Academic Editor: Peter A. McCullough
The interactions and feedback mechanisms involved in heart and renal failure are more complex than previously thought and are grouped under the term “cardio-renal axis”. In the last decades, it has always been emphasized that renal dysfunction in patients with heart failure can be attributed exclusively to low renal plasma flow resulting from reduced cardiac output. In the last two decades cardiorenal syndrome has been established to set complex and close interactions between heart and kidney. Cardiologists and nephrologist should interact in their daily clinical practice to provide better patients’ management. In this review, we will point out main features of cardiorenal axis and cardiorenal syndrome to shift into specific sets of management in Italy starting by Guyton’s hypothesis till present days.
It’s now cleared that “cardio-renal axis” grouped all pathophysiologic pathways involved in heart and renal failure. In the last decades, it has always been emphasized that renal dysfunction in patients with heart failure can be attributed exclusively to low renal plasma flow resulting from reduced cardiac output [1, 2].
On the contrary, it has been clarified how other pathophysiological pathways are involved in the genesis of renal injury secondary to heart failure.
In patients with chronic heart failure, the renal response is initially characterized by low plasma flow with a preserved glomerular filtration fraction (GFR) till to severe cardiac dysfunction because of increased glomerular hydrostatic pressure and afferent arteriolar resistance [2].
At the same time, sodium reabsorption (at the level of Henle’s loop) and several neurohormonal factors play a crucial role in maintaining renal hemodynamics; activation of the sympathetic nervous system (SNS) and the renin-angiotensin-aldosterone system (RAAS) represent the main hormonal factors and lead to the release of arginine vasopressin and endothelin resulting in systemic vasoconstriction, maintenance of GFR, and retention of sodium and water [2].
Subsequent activation of SNS and RAAS represent a compensatory response in order to preserve cardiac output, blood pressure, and GFR itself [2].
It is important to recognize how the cardio-renal crosstalk occurs, in large
part, within a clinical setting of predisposition to chronic kidney disease (CKD)
due to several risk factors such as obesity, metabolic syndrome, cachexia,
diabetes mellitus, hypertension, proteinuria, uremia and anemia. Several chronic
diseases (obesity, type 2 diabetes mellitus (DM), hypertension, obstructive sleep
apnea, atrial fibrillation, heart failure (HF), hyperuricemia, and CKD itself)
contributes to widespread chronic inflammatory status in this patients’
populations leading to production of specific pro-inflammatory cytokines [IL-6
and tumor necrosis factor-alpha] (TNF-
Hypertension and type 2 diabetes mellitus represent main risk factors for the development of CKD in Western countries. Lack of blood pressure control is directly related to accelerated nephron loss and reduced GFR [4]. Diabetes, through many mechanisms, contributes to glomerular dysfunction by accelerating the progression to CKD and end-stage chronic kidney disease (ESRD) [4].
Proteinuria represents another risk factor for the evolution towards a cardio-renal syndrome. Endothelial, mesangial and podocyte lesions are frequent in hypertensive and diabetic patients; the release of albumin in the Bowman’s space determines an increased workload for proximal tubular cells that may undergo apoptosis with further loss of nephron mass and progression of renal disease itself. Albuminuria is also a predictor of the development of HF in the general population, especially in those patients with established HF; moreover, microalbuminuria is, in turn, a risk marker for cardiovascular dysfunction (CVD) and for the progression of CKD [4].
Uremia may have a direct impact on cardiomyocyte dysfunction because of abnormal calcium displacement in the cytosol resulting in loss of contractile capacity of myocardial cells. Uremia may also contribute to the loss of skeletal and cardiac myocytes by contributing to fibrosis and unfavorable cardiac remodeling post-myocardial infarction. Actually, after renal transplantation there is improvement in left ventricular systolic function, reduction in left ventricular mass, and reduction in the size of the left ventricle itself. Hyperuricemia itself is configured as a cardiovascular risk factor as it is closely related to the processes of accelerated atherosclerosis and to the risk of death from cardiovascular causes. The reduction of uric acid levels can influence the course of CKD and the natural history of cardiovascular disease [5].
