LA, Left atrial; LAVI, Left atrial volume index; DT, Decelaration time; TDI, Tissue doppler imaging; LVEF, Left ventricle ejection fraction; CMR, Cardiac magnetic resonance; TEE, Transesophageal Echocardiography; HF, Heart Failure; HT Heart Transplantation; CRT, Cardiac resynchronization therapy; ICD, Implantable cardioverter defibrillator; PH, Pulmonary Hypertension; LGE, Late gadolinium enhancement; CPET, Cardiopulmonary excercise test; TR, tricuspid regurgitation; CW, Continuos wave; LV, Left ventricular.
Patients affected by hypertrophic cardiomyopathy (HCM) develop more frequent HF symptoms when the hypertrophic phenotype is clearly expressed and when left ventricular outflow tract (LVOT) obstruction and/or atrial fibrillation (AF) occur [24, 25]. Based on the HCM course and progression, outpatient check-up should be scheduled every year in patients with “classic” phenotypes. Holter ECG should be considered every year in all patients with HCM; consequently, in subjects with higher arrhythmogenic burden, suspected AF, unexplained syncope, a longer loop for 7 days or subcutaneous loop recorder implantation is recommended. Patients with signs and symptoms of HF due to LVOT should be evaluated every 3–6 months, to optimize medical treatment (b-blockers and dysopiramide) and re-check the indication for myectomy or a novel therapy such as mavacampten [26]. EE is commonly performed as a routine test in patients with HCM, primarily to measure the dynamic LVOT gradients provoked by physical exercise every 6 months in obstructive HCM patients and every two years in all remaining HCM patients [27, 28]. EE can add important management data and prognostic information, including functional capacity, BP response, pulmonary pressure changes and arrhythmias [29, 30]. LV diastolic pressure could markedly increase during exertion, causing exertional dyspnea and increased exercise intolerance; the diastolic function under stress should be reported in all patients subjected to EE. CMR should be recommended at diagnosis in all patients with HCM. The LGE extent and distribution significantly correlates with the prognosis and identification of typical patterns of sarcomeric forms (thick-filament involves more frequently the anteroseptal wall, with mid-wall distribution, and thin filament the mid-wall distribution, with atypical sites). CMR can provide surgical information in patient referred to myectomy, such as the exact extension of hypertrophy, the presence of mitral valve apparatus abnormalities, or accessory chordae tendineae/papillary muscles and recognized apical aneurysms and thrombi, with implications affecting the outcomes [31].
Once it has occurred, the HCM-phenotype usually has a benign course, but a small
proportion of patients, accounting for 15–20% of the total population, present
an unfavorable clinical profile, with slow and progressive adverse ventricular
remodeling, which results in 5–10% of patients with overt LV dysfunction
expressing two different morpho-functional patterns: the “hypokinetic-dilated”
variant, characterized by volume increase and LVEF impairment (
Recent data from large international registries has shown a low mortality rate, with rare occurrence of SCD compared to earlier descriptions [37], however the SCD risk score and other risk factors—such as genetic positive variants, LGE, LV apical aneurism, end-stage HCM—should be punctually reconsidered during each visit (Fig. 2).
 Fig. 2.
Fig. 2.The scheme proposes the management of patients with hypertrophic cardiomyopathy and heart failure: according to non invasive hemodynamic assessment and arrhythmic burden, the clinical evaluation may be tailored individually. Outpatient check-up should be scheduled every year in patients with “classic” phenotypes, every 3–6 months in patients with signs and symptoms of HF due to LVOT and in patients with “adverse remodeling” and “overt dysfunction phase”. EE is commonly performed to measure the dynamic LVOT gradients every 6 months in obstructive HCM patients and every two years in all remaining HCM patients. The use of serial CMR, usually every 5 years or every 2 years in patients with progressive disease, can provide valuable information to help the patient’s management, with particular regard to LGE progression and LV wall thickness reduction. CPET should be prescribed in patients with suggestion of disease progression, in order to optimize treatment and refer to HT earlier. ECG, Electrocardiogram; LA, Left atrial; LAVI, Left atrial volume index; DT, Decelaration time; CMR, Cardiac magnetic resonance; TEE, Transesophageal Echocardiography; HCM, Hypertrophic cardiomyopathy; TDI, Tissue doppler imaging; LVEF, Left ventricle ejection fraction; HF, Heart Failure; HT Heart Transplantation; PH, Pulmonary Hypertension; LGE, Late gadolinium enhancement; CPET, Cardiopulmonary excercise test; TR, tricuspid regurgitation; CW, Continuos wave; LV, Left ventricular.
