Fever of unknown origin refers to a prolonged fever with an unknown cause despite adequate medical evaluations. This condition often leads to unnecessary extensive laboratory work-ups and antimicrobial therapies. The atypical presentations often cause a delayed diagnosis and an improper treatment with an increased morbidity rate. In cardiac surgical patients, fever of unknown origin remains an intriguing problem during the diagnostic process of cardiac surgical diseases. Cardiac myxoma or aortic dissection are often misdiagnosed when patients present with fever of unknown origin as an onset symptom. Under such circumstances, medical examinations by echocardiography and chest computed tomography, particularly fluorodeoxyglucose-positron emission tomography/computed tomography, have been proved crucial for early diagnosis. A better understanding of the clinical features of cardiac surgical disorders presenting with fever of unknown origin would facilitate early diagnosis of fever of unknown origin. A further decision-making of prompt treatment of choices of a cardiac operation is important for improving patients’ outcomes.
Fever of unknown origin (FUO) was defined in 1961 by Petersdorf and Beeson as: 1) a temperature > 38.3 °C (101°F) on several occasions, 2) > 3-week duration of illness, and 3) failure to reach a diagnosis despite a 1-week inpatient investigation (Petersdorf and Beeson, 1961). FUOs are often caused by infections (30-40%), neoplasms (20-30%), collagen vascular diseases (10-20%) and numerous miscellaneous diseases (15-20%) (Knockaert et al., 2003). Durack and Street (1991) expanded the concept of FUO with three novel supplements, i.e., nosocomial, neutropenic and human immunodeficiency virus-associated FUOs, and they modified the diagnostic criterion of “a 1-week investigation” into “a 3-day in-hospital investigation” or “ ≥ 3 outpatient visits”. FUO often leads to unnecessary extensive laboratory work-ups and antimicrobial therapies (Chien et al., 2017). Due to the atypical presentations, delayed diagnosis and treatment resulting in poor patient outcome often occur (Ranjan et al., 2015). To put aside the postoperative infection complications following cardiac surgery, FUO remains an intriguing problem in the diagnosis of cardiac surgical disorders. Moreover, the decision-making of treatment of choices of cardiac operation is crucial in relation to patients’ outcomes. This article aims to address the diagnosis dilemma and surgical indications of FUO in cardiac surgical patients.
Publications were systematically searched in the PubMed, Highwire Press and the Cochrane Library databases without a limit of publication year. The MeSH terms and keywords used to identify articles included “fever/pyrexia”, “unknown/unexplained/undetermined origin/”, “persisted/prolonged/sustained/”, “cardiac surgical procedures”, “infective endocarditis”, “aortic aneurysm”, “aortic dissection”, “cardiac tumor”, “cardiac myxoma”, “inflammatory pseudotumor of the heart”, “pulmonary embolism”, “aortitis”, “Takayasu arteritis”, “Behçet disease”, “Kawasaki disease” and “aortic graft infections”. Exclusion criteria were articles describing conditions without the need of a cardiac surgical intervention for treatment, such as post-cardiac surgical infections.
