Recent studies have shown that the integrity of the gastrointestinal tract and its microbiome impact the functioning of various body systems by regulating immunological responses, extracting energy, remodeling intestinal epithelia, and strengthening the gut itself. The gastrointestinal tract microbiota includes bacteria, fungi, protozoa, viruses, and archaea which collectively comprise a dynamic community prone to alterations via influences such as the environment, illness, and metabolic processes. The idea that the host’s diet possesses characteristics that could potentially alter microbiota composition is a novel notion. We hypothesize that a high fat diet leads to the alteration of the gastrointestinal microbiota composition and that metabolic transformation of the compound trimethylamine into trimethylamine-N-oxide promotes vasculopathy such as atherosclerosis and affects cardiovascular functionality. Furthermore, we hypothesize that treatment with probiotics will restore the homeostatic environment (eubiosis) of the gastrointestinal tract.
The composition of the community of microbes in the gastrointestinal tract (GIT), known as the microbiota, is an essential indicator of the health of an organism. It has been widely understood that the microbial lining of the GIT in an infant is broadly colonized by bacteria after encountering the mother’s vaginal microbial lining. Furthermore, the composition of the mother's microbiota impacts the relative health and balance of the infant's microbiota (Rajoka et al., 2017). After this initial colonization, the microbiota is further influenced by exposure to environmental factors such as pathogens, diseases, and dietary shifts (Donaldson et al., 2016; Thursby and Juge, 2017). If the microbial environment of the GIT alters significantly, deleterious effects may follow. The eubiotic microbial environment involves a healthy balance of microorganisms in the GIT, supporting the functionality of the gut and other bodily systems. In this way, a symbiotic relationship is maintained, benefitting the host organism. In contrast, a dysbiotic environment increases the likelihood of negative consequences for the host. Dysbiosis has been related to an elevated risk of irritable bowel syndrome (IBS), diabetes, atherosclerosis, and other diseases (Fig. 3) (Falony et al., 2015; Peng et al., 2018). In this review, we focus on the impact of gut dysbiosis on the risk of cardiovascular disease due to the accumulation of trimethylamine-N-oxide (TMAO) in body tissues (Fig. 2) (Al-Rubaye et al., 2019; Velasquez et al., 2016). We summarize and critique the accumulation of research in this area.
Dietary composition has been implicated as a central factor influencing the relative composition of the gut microbiota (Brial et al., 2018; Donaldson et al., 2016; Falony et al., 2015; Martinic et al., 2018; Rajoka et al., 2017; Thursby and Juge, 2017). Indeed, it has been shown that the consumption of a diet high in saturated fats, carbohydrates, and red meats (commonly associated with the Western diet) significantly impacts the microbiota (Martinez et al., 2017; Rajoka et al., 2017). Furthermore, individuals that consume a nutrient-depleted diet (low in fiber and high in fat) have experienced the loss of some essential microbes from the lining of their GIT (Makki et al., 2018). Dietary fats are necessary for the proper synthesis of cellular structures, energy storage, and the utilization of fat-soluble vitamins (Feingold and Elias, 2014). However, fat is also the most efficient source of energy calories, with only 5-7% escaping digestion in healthy adults, and a high fat diet has been shown to negatively alter microbial composition and function. Such dysbiosis can consequently impair metabolic functions and the production of excess homocysteine (Hcy), as well as leading to the accumulation of trimethylamine-N-oxide (TMAO), remodeling of blood vessels, atherosclerosis, and cardiac dysfunction (Fig. 2 and 3) (Donaldson et al., 2016; Stremmel et al., 2017; Tang et al., 2015; Wang et al., 2011).
The microbiota lining the GIT is composed of a plethora of microbes. The composition of the microbiota changes as the organism advances in gestational age (Donaldson et al., 2016), and it is also impacted by factors such as diet, chemical exposure, and immunological physiology (Ma and Li, 2018; Nehra et al., 2016). The dominant phyla of bacteria found in the gut are the Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobiota. The GIT itself is composed of structures including a variety of chemically active components. These physiological differences within the GIT have an influence in determining which microbial species will thrive in the microbial lining of the structure. The small intestine maintains a highly acidic environment, with oxygen and antimicrobials present in abundance. The microbiota of the small intestine in mice models have been shown to be mainly composed of facultative anaerobic bacteria that readily adhere to the epithelial lining of the intestine (Jones et al., 2007, 2011). The dominant bacterial genus in the human small intestine that displays these characteristics is Lactobacillus (Thursby and Juge, 2017). Comparatively, the large intestine (colon) is composed of a diverse community of microbes that are mainly anaerobic and capable of metabolizing complex carbohydrates.