Anemia is quite common in the course of heart failure and it is associated with increased mortality, morbidity, and worsening of renal function; it is probably related to hemodilution, impaired iron transport, inflammation, cytokine-induced erythropoietin deficiency and tissue resistance to it, malnutrition, cachexia, and vitamin deficiency (cardiorenal anemia syndrome). Elevated levels of hepcidin-25 could be responsible for erythropoietin resistance, whereas increased levels of pro-inflammatory cytokines could negatively affect iron utilization by increasing the production of hepcidin-25 from the liver, which blocks the ferroportin receptor and impairs both gastrointestinal iron absorption and iron release from specific cell types (i.e., macrophages and hepatocytes) [6].
Ultimate classification of CRS was stated in a 2008 Consensus Conference (Vicenza, Italy) [7, 8] providing two main groups, cardiorenal and renocardiac. The different forms of CRS are then further classified as acute or chronic depending on the onset and duration of the underlying organ dysfunction. Type 5 CRS includes, instead, all those pathologies in which the cardio-renal involvement, induced by the systemic disease, occurs simultaneously.
In the last decades it has been cleared as hemodynamic adaptations of the kidney and related pathophysiological mechanisms may be independent of cardiac hemodynamics and estimated glomerular filtration rate (eGFR) could be preserved until cardiac function is severely due to increased efferent arteriolar resistance and glomerular capillary hydrostatic pressure [9]. At the same time, increased sodium reabsorption, activation of sympathetic nervous (SNS) and renin-angiotensin-aldosterone system (RAAS) lead to systemic vasoconstriction, preservation of GFR, salt and water retention as a compensatory response to optimize cardiac output, blood pressure and GFR [9].
In heart failure patients impaired neurohormonal and inappropriate activation of RAAS could lead to congestive state and activation of nicotinamide adenine dinucleotide phosphate (reduced) (NADPH) oxidase by angiotensin II, leading to the formation of reactive oxygen species [10, 11]. Activation of the nitric oxide (NO) results in vasodilation, natriuresis, and withdrawal of tubuloglomerular feedback mechanism together with platelets’ dysfunction and inhibition of endothelial adhesion molecule expression/leukocyte-endothelial cell interaction [10, 11]. Decreased nitric oxide and increased oxidative stress could account for higher cardiovascular events’ incidence due to atherosclerosis [10, 12]. C-reactive protein’s plasmatic levels are also elevated in end-stage renal disease contributing to progression of renal and cardiac atherosclerotic risk [13]. The sympathetic nervous system also stimulates renin release from sympathetic neurons with consequent glomerular hemodynamic changes due to cathecolamines release (elevated systemic vascular resistance and sodium retention) [14, 15].
CRS1 (acute cardiorenal syndrome) is characterized by an acute worsening of cardiac function leading to acute kidney injury (AKI). CRS1 usually occurs in the setting of an acute cardiac disease such as Acute Decompensated Heart Failure (ADHF) (25% of patients hospitalized for ADHF), often following ischemic damage (acute coronary syndrome, cardiac surgery complications) or non-ischemic cardiac disease (valvular disease, pulmonary embolism) [16, 17, 18]. Clinical presentation extends from worsening chronic heart failure to pulmonary edema, hypertensive crisis, or cardiogenic shock [19].
Obesity and metabolic syndrome also represent risk factors for developing heart and kidney disease. In obese patients fatty acid content may can contributes to endothelial damage in epicardial coronary arteries and glomerular hyperfiltration leading to CKD progression [19].