Cardiac amyloidosis (CA) has exponentially increased among patients misdiagnosed as undifferentiated HFpEF, thanks to more advanced imaging tools, that are capable of recognizing matrix extracellular deposition with higher accuracy than in the past. A remarkable concentric hypertrophy, paradoxical low-gradient aortic stenosis, or unexplained LV hypertrophy are all potential conditions associated with patchy amyloid deposition into myocardial tissue [38]. Transthyretin amyloid (ATTR) can be managed with emerging therapies, such as stabilizing molecules (tafamidis - AG10) and genetic silencers (patisiran and inotersen), which show a reduction in mortality accomplished by a relative reversal of LV mass [39]. In the meantime, the most common clinical picture of CA remains the advanced HF symptoms and recurrent congestion; the management still remains a challenge, often requiring high-dose diuretics and frequent hospitalizations, with a poor prognosis and a high healthcare burden. Thus, innovative outpatient programs and the earliest possible referral to an experienced center are crucial in order to assess the optimal treatment and patient care. Outpatient visits should be scheduled after 1 month from hospital discharge, and then every 6 months in chronic patients, including NP, troponin and Holter ECG every 6 months. 2D echocardiography is the primary imaging tool to be used in the follow-up of patients with amyloidosis. Diastolic function is invariably impaired, and the degree of dysfunction is related to the HF symptoms and the progression of the disease. Severe diastolic dysfunction, leading to the onset of AF, is the most common cause of destabilization in these patients. LVEF is not a reliable indicator of systolic function in CA, because it reflects radial contraction, which is often preserved until the end-stage disease. Longitudinal function is typically affected earlier than radial contraction, and indices of longitudinal function can be used as early disease markers. The longitudinal strain measurement shows the typical impairment of the basal segments, with sparing of the apical segments. New CMR techniques such as ECV can recognize interstitial fibrosis, a hallmark of CA. ECV level provides better prognostic information than LGE, and correlates with amyloid burden, disease severity, and with systolic and diastolic dysfunction markers [40] (Fig. 3).
 Fig. 3.
Fig. 3.The diagram proposes the management of patients with transthyretin amyloidosis and heart failure: the clinical evaluation may be settled according to disease evolution and treatment response. Outpatient visits should be scheduled after 1 month from hospital discharge, and then every 6 months in chronic patients, including NP and troponin. 2D echocardiography is the primary imaging tool to assess the common echocardiographic characteristics of the disease, to evaluate the presence of aortic stenosis and the degree of diastolic dysfunction that is related to the HF symptoms and the progression of the disease. LVEF is not a reliable indicator of systolic function, which is often preserved until the end-stage disease. The longitudinal strain measurement shows the typical impairment of the basal segments, with sparing of the apical segments. ATTR can be managed with emerging therapies, such as stabilizing molecules and genetic silencers, which show a reduction in mortality accomplished by a relative reversal of LV mass. Innovative outpatient programs and the earliest possible referral to an experienced center are crucial in order to assess the optimal treatment and patient care. TTR-CA, Transtiretin cardiac amyloidosis; ECG, Electrocardiogram; LA, Left atrial; LAVI, Left atrial volume index; DT, Decelaration time; TDI, Tissue doppler imaging; LVEF, Left ventricle ejection fraction; HF, Heart Failure; HT Heart Transplantation; CPET, Cardiopulmonary excercise test; TR, tricuspid regurgitation; CW, Continuos wave; LV, Left ventricular.
In case of amyloid light-chain (AL) amyloidosis, the main actor is the hematologist. The main role of the cardiologist is to evaluate the cardiac assessment for initial hematologic strategies, with the monitoring of HF symptoms and systolic function during chemotherapy and with the use of supportive HF treatment. Outpatient visits should be scheduled every month during the initial hematological treatment, and then every 3/6 months. Holter ECG, complete blood count, basic biochemistry, NP, troponin and serum free light chain quantification is requested upon each visit [41].