Many external factors can influence the composition of the gut microbiota. Its relative integrity may play an essential role in regulating metabolic functions within the GIT. An organism’s diet is one of the main determinants of the composition of the gut microbiota (Chen et al., 2017; Donaldson et al., 2016; Martinic et al., 2018; Nehra et al., 2016; Rajoka et al., 2017; Thursby and Juge, 2017). In mice models, it has been shown that microbiota composition is significantly different in animals fed a high-fat diet (HFD) compared to those fed a standard chow diet (Fig. 1) (Howarth et al., 2005; Rajoka et al., 2017). In a human study comparing the composition of the microbial lining of different individuals, it was found that African children with a diet mainly composed of fiber, starch, and plant polysaccharides had many bacteria from the phylum Bacteroidetes, whereas European children with a diet consisting mostly of starch, sugar, and animal proteins had significantly fewer Bacteroidetes in their microbial lining (Jama et al., 2019; Thursby and Juge, 2017). This study implies that there may be an association between the presence of certain microbes and specific dietary components. In a recent study by Jama et al. (2019) , it was found that the relative ratio of the two dominant phyla of bacteria in the gut, Bacteroidetes and Firmicutes, is dependent upon the dominant nutrient being supplied to the GIT. Individuals with a diet mainly composed of fiber-rich foods possessed greater numbers of Bacteroidetes, and fewer Firmicutes. In this study, the relative ratio of the amount of Firmicutes to Bacteroidetes was used as an indicator of the integrity of the gut microbiome (Jama et al., 2019). A population of gut microbiota containing a significantly greater number of Firmicutes than Bacteroidetes would reflect a dysbiotic environment, with several metabolic consequences.
 Figure 1.
Figure 1.(A) A schematic illustrating the effects of diet on gut microbiome and subsequent consequences. Mice that were given a regular chow diet maintained the eubiotic gastrointestinal environment. The eubiotic environment did not promote the accumulation of TMAO or significantly alter cardiovascular functionality. (B) Mice that were fed HFD developed a dysbiotic gastrointestinal environment that supported the accumulation of TMAO and altered the cardiovascular functions. Proposed treatment with probiotic (s) supplementation may decrease some harm as caused by the HFD through mitigating deleterious effects induced by an unhealthy diet by reconstituting the microbial lining of the gut with the healthy microbes.
The regulation of choline, phosphatidylcholine (PC), and other metabolic intermediates is necessary to maintain the relative health and proper functioning of the GIT and other organ systems (Romano et al., 2015; Wang et al., 2014). The accumulation of TMAO occurs in part because of the consumption of dietary PC, a type of phospholipid that has incorporated choline attached to its head group (Fig.2) (Koeth et al., 2013; Romano et al., 2015; Stremmel et al., 2017; Velasquez et al., 2016; Wang et al., 2014). A member of the lecithin group of yellow-brownish fatty substances, dietary PC is abundant in commonly consumed foods such as poultry, meat, and dairy products. In the eubiotic environment, PC is converted into choline by the enzyme phosphatidylcholine phosphatase. The newly synthesized choline is used to produce betaine, which, when combined with homocysteine (Hcy), produces methionine and dimethyl glycine. In a dysbiotic environment, however, the phosphatidylcholine phosphatase is not able to convert PC into choline, and thus the concentration of PC is increased within the GIT.
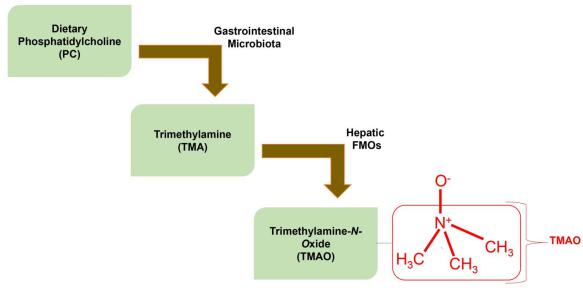 Figure 2.
Figure 2.Representation of how TMAO accumulates in the body because of the ingestion of dietary PC. The choline portion of the PC is metabolized into TMA by gastrointestinal microbes. The TMA then travels to the liver, via the bloodstream, where it is converted into TMAO by hepatic FMOs. A skeletal structure for the TMAO molecule (boxed in red color) is implicated in developing cardiovascular diseases by enhancing atherosclerotic factors. The precursor of TMAO that is TMA is produced in GIT as a byproduct of the metabolization of choline/PC by gut microbiota. The resulting gaseous TMA enters the circulatory system where it is converted into TMAO (boxed in red color) by hepatic FMOs (Eswaramoorthy et al., 2006). The FMOs are responsible for oxygenating lipophilic compounds so that they can be solubilized and rapidly excreted (FMOs selectively oxygenizes the nucleophilic nitrogen in the amine group producing the TMAO molecules) (Eswaramoorthy et al., 2006).