Hemodynamic and non-hemodynamic (SNS hyperactivation, RAAS activation, chronic inflammation, and imbalance in the proportion of reactive oxygen species (ROS)/nitric oxide (NO) production) mechanisms play an important role in CRS1 in an ADHF setting, leading to decreased renal arterial flow and decline in GFR. Four different hemodynamic profiles have been proposed in patients with acute heart failure: cold/warm and dry/wet [20]. In patients with a “cold” pattern, a reduction in extra corporeal fluid volume (ECFV) represents the main hemodynamic change along with a decrease in renal blood flow related to RAAS and systemic nervous system activation causing efferent vasoconstriction. Patients with a “wet” hemodynamic profile show increased pulmonary and/or systemic congestion. In these patients, high central venous pressures (CVP) directly affect renal vein pressure and renal perfusion pressure [21, 22]. Increased CVP also results in increased interstitial pressure with tubular collapse and progressive decline in GFR [23].
Early diagnosis of AKI in CRS1 (as in CRS type 3) still remains a challenge [24] although novel biomarkers such as serum and neutrophil gelatinase-associated lipocalin (NGAL), cystatin-C, renal injury molecule-1 (KIM-1), interleukin-18 (IL-18), and liver fatty acid binding protein (L-FABP) are promising [25, 26].
Echocardiography could reveal impaired myocardial kinetics and left ventricular
hypertrophy, valvular stenosis and/or regurgitation pericardial effusions, normal
inspiratory collapse of the inferior vena cava (excluding severe hypervolemia),
aneurysms or aortic dissection [27], while kidneys appears normal or larger (due
to edema) with increased resistance index (
CRS2 is characterized by chronic renal involvement in chronic cardiac disease’s patients although it’s difficult to state which of the two disease is primary or secondary. CKD has been observed in 45 to 63% of patients and it is not easy to differentiate these patients from those with CRS type 4 [28, 29, 30, 31].
In CRS2 patients renal congestion, hypoperfusion and increased right atrial pressure represent main features of this clinical condition [32]. More recently, there has been increasing interest regarding the role of erythropoietin deficiency [33]. Erythropoiesis-stimulating agents (ESAs) therapy in patients with HF, CKD, and anemia is controversial: according to previous evidences, it has resulted in improved cardiac function with reduction in left ventricular hypertrophy and improved New York Heart Association (NYHA) class [31]. On the other hand, the randomized heart failure trial (RED-HF) conducted with Darbopoetin found no difference in the primary endpoint (a composite endpoint of death from any cause or hospitalization for worsening heart failure) [34].
CRS3 or acute renocardiac CRS occurs when the onset of AKI contributes to and/or precipitates the development of acute cardiac injury due to volume overload, metabolic acidosis, and electrolyte disturbances (e.g., hyperkalemia and/or hypocalcemia); coronary artery disease and left ventricular hypertrophy have also been described in AKI patients with direct effects on cardiac hard endpoints [35, 36].
Defining the incidence and prevalence of CRS3 is difficult because of the lack of epidemiological data. In a population-based study from northern Scotland, the incidences of AKI and acute-on-chronic renal failure were 1811 and 336 per million population [37]. Another prospective, multicenter, community-based study in 748 AKI patients reported the incidence of the following causes of death: infection (48%), hypovolemic shock (45.9%), respiratory distress (22.2%), heart disease (15%), disseminated intravascular coagulation (6.3%), gastrointestinal bleeding (4.5%), and stroke (2.7%) [38, 39, 40].
The pathophysiological interactions between kidney and heart during AKI have been termed “cardio-renal connectors” [41]: see, for example, the activation of immune cytokines (i.e., the release of pro- and anti-inflammatory cytokines and chemokines) and the sympathetic nervous system, that of the RAAS or the hemocoagulative cascade. Hyperkalemia may contribute to the risk of fatal arrhythmias and sudden death, while metabolic acidosis could be accountable of pulmonary vasoconstriction, increased right ventricular afterload, and negative inotropic effect (Fig. 1) [42].
 Fig. 1.
Fig. 1.Cardiorenal syndrome classification. CRS, cardiorenal syndrome.
