Academic Editor: Peter A. McCullough
Renal artery aneurysms, although rare, may give rise to complications both per se (due to the risk of thrombosis and subsequent wall rupture) and by impairment of the renal function (due to extrinsic compression and high blood pressure). We describe a paucisymptomatic young patient with acute thrombosis of a massive dissecting renal artery aneurysm, for which the successful treatment was performed through ex-vivo vascular surgery followed by autotransplantation of the reconstructed kidney. The aneurysm was described through abdominal echography, computed tomography angiography, and transfemoral transcatheter arterial angiography. It originated from an atypical branch emerging at 90 degrees from the left renal artery. After a short branching off, it degenerated into a dissected aneurysmal sac as large as half a kidney (outer diameter of 60 mm), compressing the lower pole of the left kidney and delaying the lower half nephrogram. Ex-vivo surgical exclusion of the aneurysm was successfully performed. The kidney was reimplanted in the left iliac fossa (termino-lateral anastomosis between the renal artery and external left iliac artery, termino-terminal ureteric anastomosis) with excellent postoperative outcomes. For most asymptomatic aneurysms, expectant treatment is a reasonable approach. However, interventional or surgical repair is indicated in certain circumstances depending on the size of the aneurysm and its natural history, rupture risk, and interventional/surgical risks. The renovascular hypertension, dissecting and thrombotic events, its giant size, the young fertile age, and the presence of the flank pain were all indicative of the need for aneurysm exclusion in our case.
Renal artery aneurysms (RAAs), although rare, may give rise to complications both per se (due to the risk of thrombosis and subsequent wall rupture) and by impairment of the renal function (due to extrinsic compression and high blood pressure triggered by neurohormonal mechanisms) [1]. Existing medical/surgical practice guidelines recommendations are applicable under the circumstances relying on numerous parameters (e.g., aneurysm size, relationship with neighboring structures, risk of rupture, feasibility of aneurysmal exclusion through embolization or stent-graft). Thereby, therapeutic decisions are often made ad-hoc due to the collaborative effort between the medical team members (nephrologist, radiologist, angiographer, vascular surgeon, and urologist). Each treatment stage should be continuously adapted depending on the outcome. For instance, the residual risk of rupture fostered by an aneurysm left in situ after a stent-graft deployment must be taken into account by adapting the therapeutic strategy [2]. Simultaneously, the adhesions with neighboring structures (artery branches, veins, ureter, or renal capsule) found during surgical excision may entail the need for nephrectomy [3]. We describe a paucisymptomatic young patient with acute thrombosis of a large dissecting RAA for which the successful treatment of the aneurysm was performed through ex-vivo vascular surgery followed by autotransplantation of the reconstructed kidney.
We recall the case of a 24-year-old female presenting at our center with an acute onset of pain in the left flank. She had no prior medical history. Clinical examination revealed high blood pressure (150/95 mmHg) and abdominal pain in the left flank. Laboratory data were within normal range (including renal function). The routine abdominal and pelvic ultrasound investigation unveiled an apparent communicating aneurysm below the left renal artery.
Emergent computed tomography angiography (CTA) with contrast enhancement reported a partially thrombosed dissected aneurysm sac of the left renal artery with an outer diameter of 60 mm, at a distance of about 20 mm from the aortic origin. The transfemoral transcatheter arterial angiography revealed that the aneurysm originated from an atypical branch emerging at 90 degrees from the left renal artery (see Fig. 1, black arrows). After a short branching off, it degenerates into an aneurysmal sac as large as half a kidney (with an inner diameter of 52 mm), compressing the lower pole of the left kidney and delaying the lower half nephrogram (see Video 1 and 2). Otherwise, the upper half nephrogram was normal.