ICM has a spectrum of clinical changes and pathophysiological states, which
eventually lead to congestive HF, ranging from myocardial stunning, hibernation
and scarring [42]. Remodeling is primarily achieved by myocardial fibrosis, which
results in decreased cardiac function, arrhythmia, and possible cardiac
conduction system impairment, leading to HF [43, 44]. Outpatient visit should be
scheduled 1 month from the hospital discharge in patients with de novo HF,
reduced LV systolic function and LV remodeling, or in patients with complex
anatomy and multivessel disease and further evaluation of revascularization after
imaging test for inducible myocardial ischemia [45]. Data from the STICH (Surgical Treatment for Ischemic Heart Failure) trial show that a
In chronic patients with preserved LVEF and without LV remodeling, outpatient
visits should be scheduled every 6 months to assess clinical status, NP, LVEF and
the optimization of the treatment of risk factors, as well as to further evaluate
myocardial revascularization. Scar identification is important not only in
patients with impaired systolic function, but also in patients with preserved
contractility [51]. This observation agrees with another study evaluating scar
and wall motion in patients with healed MI, identifying scar as a better
predictor of all-cause mortality than LVEF or LV size. In patients with ICM, HF
and smaller sub-endocardial scar, the prognosis is more frequently related to
non-cardiac comorbidities, such as older age, diabetes, and chronic kidney
disease, with less frequent hospitalizations for cardiac causes. EE is a
well-established technique to assess myocardial ischemia/viability, functional
evaluation, and MR under stress. EE represent the most cost/effective imaging
tool to evaluate inducible ischemia, with important data (large area of ischaemia
The prompt recognition and effective treatment of congestive HF in patients with
valvular disease are of the utmost importance for the practicing physician.
Aortic stenosis (AS) is a progressive disease that characteristically remains
asymptomatic for decades, but once its symptoms occur, survival is severely
compromised. Historical data have shown that the time from the onset of the
symptoms to death is about two years in patients who develop HF symptoms, three
years in those who present with a syncope, and five in those presenting with
anginal symptoms [53]. The continued improvement in transcatheter heart valves
and implantation techniques resulted in a consistent decrease in the overall
rates of all-cause death at 1 year among 31% of patients in the inoperable
cohort of the PARTNER IB trial treated with TAVR [54], to 7% in SURTAVI
trial targeting patients with an intermediate risk [55], and 5% in the
all-comers NOTION trial [56]. The survival rate in patients with asymptomatic
severe AS and preserved LVEF is similar to that of age-matched controls, with a
low risk of sudden death. However, few echocardiographic parameters are
associated predictors of symptoms development and adverse outcomes such as peak
aortic jet velocity, severity of valve calcification, LV hypertrophy and LVEF. EE
is useful to clarify the symptoms status under effort in patients with
asymptomatic severe AS, and to suggest some prognostic indicators that could
impact the decision of surgery, such as an increased peak aortic gradient during
exercise, PH and a
Low-flow low-gradient (LF-LG) AS is a complex scenario, with diagnostic pitfalls and uncertainty about stenosis severity and challenges in differentiating a truly severe AS that benefits from aortic valve replacement from only moderate AS. Low-dose dobutamine stress echocardiography is indicated every year in asymptomatic patients with LF-LG AS when attempting to differentiate a truly severe AS from pseudo-severe AS and to assess contractile reserve. Moreover, in patients with suspected LF-LG AS, the calculation of the ratio of the outflow tract to aortic peak velocity and measurement of aortic valve calcium score by computed tomography would be reasonable, in order to further define the severity [59]. Although symptomatic patients with LF-LG severe AS show worse outcomes than those with high-gradient AS following aortic valve replacement, survival analyses highlight a significant benefit with intervention [60].
Organic and functional MR are both closely related to HF. Hemodynamically
significant MR may exacerbate the hemodynamic strain on the failing LV, thus
contributing to a worsening of symptoms and survival. In patients with moderate
to severe MR, outpatient visit may be scheduled every 3–6 months. The onset of
HF symptoms in severe MR represents the indication for mitral intervention,
regardless of the LV systolic function. However, symptom onset is frequently
associated with advanced MR, thus prompting the identification by
2D-echocardiography of increased LV size (LV end systolic diameter
Outpatient visits of secondary MR should follow the same schedule of primary MR.