Choline, along with betaine, PC, L-carnitine, γ-butyrobetaine, and TMAO can be utilized to synthesize trimethylamine (TMA) in the GIT (Fig. 2) (Chen et al., 2017; Koeth et al., 2013; Wang et al., 2014). Ingested and accumulated PC is metabolized by the intestinal by the Enterobacteriaceae into gaseous TMA, which is absorbed into the bloodstream (Bu and Wang, 2018; Chen et al., 2017; Gallistl et al., 2000; Tang et al., 2013; Wang et al., 2011). It is then metabolized into trimethyl-N-amino oxide (TMAO) by flavin-containing monooxygenases found in the liver (Fig. 2) (Eswaramoorthy et al., 2006). An increased concentration of TMAO in the plasma has been correlated with the accumulation of fatty deposits in the blood vessels and the development of atherosclerosis, as well as an increased risk of major adverse cardiovascular events such as myocardial infarction and stroke (Fig. 3) (Randrianarisoa et al., 2016; Stremmel et al., 2017; Tang et al., 2015; Velasquez et al., 2016; Wang et al., 2011) (Fig. 2).
 Figure 3.
Figure 3.A visual depiction of the stepwise mechanism connecting TMAO and the enhancement of atherosclerotic factors. TMAO is produced in the liver by FMOS. The increased plasma concentration of TMAO promotes the upregulation of macrophage scavenger receptors. The stimulated scavenger receptors promote inflammation and gobble-up the macrophages. Furthermore, the accumulated macrophages produce foamy cells because of their inability to properly metabolize lipids inside them. In summary, the accumulation of macrophages, foamy cells, and inflammation together promote the development of atherosclerosis.
It has been proposed that TMAO contributes fundamentally to the pathogenesis of obesity and metabolic syndrome. Metabolic syndrome refers to a cluster of conditions including high blood sugar, elevated blood pressure, the accumulation of fat around the waist, which increase the risk of type 2 diabetes and cardiovascular disease. Barrea et al. (2018) found plasma concentration of TMAO to be positively correlated with body mass index (BMI). These factors were also positively associated with visceral adiposity index (an indicator of adipose dysfunction) and fatty liver index (a predictor of non-alcoholic fatty liver disease (NAFLD), which increases the risk of developing metabolic syndrome). The researchers concluded that TMAO may be utilized to determine individual risk of developing NAFLD and subsequent metabolic syndrome (Barrea et al., 2018). These findings suggest that the promotion of metabolic syndrome and type 2 diabetes by TMAO may increase the risk of adverse cardiovascular events. In this context, TMAO is a potential early biomarker of metabolic syndrome. However, mechanistic links between these variables have yet to be established. (Al-Rubaye et al.,2019).
It has also been shown that elevated plasma levels of TMAO remain a significant indicator of cardiovascular risk even after controlling for traditional risk factors such as diabetes, age, obesity, and cigarette smoking (Nam, 2019; Tang et al., 2013, 2015). In mice models, it has been shown that the accumulation of TMAO in the circulatory system may contribute to the pathogenesis of atherosclerosis (Fig. 2) (Howarth et al., 2005; Leustean et al., 2018). The GIT microbiota is thought to play an essential role in the enhancement of atherosclerotic factors (Jonsson and Backhed, 2017; Nam, 2019; Romano et al., 2015). In a study aimed to determine the role of the microbiota in the metabolism of PC and the subsequent production of TMA, Wang et al. (2011) found that mice with completely suppressed intestinal flora (achieved with antibiotics) showed no enhancement of atherosclerotic factors with the addition of choline supplements. Conversely, in the absence of antibiotics a three-fold increase in the accumulation of fatty plaques was measured following choline supplementation (Wang et al., 2011). This study provides a strong argument that PC/choline metabolism mediated by intestinal microbiota is an important link in the chain of events leading to the increased atherosclerotic risk (Fig. 3).