Kidney size and echogenicity provide primary features to distinguish between acute and chronic renal disease [43, 44]. A hyperechogenic renal cortical with low cortico-medullary ratio are suggestive of chronic renal disease [43, 44]. The echocardiographic pattern is not diagnostic, showing increased atrial volumes, pleural and/or pericardial effusion, and is often associated with the presence of “pulmonary comets” or “B-lines” on chest ultrasound examination [27]. Pulmonary comets, sonographically, present as hypercogenic striae (similar to cometary star tails) originating from water-thickened interlobular septa and fanning out from the lung surface [45].
Over the past 5 to 10 years, several potential biomarkers have been proposed for
AKI diagnosis: neutrophil gelatinase-associated lipocalin (NGAL), KIM-1,
interleukin-18 (IL-18), interleukin-6 (IL-6), cystatin C (CysC),
N-acetyl-
CRS type 4 (CRS4) defines cardiovascular comorbidities in patients with chronic kidney disease (Fig. 2) [16] with an adjusted risk for cardiovascular events increases inversely with the estimated value of GFR [47].
 Fig. 2.
Fig. 2.AICN (Italian Association of Cardionephrology) Scientific Committee (from left da right: Dr. Antonio De Pascalis, Dr. Fulvio Floccari, Dr Luca Di Lullo, Prof. Claudio Ronco, Prof. Paolo Raggi, Dr. Antonio Bellasi).
GFR is an independent cardiovascular morbidity and mortality risk factor, especially in patients with stage 3B to 5D CKD [48].
Hyperphosphatemia and secondary hyperparathyroidism are associated both with
calcification of cardiac vessels and valves because of “osteoblastic”
transformation of vascular smooth muscle cells, while hypertension may contribute
to pressure overload [49, 50]. Chronic inflammation, insulin resistance,
hyperhomocysteinemia, and lipid dysmetabolism to cardiovascular disease in CKD
patients due to accumulation of
Chronic hyperkalemia, if not treated, is mainly accountable for Sudden Cardiac Death (SCD), while several biomarkers plasmatic levels increase as GFR decreases: troponin, asymmetric dimethylarginine (ADMA), plasminogen activator inhibitor type I, homocysteine, natriuretic peptides, C-reactive protein (CRP), Amyloid AA, and ischemia-modified albumin [53, 54, 55, 56].
Renal ultrasonography shows thin and hyperechogenic cortex, a reduced cortical to medullary ratio, together with paraphyelal or sub-cortical cysts [27].
Echocardiography may reveal signs of volume overload and right and left ventricular dysfunction, especially in patients undergo renal replacement therapy [57]. It is common to observe valvular calcifications together with right heart dysfunction (elevated pulmonary artery pressure, reduced tricuspid annulus systolic excursion (TAPSE), right atrial dilatation) [57].
CRS5 is characterized by the simultaneous involvement of the heart and kidneys within different clinical contexts (i.e., sepsis, hepatorenal syndrome, Fabry disease, autoimmune disorders). In septic patients, inflammation and capillary involvement lead to cellular ultrastructural damage and organ dysfunction [58, 59]. As in sepsis-related AKI, ischemia and inflammatory mediators are accountable for disease’s development: elevated thromboxane and/or prostacyclin plasmatic levels could affect coronary circulation and endothelial function together with elevated levels of TNF (Tumor Necrosis Factor) and interleukin-1 (IL-1) [60, 61]. AKI occurs in 20% of critically ill patients and 51% of patients with septic shock and positive blood cultures and it was previously considered secondary to renal ischemia due to septic shock. Current opinion suggests that the pathogenesis of septic AKI is based on hemodynamic factors and inflammatory mediators [62, 63, 64].
CRS5 diagnosis is mainly based on clinical setting, then on serological pattern:
lipopolysaccharide binding protein, procalcitonin, C-reactive protein,
pro-inflammatory cytokines (IL-6, TGF-
Concept of organ cross-talk is not a real new aspect of internal medicine since it was even postulated in past two centuries especially when Richard Bright was first physiologist to state a close interaction between heart and kidney as far as, on the other hand, many years later Sheila Sherlock wrote her first manuscript about hepatorenal syndrome.