However, the indication for intervention in patients with chronic severe
secondary MR related to LV systolic dysfunction is more questionable and should
be proposed to patients with persistent HF symptoms despite optimal medical
therapy for HF [63, 64]. Percutaneous valve repair is reasonable in patients
with appropriate anatomy with LVEF between 20 and 50%, LV end systolic diameter
| Visit, EKG, echocardiography | Holter EKG | Exercise/stress echocardiography | Cardiopulmonary exercise test | CMR | Transesophageal echocardiography | Right Heart catheterization | Electrophysiological study | |
| DCM | - 1 month since hospital discharge | - Every 6– 12 months based on arrhythmic burden | - At the time of first diagnosis, to evaluate functional capacity and arrhythmic burden | - Patients with equivocal symptoms or HF progression | - At the time of first diagnosis and then every 5 years | - To evaluate primary/secondary MR for surgical or percutaneous repair | - In advanced HF patients prior to mechanical circulatory support and/or HT | - EPS and eventually catheter ablation of VT refractory to medical therapy |
| - 1–3 months if therapy up- titration or in candidates to CRT/ICD | - In patients with equivocal symptoms | - HT candidates | - Repeat early if HF progression and worsening of LV/RV EF | - In case of advanced HF and unclear hemodynamic status despite | - EPS and eventually catheter ablation in case of high arrhythmic burden or | |||
| - 6 months in stable patients and in “recovery” LVEF | - In patients with HF symptoms, to assess worsening of MR and PH during exercise | optimal medical therapy | tachycardiomyopathy with HF symptoms | |||||
| HCM | - 1 year in classic phenotype | - 12 months or earlier in case of suspected AF or high ventricular burden | - At the time of first diagnosis, to evaluate functional capacity, arrhythmic burden and LVOT obstruction | - Patients with equivocal symptoms and HF progression | - At the time of first diagnosis | - In patients candidate to myectomy to assess MR | - In advanced HF patients prior to mechanical circulatory support and/or HT | - EPS is indicated in patients with HF symptoms and supraventricular arrhythmias to establish the correct diag- |
| - 3–6 months in HF due to LVOT obstruction | - In patients with equivocal symptoms | - HT candidates | - Every 5 years in stable patients | - In case of advanced HF and unclear hemo- | nosis and guide the catheter ablation procedure | |||
| - 3–6 months in adverse remodeling and overt dysfunction phase | - In patients who became symptomatic or worsening dyspnea (check inducible ischemia, MR, | - Repeat early if HF progression (LV wall thickness reduction, progression of | dynamic status despite optimal medical therapy | |||||
| CA | - 1 month since hospital discharge | - 12 months or 3–6 months in case of suspected AF | PH and diastolic dysfunction under stress) | - At the time of first diagnosis, to evaluate functional capacity or in case of worsening of symptoms | LGE) | |||
| - 3 months in decompensated patients with |
- In HT candidates | |||||||
| - 6 months in stable patients | ||||||||
| ICM | - 1 month since hospital discharge | - every 12 months in the presence of high arrhythmic burden | - During follow- up to evaluate functional capacity and arrhythmic burden | - HT candidates | During follow- up to assess biventricular function (i.e., CRT/ICD) and LGE amount for prognostic | - To evaluate primary/secondary MR for surgical or percutaneous repair | - In case of advanced HF patients prior to mechanical circulatory support and/or HT | - ES and eventually catheter ablation of VT refractory to medical therapy |
| - 1–3 months in HFrEF and LV remodeling to optimize therapy or candidates to CRT/ICD | - In patients who became symptomatic, to assess inducible ischemia to guide myocardial revascularization | stratification | ||||||
| - 6 months in HFpEF without LV remodeling | - In patients with HF symptoms, to assess worsening of MR and PH during exercise | - In case of advanced HF and unclear hemodynamic status despite optimal medical therapy | ||||||
| Valvular heart disease | - 1 month since hospital discharge | - 6–12 months if arrhythmic burden is present | - In case of moderate/severe valvular dysfunction, to evaluate functional capacity, arrhythmic burden, PH and behavior or valvular dysfunction | - HT candidates | - To study the mechanism of MR | - In patients with PH, to confirm its reversibility before valve intervention | ||
| - 3–6 months in severe valvular dysfunction without HF symptoms | - In patients with equivocal symptoms or worsening of dyspnea | - To assess the feasibility of percutaneous repair or surgery | ||||||
| - Low- dose dobutamine stress in low- flow- low- gradient AS to differentiate true severe AS from pseudo- severe AS |
The COVID-19 outbreak has been associated with a 40–60% decrease in emergency
department visits, with a nearly 3-fold increase in mortality among hospitalized
patients, highlighting the need for the remote management (RM) of patients with
HF [66]. The results concerning the transmission of vital signs are ambiguous: in
the BEAT HF study, the combination of health coaching telephone calls and
telemonitoring did not reduce the number of 180-day re-admissions for HF [67]. In
the SUPPORT HF trial, the digital home monitoring with centralized specialists
showed positive results in terms of efficacy, but no improvements in the use of evidence-based treatments have been found in digital home monitoring alone [68].