Recent evidence supports the notion that increased TMAO concentration contributes to the promotion of cardiac dysfunction and atherosclerosis (Fig. 2 and 3) (Al-Rubaye et al., 2019; Kanitsoraphan et al., 2018; Koeth et al., 2013; Li et al., 2018; Randrianarisoa et al., 2016; Stremmel et al., 2017; Tang et al., 2015; Velasquez et al., 2016; Nam, 2019). Atherosclerosis is a chronic inflammatory disease. TMAO in the blood has been shown to upregulate the production of macrophage scavenger receptors (CD36 and SR-A1) associated with promoting atherosclerotic risk (Falony et al., 2015). Circulating plaque macrophages are one of the two primary cells from the innate immune system that are involved in the production of plaque. The regulation and processing of modified lipoproteins is, in part, due to the involvement of scavenger receptors. These receptors are responsible for internalizing extracellular components and marking them for degradation in the lysosome. Since there is a broad range of scavenger receptors, many of them respond to different signals. Some scavenger receptors respond to modified lipoproteins by amplifying the accumulation of macrophages in the plaque and promoting inflammation, culminating in an increased risk of atherosclerosis (Fig. 3). Comparatively, macrophages do not obtain the ability to process modified lipoproteins effectively, resulting in the production of foam cells which are one of the main components of atherosclerotic plaques (Kzhyshkowska et al., 2012). Even though studies have highlighted the role of TMAO in the accumulation of macrophage scavenger receptors as a mechanism for enhancing atherosclerotic risk, the specific role of scavenger receptors in this context is still being explored (Velasquez et al., 2016). The connection between an individual’s diet and its metabolic consequences in the lining of the GIT also continues to be explored. A potential risk factor for the development of atherosclerosis is a diet high in lipids/fat, which may lead to the accumulation of TMAO (Fig. 3).
High fat diet-induced accumulation of TMAO also increases the concentration of pro-inflammatory cytokines in the bloodstream, which leads to a marked increase in cardiac inflammation and fibrosis (Fig. 1, 2 and 3) (Chen et al., 2017). A dysbiotic environment in the GIT has metabolic consequences, including the production of pro-inflammatory factors, breaching of the endothelial layer of the intestine, and the accumulation of TMAO. Increased plasma levels of TMAO is implicated in promoting endothelial dysfunction and modulating lipid homeostasis enhanced atherosclerosis.
In a study by Seldin et al. (2016) investigating the impact of TMAO on endothelial functionality, mice fed a diet rich in choline displayed an elevated expression of inflammatory genes. Furthermore, the accumulation of TMAO in the plasma was associated with the movement of activated leukocytes to endothelial cells (Seldin et al., 2016). This is significant because the pathogenesis of atherosclerosis involves a series of transcriptional and functional changes occurring within vascular endothelial cells. In subsequent human study, Al-Obaide et al. (2017) evidenced that an increased abundance of TMA-producing bacteria in the gut microbiota, increased plasma TMAO levels, and increased gut permeability increased the risk of cardiovascular disease due to the presence of chronic inflammation and endothelial dysfunction. These and other alterations were instigated via the buildup of low-density lipoproteins. The mechanisms by which TMAO activates inflammatory pathways leading to endothelial dysfunction and atherosclerotic plaque formation is still under study. However, nuclear factor-κB (NF-κB) is known to be involved in an inflammatory pathway activated in human aortic endothelial and smooth muscle cells following an increased plasma concentration of TMAO (Ma et al., 2017; Seldin et al., 2016). The involvement of the activated NF-κB pro-inflammatory pathway is significant in playing a crucial role in the cellular inflammatory response, a precursor to the development of atherosclerosis.
It has also been found that increased plasma TMAO levels induce the endothelial recruitment of leukocytes (Seldin et al., 2016). The TMAO-induced increase in the adhesion of leukocytes in the vasculature in turn promotes endothelial cell activation, specifically in the context of the pathogenesis of atherosclerosis. Activation of the NF-κB pro-inflammatory pathway is required for TMAO to promote the inflammatory response within endothelial cells. While TMAO significantly increases the adherence of leukocytes to endothelial cells, the addition of an NF-κB inhibitor negates the TMAO-induced increase in adhesion of leukocytes to the endothelium (Seldin et al., 2016).
Furthermore, TMAO has been implicated with contributing to the pathogenesis of atherosclerosis by decreasing the self-repair capabilities of the endothelium (Li et al., 2017). In a study investigating the impact of TMAO treatment on human umbilical vein endothelial cells (HUVECs), TMAO was found to significantly decrease the proliferation and migration of HUVECs (Ma et al., 2017), due to TMAO-induced up-regulated expression of the vascular cell adhesion molecule (VCAM-1). VCAM-1 expression promotes monocyte adhesion in HUVECs (Ma et al., 2017).