Arthur Guyton himself, in 1955, published his data in which he explained how heart failure could involve renal failure by volume expansion, increased cardiac output and blood pressure together leading to renin-angiotensin system activation, renal vasoconstriction and finally to kidney failure (Fig. 3).
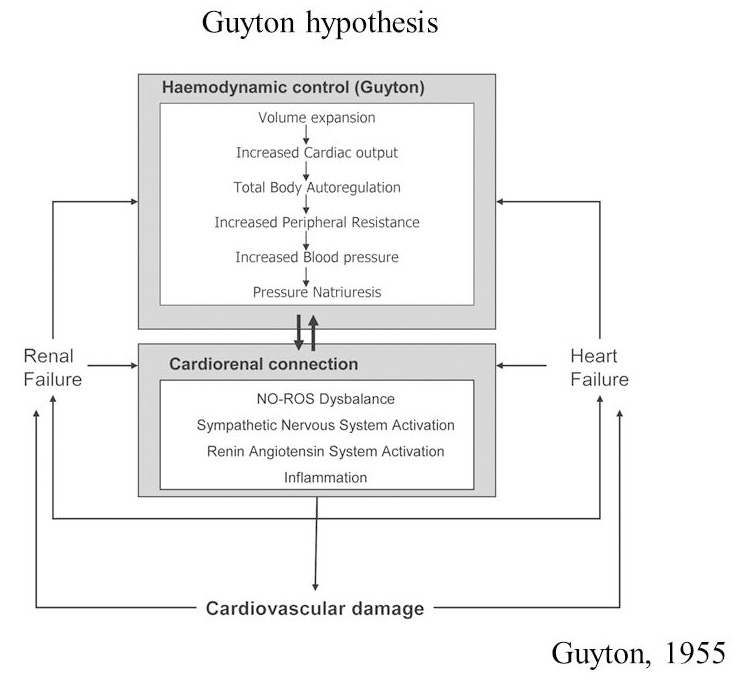 Fig. 3.
Fig. 3.Guyton original hypothesis on cardiorenal cross-talk. NO-ROS, nitric oxide reactive oxygen species.
In 2005, Bongartz [68] published his revised version of Guyton’s hypothesis adding data on inflammation and oxidative stress role in developing of cardiorenal disease (Fig. 4). In the meanwhile, new and large amount of papers have been published on national and international scientific journals about cardiorenal cross - talk. In latest centuries, term “cardionephrology” was first used in Italy by Professor Mario Timio with its international meeting held in Assisi (first Edition 1987) and then by Prof. Ronco [69] who created a new definition of Cardiorenal Syndrome by assembling an international consensus conference held in Vicenza in 2008 (Fig. 3). Despite of some doubts in scientific community, the term of Cardiorenal Syndrome is a worldwide accepted classification and more than 2000 papers are available.