Favorable results come from a selected group of patients with HF (New York Heart
Association [NYHA] class II–III, LVEF
The arrangement of a “virtual visit” should include: (1) a reimbursement by the Italian ministry of health; (2) an easily downloadable PC program for video-calls; (3) digital medical systems able to record HF parameters; (4) dedicated slots for HF visit. The first step is to identify patients who live alone or with family members or caregivers able to connect by remote modality and exclude vulnerable patients at risk of decompensation or with advanced HF [78]. The aim is to identify any symptom variation, transmit the vital signs or the hemodynamic data (only in patients with a device), up-titrate the therapy and identify earlier patients at risk of decompensation. At the end of the visit, the clinician should write a brief report with instructions regarding laboratory testing and drug adjustments, schedule imaging/laboratory testing and organize the next follow-up (specifying if in person or virtual). In this context, a multidisciplinary link between the HF specialist and the general practitioner (GP) is crucial, with a pivotal role for the latter to recognize the early symptoms of congestion and monitor any potential side effects of the HF medication. A self-monitoring evaluation—mediated by a specialized nurse—would also be useful, and patients could gain confidence with their daily physical activity, diuresis and body weight measurement. The identification of patients at risk for HF readmission may be accomplished by using clinical and laboratory parameters, easily assessed by the GP [79] (Fig. 4).
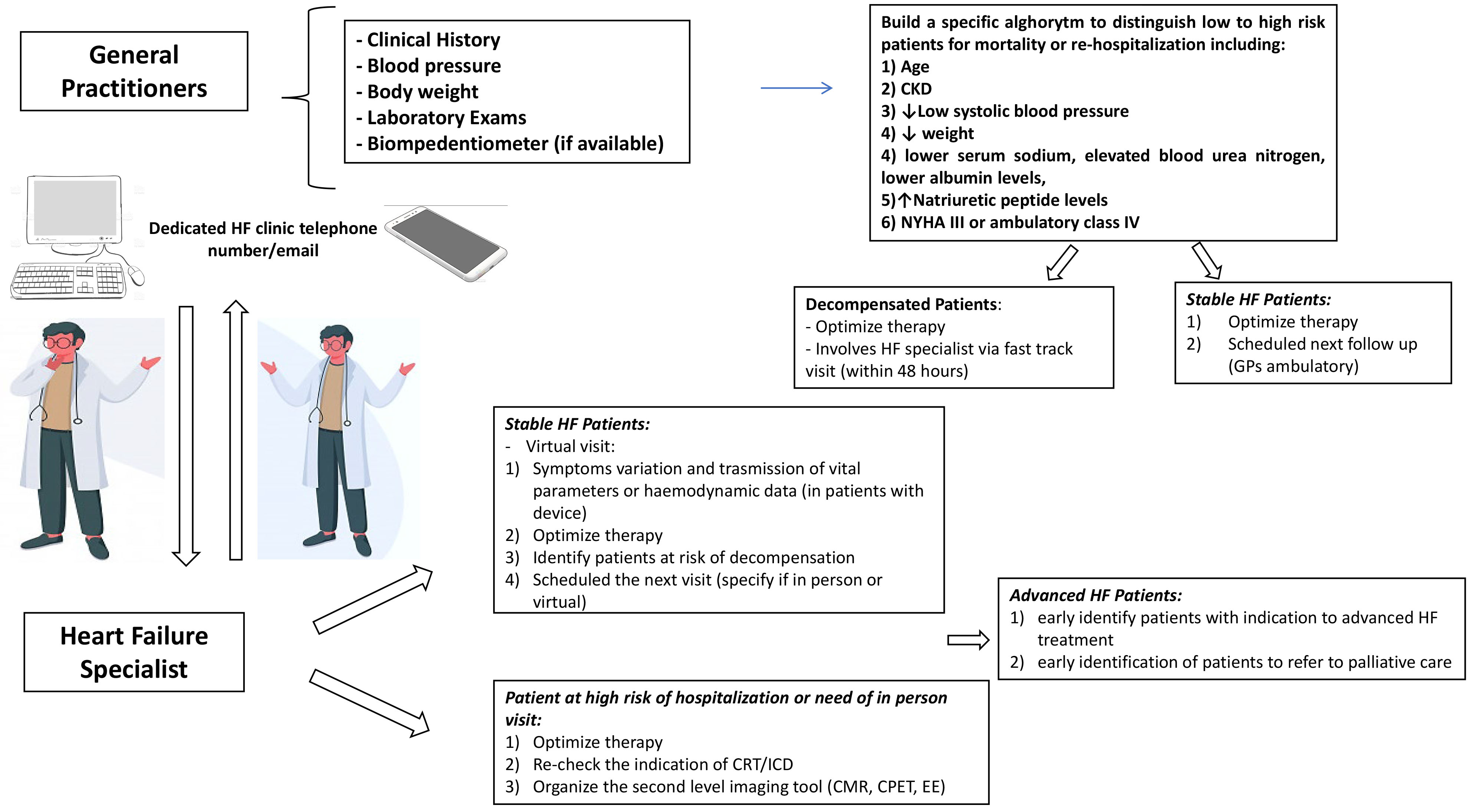 Fig. 4.
Fig. 4.Cross discussions between the General Practitioner and the Heart Failure specialist: check up schedule may be planned in relation to clinical stability, occurrence of other CV and non CV diseases. CKD, Chronic kidney disease; NYHA, New York Heart association; HF, Heart Failure; GPs, General practitioners; PH, Pulmonary Hypertension; CPET, Cardiopulmonary excercise test; EE, Exercise echocardiography; CMR, Cardiac magnetic resonance; CRT, Cardiac resynchronization therapy; ICD, Implantable cardioverter defibrillator.