IE is an important cause of FUO and accounts for 1-5% of all causes (Mourad et al., 2003). IE most commonly involves the aortic valve, then the mitral valve, and then the tricuspid and pulmonary valves, which are the least common (Yuan, 2014a). In the tricuspid valve endocarditis in non-drug addict complicated with septic pulmonary embolism, fever persisted despite prolonged antibiotic use for 5 weeks (Iwama et al., 1996). Patients with right-sided IE with vegetations < 1.0 cm on echocardiography usually did not warrant surgical operation (Iwama et al., 1996). Persistent fever could be caused by vegetations ≥1.0 cm, which may account for 36% of the patients (Iwama et al., 1996). Clinical presentations of fungal endocarditis can be more complex than those of bacterial endocarditis in terms of more common congestive heart failure, embolic events and metastatic abscesses (Kaygusuz et al., 2003). A 71-year-old male non-drug addict had tricuspid valve endocarditis caused by Candida colliculosa and suffered from a 7-month fever before the diagnosis was made. The surgical operation was arranged after 1-2 week full dosage amphotericin B therapy. It demonstrated that early antifungal treatment and valve replacement might lead to an improved outcome (Kaygusuz et al., 2003). Patients with pacemaker led endocarditis may present with FUO. In the diagnosis of IE, erythrocyte sedimentation rate was less sensitive than C-reactive protein (CRP). IE of the native valves is associated with higher CRP than that of prosthetic valves. Higher CRP levels appear with a Staphylococcal origin, short duration of symptoms (including fever), and higher temperature (Hogevik et al., 1997). Blood cultures are a golden standard for microbial diagnosis of IE, and histological and molecular studies are helpful diagnostic methods for resected valves (Liesman et al., 2017). Echocardiography helps identify the vegetation, and computed tomography (CT) confirms complicated pulmonary infarction. Surgical complete or percutaneous partial extraction of pacemaker lead should be performed. Usually, the tricuspid valve is excised and replaced by a prosthetic valve; however, tricuspid valve repair is advocated for the preservation of valve structure. The timing of a new pacemaker lead insertion should be done 2 weeks after surgical operation (Simon et al., 2008).
Culture-negative endocarditis is reported in 5-10% of IE patients. The HACEK group is responsible for 5-10% of IE cases and is the most common cause of Gram-negative bacilli (Haemophilus spp., parainfluenzae, aphrophilus and paraphrophilus), Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella spp. endocarditis among non-intravenous drug users. IE with negative cultures sometimes presents with FUO and complaints of deterioration of cardiac function, thus constituting a challenging differential diagnosis (Rocha et al., 2014). The risk factors for blood culture-negative IE include previous antibiotic administration, exposure to slow-growing and fastidious non-bacterial microorganisms, underlying valvular heart disease and IE in patients with vascular or intracardiac devices (Rocha et al., 2014). Fever, multiple pulmonary emboli and sustained bacteremia by Staphylococcus aureus are signs of clinical alert for right-sided IE (Yuan, 2014a). Pulmonary valve gonococcal IE patient was also reported to show FUO (Rosoff et al., 1983). The diagnosis and treatment of Brucella endocarditis in a non-endemic area was even more challenging due to the rarity of the pathogen and poor response to empirical antibiotic therapy. The diagnosis might rely on blood culture after 1-week antibiotic cessation, and antibiotic changes into streptomycin, doxycycline, co-trimoxazole, and rifampicin are warranted (Manade et al., 2014). In a similar case, the diagnosis was made by positive blood culture of Brucella abortus and paravalvular abscess formation after 3.5-month fever. The patient had a satisfactory outcome after aortic valve replacement (Pilar, 2010). Fungal endocarditis and mycobacterial endocarditis are also causative sources of FUO (Yuan, 2015a; , 2016). Yuan (2016) summarized the clinical features of fungal endocarditis, and found all patients presented with fever and 2 of them had FUO, eventually requiring surgical treatment. Additionally, Yuan (2015a) described mycobacterial endocarditis, noting 76.1% of patients had fever with a mean body temperature of 38.9 °C and a mean CRP of 6.8 mg/dL, and 2 of them had FUO. In spite of adequate antibiotic use, worsening symptoms including uncontrolled fever and infection, congestive heart failure, and enlargement of vegetations might occur and then, the patient may be indicated for surgical treatment (Kietselaer et al., 2007).