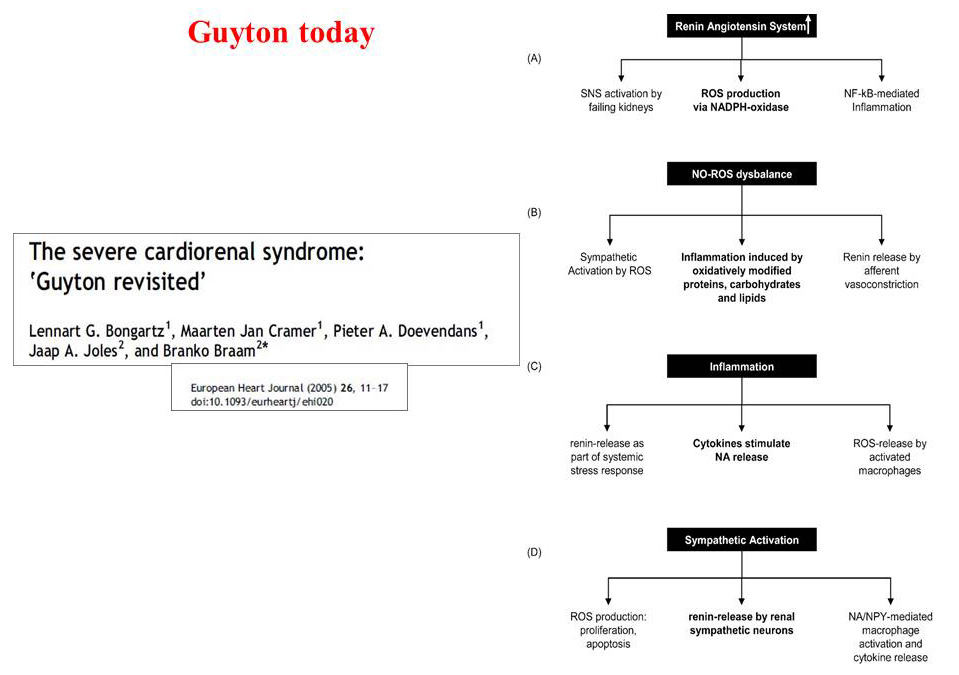 Fig. 4.
Fig. 4.Guyton’s hypothesis revised in 2005. SNS, sympathetic nervous system; ROS: reactive oxygen species.
It’s well-known that, among chronic kidney disease patients, cardiovascular involvement represents main comorbid and risk factor for death in such patients’ population that absolutely needs of a patient – tailored clinical approach.
As previously indicated, heart and kidney are closely related and dependent each one from the other ‘till dysfunction or injury of one organ may result in an engagement on the other in a bidirectional process. As a consequence a new kind of relationships and understanding between nephrologists and cardiologists has to be established all around the world.
Few months ago a new paper by Claudio Ronco [70] pointed out as cardiorenal medicine should became part of physician’s knowledge, especially for all people working in internal medicine’s units.
Usually medical and post-graduate schools focus their curricula on single discipline instead to promote an “us and them” culture; this point of view has often led specialist to provide opposite view in treating their patients.
Since the beginning of Italian nephrology, special attention to the relationship between heart and kidney has always been evident. As previously told, Professor Timio has organized with persevering interest the first conferences on cardionefrology that have since become a classic appointment not only for experts in the field but also for experts from various disciplines, residents and practicing physicians. This legacy has been collected in a mass of scientific works that had their turning point in the 2008 publication in Journal of American College of Cardiology (JACC) that led to the new definition and classification of cardiorenal syndrome. The modern nosography has been adopted by several consensus groups and among them ADQI (Acute Disease Quality Initiative), the European Society of Cardiology ESC (Publication of the results of the consensus conference in Venice) and the Cardiorenal Society of America [71]. Today, all this has been recovered and proposed again by the newly founded Italian Association of Cardionephrology of which Prof. Claudio Ronco is actual president. Far from being an entity in opposition to already existing and renowned scientific societies, this new association aims to bring together the lovers of the multidisciplinary approach to the cardio-renal patient, trying to break down preconceived barriers of training or hospital organization that see only one point of view in the path of diagnosis and treatment of the complex patient, without evaluating the benefits of a multidisciplinary approach as appropriate as necessary. Nephrology and Cardiology are complex disciplines that find even greater dignity in mutual collaborations and interactions. The nephrologist can represent a reference figure in the case of cardiological patients in which the interaction between heart and kidney is the focus of pathophysiological alterations worthy of study and research. The skills of a single specialist are not sufficient to treat such complex patients and, despite the fact that training in the past has been based on “organ” criteria, the need for a shared and multidisciplinary medicine is becoming increasingly evident [72].