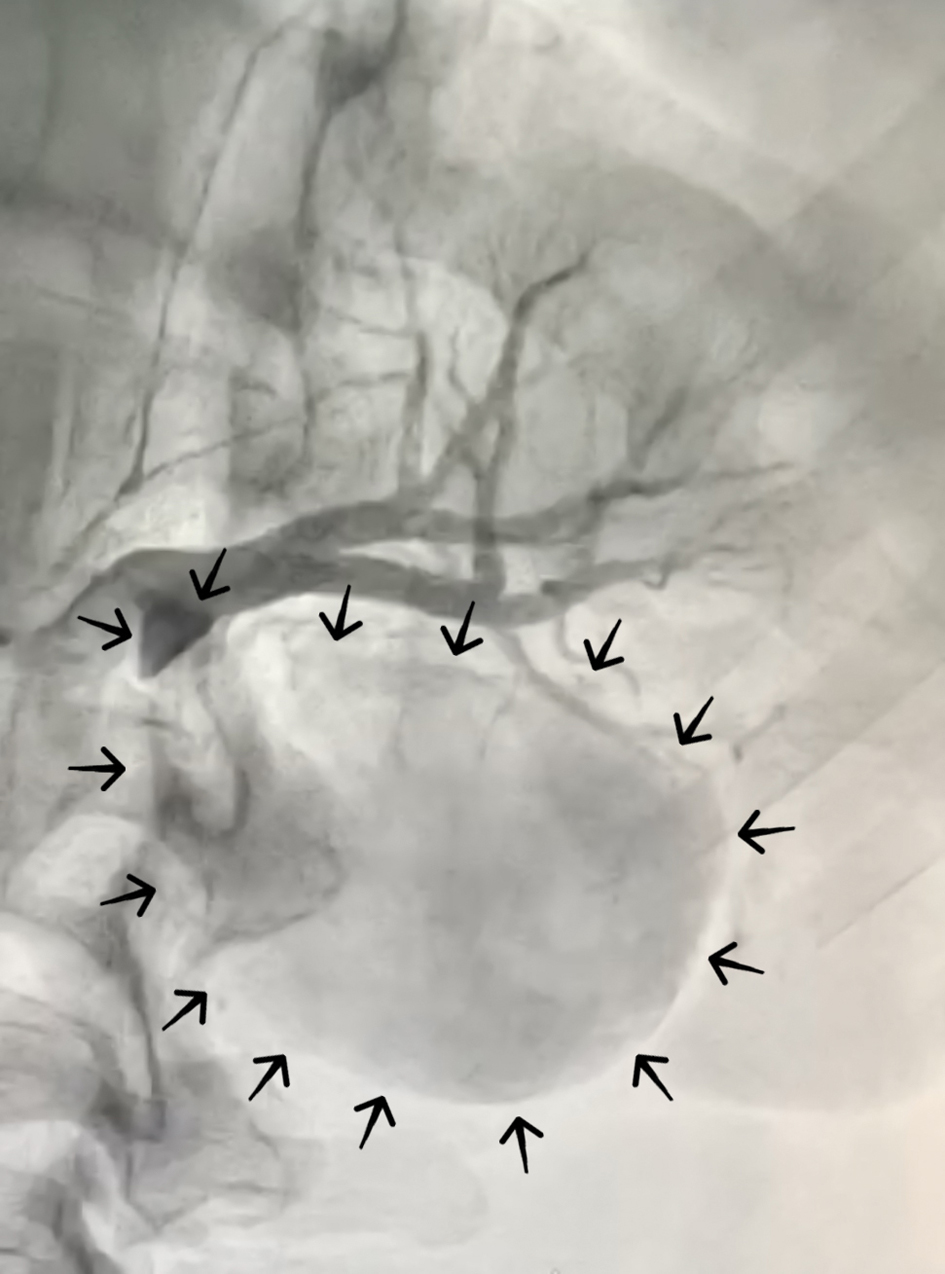 Fig. 1.
Fig. 1.Preoperative percutaneous renal angiography showed a giant aneurysm originating from the renal artery emerging at 90 degrees from the left renal artery. After a short branching off, it degenerated into a dissected aneurysmal sac as large as half a kidney, compressing the lower pole of the left kidney and delaying the lower half nephrogram (black arrows).