3.2.1 Parietal aortic aspergillosis
Aspergillus species are angioinvasion. Parietal aortic aspergillosis often occurs in immunocompetent patients who may present with FUO (Abenza-Abildua et al., 2009). It is also caused by invasive pulmonary aspergillosis (Verea-Hernando et al., 1989). Aspergillus aortitis may be presented with aortic pseudoaneurysm on CT, but transthoracic and transesophageal echocardiograms showed no major cardiac findings and no evidence of valvular vegetations (Elmezayen and Youssef, 2016). Prosthetic vascular graft infection caused by Aspergillus species is a rare condition (Liu et al., 2015). Diagnostic work-ups may include laboratory tests, cranial CT and echocardiogram due to the possible sequelae like cerebral infarction, embolic events and cardiac dysfunction (Abenza-Abildua et al., 2009). Perigraft air, fluid, soft tissue attenuation and pseudoaneurysm can be observed by CT scans of patients with aortic graft infection. Most cases of vascular graft infection caused by Aspergillus species are believed to originate from contamination by fungal spores during operation (Liu et al., 2015). In severely infected patients, therapy with intravenous amphotericin B was initiated and followed by oral itraconazole (100 mg/day) should be undertaken before surgical operation is performed (Ramos Ade et al., 2003). The surgical operation is determined by extent of aorta involvement, and postoperative antifungal therapy should be continued.
3.2.2 Mycotic aortic aneurysm
Mycotic aortic aneurysm is an uncommon cause of FUO and is often associated with a high mortality (Asthana et al., 1989). Streptococci viridans, Staphylococcus aureus, enterococci, gram-negative bacilli, and Staphylococcus epidermidis are common infecting organisms (Asthana et al., 1989). The disease course can be insidious, and persistent fever ranging 37.5 - 38.5 °C may last for a few months (Asthana et al., 1989). Pathological studies revealed that the aortic wall was characterized by complete destruction of elastic fibers. The mycotic aneurysms may develop secondary to IE, or following invasive cardiovascular procedures, such as aortic operation, coronary artery revascularization, or percutaneous transluminal coronary angioplasty. The growth of mycotic aneurysm is rapid at several centimeters/month. It is recommended in febrile patients with previous cardiovascular procedures that careful examinations be made with echocardiography, ultrasonography, digital subtraction angiography, CT and magnetic resonance imaging (MRI) (Asthana et al., 1989; Prech et al., 2000). Early diagnosis and treatment with proper antibiotics and timely surgical repair are determinants of patients’ survival (Manade et al., 2014). Surgical treatment includes in situ or extra-anatomical graft placement (Asthana et al., 1989).
FUO is a rare primary presentation of aortic dissection (Yuan et al., 2009a). Thirty-seven (74%) patients had a pure aortic dissection responsible for FUO, whereas 13 (26%) patients had aortic dissection associated with an infectious disorder (Yuan, 2017a). The fever type in patients with aortic dissection with FUO can be persistent (76.3%), episodic (10.5%), intermittent (5.3%) and recurrent (7.9%). The patients’ temperature on admission was 38.2 ± 0.6 °C, and the maximal temperature was 38.8 ± 0.4 °C (n = 12) during the course (Yuan, 2017a). Such patients often had fever ahead of chest pains, therefore leading to missed or delayed diagnosis. More pronounced laboratory findings of the infectious type than the inflammatory type aortic dissection could be helpful for differential diagnosis. Half patients warrant aortic repair with or without valve replacement, less than half of patients were conservatively managed, and a few were interventionally treated or under follow-up. The mortality rate was 9.5% (Yuan, 2017a).
3.4.1 Cardiac and heart valve myxomas
Symptoms of patients with a cardiac or heart valve myxoma could be constitutional including fever, weight loss and night sweat (Yuan, 2012a), but on rare occasions, patients may present with FUO (Mroziński et al., 1997; Yuan et al., 2017; Yuan, 2017b). The tumors had small sizes, the predilection of mitral leaflet location, solitary and pedicled nature, and good response to surgical resection (Yuan and Sternik, 2012). In a recent review, clinical features of 62 patients with cardiac myxoma with FUO were analyzed. Fever in these patients during hospitalization was 38.8 ± 0.7 °C with a fever duration of 6.4 ± 14.6 months. The time interval from onset to cardiac myxoma diagnosis was 6.0 ± 6.1 months, and from physician consultation to diagnosis was 1.4 ± 2.6 months. Poor responses to antimicrobial therapies were observed in > 35% patients and 4.9% were otherwise managed. Surgical resection of cardiac myxoma was performed in 48 (88.9%) patients. Kaplan-Meier survival analysis revealed an overall survival of 95.5% in surgical versus 16.7% in nonsurgical patients (Yuan, 2017c).