The various models proposed over the last years, during the pandemic, may became an opportunity for a novel practical HF approach, and may prevent the need for many redundant visits and outpatient accesses. Accordingly, the above experiences could lead to a lower burden for the Health System, together with quality-of-life improvement in stable patients who do not require repetitive check-ups. Program goals can be tailored according to the geography and location: especially for patients who live in geographic areas with difficult access to HF medication-assisted treatment. In this context, the GP needs dedicated outpatient HF program and a simple laboratory (NP measurement) and imaging ultrasound instruments to measure the inferior vena cava and detect lung ultrasound to assess pulmonary congestion by imaging B-lines. Several telemedicine programs have proven their feasibility and effectiveness in the HF populations, mostly in frequently overloaded Health Systems [80]. This approach could be suggested in situations in which physical consultation is difficult, due to logistical constraints. Several National Programs suggest a specific algorithm to distinguish from low to high risk mortality or re-hospitalization and to identify those patients that may benefit from a closer monitoring and an aggressive evidence-based treatment [81]. Ongoing experience will evaluate the availability and usefulness of different models, recognizing the more practicable ones in relation to the health resources and the local opportunities.
The HF clinic is becoming an important tool in the decision-making process aimed at avoiding the excessive rates of re-hospitalization and adverse event during the early and late post-discharge phases. At this point, there is no universal HF algorithm codifying for primitive diseases, etiology, evolution and severity. New outpatient clinic models should be organized following these criteria, in order to focus the efforts towards a significant reduction in the re-hospitalization and mortality rates. New, non-invasive telematic processes monitoring vital parameter, congestion and ECG criteria may be promoted, especially during this pandemic, in order to minimize hospital access among stable patients and to optimize treatment in those with recurrent worsening episodes.
MB and AP conceived the idea presented, wrote the manuscript and supervised the work. SB and MM collected figures and tables and performed the literature research. All authors contributed to the editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
The authors would like to thank all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