Considering that our patient met six of the nine indications for an
invasive (interventional or surgical) repair of RAAs (Table 1), two distinct
therapeutic approaches were discussed by weighing the risks and benefits. The
first strategy was to percutaneously deploy an endovascular stent-graft of 6.0 mm
diameter by 20 mm length (Celosia
| 1 | Aneurysmal rupture |
| 2 | Renovascular hypertension |
| 3 | Dissecting aneurysm |
| 4 | Expanding aneurysm |
| 5 | Thrombotic aneurysms |
| 6 | Maximal diameter |
| 7 | Women who are pregnant or of childbearing age |
| 8 | Significant stenosis, flank pain, or hematuria |
| 9 | Patients with hypertension and a solitary functional kidney |
Secondly, an alternative option was to practice open vascular reconstructive surgery. In this case, a total nephrectomy is performed as for standard transplantation. The decision whether the aneurysm could be safely removed was taken ad-hoc and ex-vivo. Open surgery for RAAs presents the same risks of any major abdominal surgery, including the RAA-specific complications of renal artery/graft occlusion, prothrombotic nature of graft material, emboli migration, and diminished renal function secondary to prolonged warm ischemia time [7]. However, Tsilimparis et al. compared the percutaneous approach versus reconstructive surgery and described similar perioperative morbidity without mortality and a similar risk for cardiac, respiratory, and renal complications at 30 days [8]. Thereby, considering the current alignment of advantages and disadvantages and the risk of subsequent rupture, the medical-surgical team comprised of the vascular surgeon, urologist, nephrologist, and angiographer finally opted for a surgical approach (see Fig. 2A, B).
 Fig. 2.
Fig. 2.Intraoperative images of the aneurysm (before resection). The size of the aneurysm in relationship with the kidney (almost half of its size) can be noted. The aneurysm had an outer diameter of 60 mm and compressed the lower pole of the left kidney. The partially thrombosed dissected aneurysm sac emerged at a distance of about 20 mm from the aortic origin.
Surgical exclusion of the aneurysm was successfully performed (see Fig. 3A, B), and the kidney was reimplanted in the left iliac fossa
(termino-lateral anastomosis between the renal artery and external left iliac
artery, termino-terminal ureteric anastomosis) with excellent postoperative
outcomes (see Fig. 4). Up until 30 days, the patient was asymptomatic. No
postoperative complications have occurred. Serum creatinine was within normal
range immediately after surgery and at 30 days follow-up. Similarly, a
substantial decrease in blood pressure (
 Fig. 3.
Fig. 3.Intraoperative images with the ex-vivo resected aneurysm and the thrombus inside. Surgical exclusion of the aneurysm was successfully performed. No perioperative complications have been reported.
 Fig. 4.
Fig. 4.Intraoperative image with the autotransplanted kidney and the left external iliac artery. The kidney was reimplanted in the left iliac fossa (termino-lateral anastomosis between the renal artery and external left iliac artery, termino-terminal ureteric anastomosis). Up until 30 days, the patient was asymptomatic. No postoperative complications have occurred.
This case’s peculiarity lies primarily in the ad-hoc manner to treat a giant left renal artery aneurysm. Ex-vivo vascular reconstruction by excision of the aneurysm (with its internal thrombus) was preferred (Fig. 3B) due to the high risk of rupture. Given the kidney’s compression damage, there was the problem of choosing between total nephrectomy and reimplantation by autotransplant. Another notable feature is the paucisymptomatic presentation (minimal symptoms usually being considered by the patient in a premenstrual context), the lack of feasibility, and timeliness for the interventional stent-graft treatment (owing to the high risk of fragile wall rupture).
An RAA is defined as a dilated segment of the renal artery or a sizeable segmental branch and exceeds twice the renal artery’s physiological diameter [9]. RAAs are rare, and their prevalence remains unknown, although various studies estimated their occurrence in approximately 0.01-0.1% cases relative to the general population and 1% of cases relative to the number of all aneurysms [10]. Giant RAAs are even rarer, and their documented size range from 5 to 12 cm [11]. In our case, the young’s patient aneurysm was reported as having 60 mm in diameter (Fig. 3A).