3.4.2 Infected cardiac myxoma
Infected cardiac myxoma is a rare condition that incorporates infection, immune and neoplasm (Bhanot et al., 2010). Infected cardiac myxomas illustrated some aggravated clinical manifestations, including relative more occasions of high-grade fever, multiple embolic events and presence of refractory microorganisms (Yuan, 2015b). Most (97.3%) patients with infected cardiac myxoma had fever (Yuan, 2015b) and 14% with FUO (Yuan et al., 2017). Accordingly, the diagnosis seemed more difficult. A special sign of “finger-like projections” on tumor surface on echocardiography or MRI might suggest infected cardiac myxoma (Puvaneswary and Thomson, 2001). Cardiac myxoma resection may relieve fever; however, a delayed operation for 18 days was sometimes necessary to allow for sufficient antibiotic treatment and stabilization of patients’ conditions (Yuan, 2015b).
3.4.3 Cardiac hemangioma
Cardiac hemangioma is a rare primary benign tumor. Most cardiac hemangiomas are asymptomatic and are discovered incidentally at autopsy, or by echocardiography, CT, or MRI check-up. Symptomatic patients may manifest arrhythmias, pericardial effusions, congestive heart failure, embolic events, or even sudden death (Yuan et al., 2008). Patients with cardiac hemangioma may have a fever and mistaken as sepsis or disseminated intravascular coagulation. The mechanisms of fever in such patients were considered to be feeding coronary artery occlusion by tumor emboli, concomitant coronary disease, tumor invasion, or infected cardiac hemangioma. Enhanced contrast CT or MRI may show vascular nature of the tumor, and coronary angiography often shows tumor blush. Complete resection is recommended (Tse et al., 2005). The accurate preoperative imaging assessment of cardiac tumors necessitating surgical resection become increasingly important in the decision-making process of a surgical approach (Yuan et al., 2009b).
3.4.4 Inflammatory pseudotumor of the heart
Inflammatory pseudotumor may involve any location of heart, with atria being the most common (Anvari et al., 2009). Anvari et al. (2009) reported an 18-year-old male with this lesion had 1-month fever, during which some unnecessary laboratory investigations were made. Echocardiography and CT angiogram demonstrated an intracardiac space-occupying lesion. The patient’s fever subsided following tumor resection. Histological studies revealed the tumor caused predominate chronic inflammatory cell infiltration. Immunohistochemical staining also showed CD68 reactivity.
3.4.5 Primary cardiac lymphoma
Patients with a primary cardiac lymphoma may present with FUO. The diagnosis could be established by transesophageal echocardiography-guided biopsy. Complete remission of the tumor could be obtained by immunochemotherapy, and a surgical resection becomes necessary when immunochemotherapy is ineffective (Groebner et al., 2014).
3.4.6 Acute pulmonary embolism
Pulmonary embolism is an important cause of nosocomial FUO (Roth and Basello, 2003). Wilson and Tuazon (1980) reported a patient with pulmonary emboli 5 days post trauma had FUO. Fever in acute pulmonary embolism is probably due to an acute inflammatory response triggered by endogenous chemotactic factors via tissue damage or complement activation processes (Boos et al., 2003). Anticoagulation therapy for such patients may lead to rapid fever resolution, and this was explained as a result of the anti-inflammatory and antipyretic properties of heparin (Boos et al., 2003). It was also proposed that in the anti-inflammatory process, activity or synthesis of tissue necrosis factor and inflammatory cell adhesion were inhibited (Okajima, 2001). In patients with contraindication for thrombolysis, surgical pulmonary embolectomy can be performed with low mortality (Lehnert et al., 2012).