With this in mind, the Italian Association of CardioNephrology (AICN) has been created, following what has already happened in other countries in the world (see Cardio-renal Society of America). This is a technical-scientific non-profit association that aims to collect enthusiasts, experts and lovers of cardio-renal issues, with the free spirit of scientific knowledge. AICN has no purpose of opposition with other scientific societies and even less with the Italian Society of Nephrology (SIN) but it can integrate and overcome some structural limits of scientific societies imposed by the current legislation [73]. The Gelli Law has revolutionized the world of scientific societies and has imposed that scientific societies can count among their members only the specialists of reference.
In this context, AICN can represent a resource and an opportunity for many nephrologists who, without underestimating the important contribution that the SIN offers to their careers and their scientific development, want to engage beyond the artificial barriers of the discipline in collaborations and studies of multidisciplinary interest (Fig. 4).
During last Cardionephrology Meeting held in Rome on March 2019 (4th Edition since 2013 with 5th Edition planned for upcoming November), more than five hundred participants attended a series of lectures, seminars, exercises and discussions that took stock of what everyone now calls “the talk” between heart and kidney. A virtuous relationship in optimal physiological conditions that becomes dangerous when one of the two organs begins to dysfunction. A syndrome that becomes a vicious circle in which the skills of the nephrologist and the cardiologist must merge in order to make the best combined skills available to the patient and the clinical case. Many of the patients admitted to the hospital, especially in intensive care units, present with renal or cardiac dysfunction of varying degrees. A primary pathology affecting one of these two organs often ends up involving secondarily the other system, damaging it or altering its physiological mechanisms. These interactions represent the physiopathological basis for the clinical entity that takes the name of Cardio-renal Syndrome (CRS).
Although there is a general consensus on the need for a multidisciplinary approach, in practice little or nothing is done at the level of hospital organizations and graduate schools to form mist teams with diversified but at the same time integrated skills.
This lack of perspective is all the more evident in light of recent research that has begun to understand the interactions between organs (“crosstalk”) and has demonstrated how some therapies can be effective in treating both cardiac and renal problems at the same time.
The increase in the average age of the world’s population has led to a substantial improvement in the clinical outcomes of patients with cardiopathy and/or cerebrovasculopathy on an ischemic basis, but, at the same time, has increased the number of patients with cardiovascular disease: this is the case, for example, of heart failure for which we are witnessing a steady increase in the development of new drugs (see guanylate cyclase and neprilysin inhibitors) for the treatment of the acute phase and chronic therapy. All this has not only led to a greater awareness of the pathophysiological mechanisms underlying cardio-renal cross-talk, but also stimulated the nephrologist to study more closely the role of the kidney in the different clinical “settings” of cardiovascular disease.
Fortunately, several new tools have been added to those already available to nephrologists and cardiologists, including new instruments for the correct determination of intra- and extra-cellular volumes, new technologies in the field of bio-impedance analysis and, above all, new devices integrated in dialysis monitors capable of capturing real-time changes in intravascular volume.
In light of all this, the need arises for new proposals from and for cardionefrologists who must always acquire new skills in a work scenario that changes day by day and must adapt to the needs of increasingly complex patients. What is required by 21st century cardiology is a wealth of knowledge that goes well beyond the current skills of the nephrologist as we mentioned in the column of the previous issue of the journal in which we discussed the basic ideas for a new training curriculum for cardiologists.
The main barriers to further development of cardionephrologic competencies are essentially economic (lack of funding) and cultural limitations. The new proposal of a curriculum oriented to the needs of cardiology wants to disassociate itself, first of all, from the need to recover additional funds for training and find its natural outlet in the creation of a working group that brings together cardiologists, nephrologists and intensivists with cardio-nephrological skills.
The key point around which revolves the ability to build a curriculum in
cardionephrology is called “training” to 360
Clinical experience in the field should be the cornerstone of the entire training experience and, also in order to develop optimal skills in the academic environment, it would be good to employ future cardiologists in research projects related to various clinical aspects in the field of cardiology (under the direct supervision of experts) and, above all, in clinical meetings in which to discuss new diagnostic and therapeutic approaches.