According to the extensive literature review of currently published case series and case reports of patients with RAA [12, 13, 14, 15, 16, 17, 18, 19, 20], there is still considerable controversy surrounding the most appropriate treatment: what size of RAA needs surgery, how and when is the most opportune moment for repair interventions, how should patients that underwent endovascular repair should be followed, and whether RAAs indeed cause hypertension [21]. To discover the most appropriate scientific answers, the level of evidence must be raised by publishing cases of resolved patients until randomized studies. Our paper’s value consists of the fact that it adds new evidence to the currently available literature on this rare disease and increases the size of the publicly available database of patients with RAA.
There are two leading causes of RAA: atherosclerosis and fibromuscular dysplasia. Considering that fibrodysplasia is the most frequent cause of extrarenal artery aneurysms [22], the patient’s young age and the absence of any risk factors for atherosclerosis (except hypertension), the most likely cause would have been the fibromuscular dysplasia. An anatomopathological examination has been performed. Unexpectedly, the pathological exam was conclusive for atheromatosis: “Arterial aneurysm with numerous fibro-lipid atheroma plaques, complicated by calcification, intra-plaque hemorrhage, and thrombosis”. The giant RAA caused by atherosclerosis in a 24-year-old female is a rare encounter and constitutes an important particularity of the reported case.
Hypertension is the most common finding due to increased renin production [22], and was also present in our patient. The disease is frequently asymptomatic, yet the young female patient complained of left flank mild abdominal pain. Additionally, despite its benign natural history, aneurysm dissection, thrombosis, and rupture may occur (especially during pregnancy), posing severe, even fatal, massive hemorrhagic consequences [1]. The medical team acknowledged the dissected and thrombosed RAA of our patient at a childbearing age.
For most asymptomatic aneurysms, expectant treatment is a reasonable approach. However, interventional or surgical repair is indicated in certain circumstances (as described in Table 1) depending on the size of the aneurysm and its natural history, rupture risk, and interventional/surgical risks [6]. The renovascular hypertension, the dissecting and thrombotic aneurysm, its giant size, the young fertile age, and the presence of the flank pain were all indicative of the need for aneurysm exclusion in our case.
Complex criteria for selected cases recommend either endovascular repair (stent-grafts or aneurysm embolization) or open repair (vascular reconstructive surgery or in extremis, nephrectomy) in order to prevent significant complications [23]. Even if therapeutic strategies have been recommended for the management of RAAs, giant RAAs management is less discussed. Although no consensus currently exists, an open approach (arterial reconstruction or planned nephrectomy) has been advocated for giant RAA cases, depending on the vessels anatomy, location of the aneurysm, and patients’ particular context (as arterial reconstruction may not be an option for elderly patients with significant comorbidities) [11].
The endovascular approach is still the first-line therapy for RAAs in anatomically suitable cases [6]. In our case, deploying a stent-graft and leaving the aneurysm on-site determined a high risk of perforation due to the impending dissection. Nevertheless, surgery remains the best treatment option for young patients as it provides a long-lasting resolution of the RAA (essential where an average life span is anticipated) [6]. The best open repair option is resectioning the aneurysm and reconstruction of the native artery [6]. Ex-vivo reconstruction followed by the kidney autotransplantation strategy chosen in our patient proved several advantages in terms of the ease of repair and sutures, the excellent renal function preservation with satisfactory long-term patency, rupture risk elimination, and beneficial effects on hypertension [24].
CM, AB and AM conceived and designed the surgical protocol; CM and AM performed the surgery; AB analyzed the data; AB and IVP wrote the paper; AC and AB revised the paper.
Patient consent has been obtained for publication. The study was approved by the regional Romanian institution “C. I. Parhon” Hospital Ethics Committee and all procedures were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
We thank “C. I. Parhon” Hospital staff that is responsible for the good quality of the photos and videos related to the case presentation.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
The authors declare no conflict of interest.