3.5.1 Takayasu arteritis
Takayasu arteritis is a rare cause of FUO. In some Takayasu arteritis patients, the etiology of FUO could only be verified after patients developed pulselessness (Vaz et al., 1988; Wu et al., 1989). Noninvasive diagnostic techniques, such as MRI, CT, gallium-67 scintigraphy and ultrasonography, may help the diagnosing of Takayasu arteritis in a pre-pulseless stage (Uthman et al., 1999). Fluorodeoxyglucose-positron emission tomography/CT can assist early diagnosis of Takayasu arteritis with FUO (Kim and Oh, 2015). Patients with Takayasu arteritis who undergo aortic valve replacement may develop severe complications including detachment of the prosthetic valve and pseudoaneurysm formation. With everting mattress suture techniques along with postoperative prednisolone intake, these complications might be avoided (Sakakibara et al., 1998).
3.5.2 Behçet disease
Behçet disease is a chronic inflammatory disorder with multisystem involvements. Cardiac involvement accounted for 7-29%. It showed a high propensity to develop intracardiac thrombus with half number in the right ventricle, and thrombus in other cavities or multiple sites. The thrombus attached tightly to the myocardium is still considered a source of distal embolization. Prognosis of heart involvement of Behçet disease was poor and the mortality was high. The diagnosis of Behçet disease is challenging when the onset of symptoms, signs, or laboratory findings are nonspecific. FUO are frequent at onset and difficult to distinguish from other inflammatory disorders (Koné-Paut, 2016). Erkek and Ayaslioglu (2006) reported a patient with Behçet disease presenting with 1-month fever with chills predominantly occurring at nights. Gotfried et al. (1985) reported a young female presented with 12-week FUO before pathognomonic presentations appeared. Her fever subsided 48 hours after attempted treatment of colchicine 1.5 mg/day. In Behçet disease, chest CT or MRI is commonly used to detect pulmonary and intracardiac thromboembolism. Echocardiography is widely used to evaluate the efficacy of treatment and progress of patients (Xing et al., 2013).
Cardiac operations performed in Behçet disease were valvular 42%, aneurysmal 33%, thrombotic 13.1%, coronary 5.7% and miscellaneous 6.3%. Aortic valve regurgitation, pulmonary artery aneurysm and intracardiac and great vessel thrombosis were the most common indications for a cardiothoracic intervention. Dehiscence of the prosthetic valve was the main cause of death. Intense immunosuppressive treatment may reduce postoperative complications and need for reinterventions (Yuan, 2014b; , 2014c). Pulmonary artery aneurysms are the most common type of pulmonary involvement in Behçet disease. Conservatively treated patients were prone to pulmonary artery aneurysm progression; interventional embolization was associated with higher risks of recurrence and reintervention, and surgically treated patients exhibited the highest mortality rates (Yuan, 2014d).
3.5.3 Kawasaki disease
Kawasaki disease is an acute inflammatory vasculitis of the medium-sized arteries. Coronary artery aneurysms/arteritis was found in 20-25% of Kawasaki patients (Kato et al., 1982). FUO was attributable to myocarditis and coronary panarteritis of an acute or subacute phase of the disease. CD68 and CD45RO-positive immunohistochemical reactions were noted in the myocarditis foci of heart tissue specimens (Bartoloni et al., 2002). Two infants at the age of 3 and 4 months, respectively, presented with FUO lasting for > 12 days in both before atypical Kawasaki disease was diagnosed. Cardiac echocardiograms showed dilation of the coronary arteries in both patients and confirmed the diagnosis. Immediate therapy with intravenous immunoglobulins and acetylsalicylic acid was given, whereupon fever subsided within 24 hours (de Ruijter et al., 2004). Kawasaki disease should be suspected in any child with persistent fever with no other likely explanations. The pyrexia associated with Kawasaki disease is typically high (often > 39 °C), remittent and unresponsive to antibiotic therapy, and usually also unresponsive to antipyretics (Golshevsky et al., 2013). Echocardiography is recommended to perform on admission to detect the dimensions and morphologies of the coronary arteries. However, within 10 days of admission, coronary artery aneurysm may not be evident by echocardiography (Golshevsky et al., 2013), but coronary ectasia, perivascular brightness, or lack of normal coronary tapering could be seen instead (Crystal et al., 2008). Intravenous immunoglobulin (IVIG) is an effective therapy to improve coronary artery pathologies, and to reduce incidence of coronary artery aneurysms to 2-5%. Kawasaki disease unresponsive to initial IVIG is usually treated with a further IVIG dose, and with pulsed methylprednisolone or anti-tumor necrosis factor therapy (Golshevsky et al., 2013). Approximately half of coronary artery aneurysms resolve within 1-2 years (Bartoloni et al., 2002), but stenosis of the affected vessel might develop with healing (Mueller et al., 2009). Kawasaki patients with coronary aneurysm and/or calcification/thrombus requires coronary artery bypass at a very young age (Yuan, 2012b).