Several “topics” can be part of the cultural background of the cardiologist, whether they are related to specific skills in acute or chronic cardiorenal syndromes, both in outpatients and inpatients clinics.
An essential component of the educational program lies in learning and practicing the guidelines of the scientific and professional societies involved (such as the American Heart Association guidelines for heart failure). And just as the cardiologist must acquire the basics of nephrological know-how (e.g., the management of extracorporeal purification techniques, as well as that of the renal transplant patient), the nephrologist must acquire skills in the field of electrocardiography, echocardiography, vascular echo-Doppler and the management of impedance testing and related techniques.
It is therefore important, in the process of cardionephrologists’ growth, the role of the medical societies involved such as, for example, the Cardiorenal Society of America (CRSA), the American Society of Nephrology, the National Kidney Foundation, the American College of Cardiology, the American Heart Association, the International Society of Nephrology and the European Society of Cardiology to mention examples of scientific societies that have shown attention to these interdisciplinary professional developments.
The CRSA has begun to propose “Cardiorenal Universities” to test the feasibility of a project that manages to bring together the expertise of nephrologists and cardiologists by contaminating their fields of action: at the moment the feedback is highly positive and all this opens up interesting prospects for the future.
In 21th century clinical approach requires more than one specialist to take care of more complex diseases and syndromes. Pharmacological approach itself needs to be shared by several different clinicians and cardiorenal disease is a typical example in that way.
Vicenza’s experience and IRRIV (International Renal Research Institute of Vicenza) project was first step of this new project in Italy with Prof. Ronco that created an international group of residential clinicians together with fellows from all over the world with the aim to collect best minds in cardiorenal field (Fig. 5). Together with Vicenza’s experience, Dr. Luca Di Lullo has created, together with his colleague Dr. Vincenzo Barbera, in Colleferro, near Rome, an outpatient clinic in which heart diseases’ patients (especially those with heart failure and cardiac arrhythmias such as atrial fibrillation) can be also followed by nephrologists who take care of integrated pharmacological approaches. Nephrologic outpatient clinic in Colleferro directly provides (and it’s the only one experience in Italy) to anticoagulant therapy (with new direct oral anticoagulant drugs) together with therapy with anti-PCSK9 inhibitors (for patients with familiar and non-familiar hypercholesterolemia) and sacubitril/valsartan association for people with heart failure and reduced ejection fraction also affected by moderate to severe chronic kidney disease. Nephrologists themselves will also provide to ultrasound evaluation of those patients since they are accounted by Italian Association of Ultrasonography in Medicine and Biology (SIUMB) (Fig. 6).
 Fig. 5.
Fig. 5.IRRIV’s Director Prof. Claudio Ronco with his collaborators and fellows.
 Fig. 6.
Fig. 6.Dr Vincenzo Barbera (left) and Dr Luca Di Lullo in Ultrasound outpatient clinic in Colleferro (Italy).
Cardiorenal syndromes and cardionephrology itself needs to be closely shared by several clinicians such as cardiologists, nephrologists, critical care practioners as dedicated nurses. We hope that cardionephrology could include more followers in next future to provide better standard of integrated care for our cardiorenal patients, together with new scientific issues. Field of cardiology itself appears to be extremely broad and rapidly expanding, but it needs training programs and multidisciplinary international groups adequate to fully explore the skills and peculiarities of both specialists. It’s desirable that all new training’s programs for young specialists take care of this “unmet need” because we have to avoid that medical discipline could live in single closed “boxes”.
LDL—involved in chapter dedicated to the history of Cardionephrology in Italia and overview of Cardiorenal syndrome’s sections. AB—involved in writing chapters dedicated to CRS type 1 and type 2. VB—involved in writing chapters dedicated to CRS type 3 and type 4. CR—involved in whole project, especially on language’s editing.
Not applicable.
We would like to thank you all contributing Authors and peer reviewers for their comments and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