Graft infections should be included in the differential diagnosis of FUO. Thoracic graft infection is rare but life-threatening with an incidence of 0.9-1.9% (Aizawa et al., 2008). Raffetto et al. (2004) reported a case of aortic graft infection with Mycobacterium tuberculosis presenting with relapsed pronged fever, and was cured by multiple antimycobacterial drugs and surgical debridement. Matsuhisa et al. (2009) reported an infant with modified Blalock-Taussig shunt using a 4 mm expanded polytetrafluoroethylene graft developed FUO due to anastomotic pseudoaneurysm and shunt infections. He underwent aneurysmal wall and graft resections and had an uneventful recovery.
The paradoxical manifestations of FUO may perplex physicians, and lead to a delayed diagnosis and an incorrect management (Yuan, 2017c). Causes of FUO can be categorized as infection (tuberculosis, mononucleosis, Lyme disease, cat scratch fever and endocarditis), inflammation (lupus, rheumatoid arthritis and inflammatory bowel disease), malignancy (lymphoma, leukemia, pancreatic carcinoma and sarcomas) and miscellaneous (fevers caused by drug use or abuse, hyperthyroidism, hepatitis and factors unfitting into other categories).
Differential diagnosis of etiologies of FUO is crucial for avoiding excessive work-ups and timely proper treatment (Cunha et al., 2015). Serum D-dimer is a specific diagnostic indicator of pulmonary embolism and interleukin-6 is specific for cardiac myxomas. Blood cultures are valuable means for diagnosis of IE caused by various pathogens. Medical imaging techniques (echocardiography, CT, fluorodeoxyglucose-positron emission tomography/CT and MRI) are of great diagnostic importance. Gallium-67 or indium-111 scan is helpful for etiological diagnosis of FUO for infective or malignant natures (Arnow and Flaherty, 1997). In the cardiac surgical field, an early diagnosis may warrant a prompt surgical treatment for patients with FUO. A flow chart showing the suggested diagnostic work-up for a case of FUO in cardiac patients, and the diagram of the screening and management protocols of FUO for a cardiac operation were shown in (Fig. 1 and 2).
 Figure 1.
Figure 1.A flow chart showing the suggested diagnostic work-up for a case of fever of unknown origin in cardiac patients.
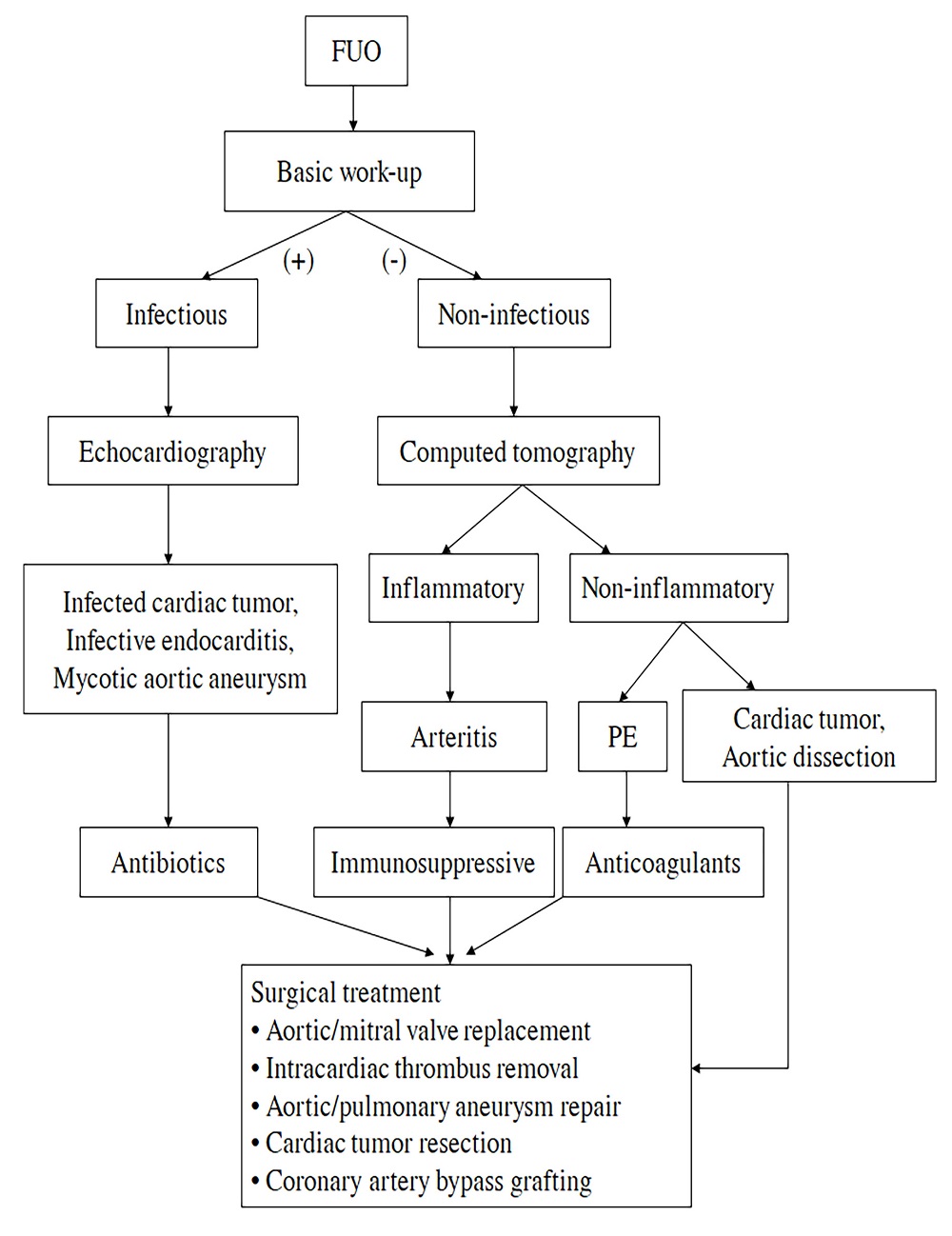 Figure 2.
Figure 2.The diagram of the screening and management protocols of fever of unknown origin in cardiac surgery. FUO: fever of unknown origin; PE: pulmonary embolism.
The surgical indications for patients of FUO requiring a cardiac surgical intervention are: 1) treatment of the primary cardiac disorders (aortic dissection, pulmonary embolism, or cardiac tumors), 2) removal of sources of infections, 3) avoidance of potential embolic events and other comorbidities, 4) removal of pyrogens, and 5) surgical treatment of cardiovascular complications of arteritis/vasculitis/aortitis (aortic regurgitation, intracardiac thrombus, aneurysms of the pulmonary artery, coronary artery, or aorta). Immediate defeverence and significant decrease of inflammatory indicators have been observed after surgical treatment ( Lin et al., 2011; Yuan, 2017a).
FUO often leads to unnecessary extensive laboratory work-ups and antimicrobial therapies. Due to atypical presentation, delayed diagnosis and treatment are often seen. In cardiac surgical patients, FUO remains an intriguing problem in diagnosis of cardiac surgical disease. The decision-making of cardiac operation is crucial for improving patients’ outcomes. A better understanding of clinical features, surgical indications, and surgical timing of these disorders could facilitate the etiological diagnosis and treatment of FUO.
Substantial contribution to the conception and design of the work; and the acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Thank numerous individuals participated in this study.
Author declares no conflict of interest.