The pathogenesis of atherosclerosis involves the accumulation of lipids in the vascular wall, increasing the risk of adverse cardiovascular events such as a myocardial infarction or stroke. Cholesterol, a lipid sterol, is one of the main components of atherosclerotic plaques. The accumulation of cholesterol in the bloodstream is hailed as an important indicator of CVD risk. The mechanisms that contribute to the buildup of cholesterol within arterial walls involve four processes: increased cholesterol influx to the arterial wall, increased cholesterol synthesis, decreased cholesterol efflux from the arterial wall, and/or a decreased bile acid excretion (Koeth et al., 2013). TMAO, and other metabolites of phosphatidylcholine and L-carnitine, have been implicated in the upregulation of some of the mechanisms associated with cholesterol accumulation (Janeiro et al., 2018).
A key indicator of CVD risk is the functioning of intestinal cholesterol transporters, which are responsible for moving cholesterol into or out of the gastrointestinal lumen. In a study investigating the impact of TMAO supplementation on the expression of key cholesterol transporters, it was found that TMAO reduced the expression of both the Niemann-Pick c1Like 1 (Npc1L1) and the Abcg5/8 transporter (Janeiro et al., 2018). Significantly, the Abcg5/8 transporter is responsible for transporting cholesterol out of the lumen (Stender et al., 2014). Npc1L1 is responsible for the influx of cholesterol and is the target of ezetimibe, a potent inhibitor for cholesterol absorption (Jia et al., 2011). Furthermore, TMAO is also responsible for inhibiting reverse cholesterol transport (RCT) (Janeiro et al., 2018; Koeth et al., 2013). Koeth et al. (2013) explored the degree of inhibition induced by TMAO, finding that mice supplemented with TMAO experienced a 35% decrease in RCT activity. In addition to this, TMAO has been associated with increasing macrophage surface expression of scavenger receptor A (SRA) and cluster of differentiation 36 (CD36) and, hence, foam cell formation (Koeth et al., 2013). The additive outcome of these mechanisms explains the observed positive correlation between plasma levels of TMAO and approximate atherosclerotic plaque size (Huc et al., 2018; Wang et al., 2011).
One of the crucial mechanisms by which TMAO increases atherosclerotic risk is via sterol metabolism, specifically that of cholesterol (Koeth et al., 2013). Cholesterol is excreted from the body (via the liver) principally via the activity of bile acids (BAs) (Charach et al., 2012; Lu et al., 2010). Once BAs enter the lumen of the small intestine, they induce the absorption of fats and fat-soluble vitamins (A, D, E, K) (Lu et al., 2010). A relationship has been established between elevated TMAO levels, vitamin D deficiency, and the development of NAFLD associated with cardiovascular disease (Barrea et al., 2019). In laboratory trials, TMAO was shown to decrease the expression of CYP7A1, which is one of the main bile acid synthetic enzymes responsible for catalyzing the rate-limiting step in cholesterol catabolism (Janeiro et al., 2018). Furthermore, TMAO has been known to decrease the expression of Cytochrome P450, an important BA synthetic enzyme responsible for maintaining cholesterol metabolism homeostasis (Koeth et al., 2013; Pikuleva, 2006). Reducing the ability of the liver to convert cholesterol to BAs is associated with hypercholesterolemia and an increased atherosclerotic. As such, it has been suggested that the ability to synthesize and secrete enough BAs may be an adaptive mechanism protective against hypercholesterolemia and atherosclerosis (Charach et al., 1998). Recent studies have found a direct association between BA secretion and cardiovascular risk. In human studies, patients with coronary artery disease are unable to effectively excrete enough BAs to prevent the development of hypercholesterolemia (Charach et al., 1998). Interestingly, it has been found that rodents do not readily develop atherosclerosis because of their ability to excrete large amounts of BAs, thus preventing the harmful accumulation of cholesterol.
The health and composition of bacteria in the GIT microbiota is known to influence other areas of the body, including the immune and cardiovascular systems. The microbiota also plays an integral role in the metabolism of PC/choline and dietary lipids, leading to the accumulation of plasma TMAO (Fig. 2) (Kzhyshkowska et al., 2012; Rajoka et al., 2017; Wang et al., 2011). These findings support the notion of a global impact of dysbiosis on immunological and cardiovascular functionality (Ma and Li, 2018). An established indicator of a dysbiotic gastrointestinal environment that is broadly defined as the failure of the microbial community to satisfy the metabolic and functional needs of the host - is the relative ratio of bacteria from the Firmicute and Bacteroidetes phyla (Thursby and Juge, 2017). One proposed model of preventing the HFD-induced accumulation of TMAO is probiotic treatment. Supplementation with specific probiotic strains of Lactobacillus has been found to mediate some of the negative consequences associated with consuming a high-fat diet such as phenotypic alterations, hindered immune system functioning, and increased blood glucose levels (Fig. 2) (Martinic et al., 2018).
Studies investigating the advantageous effects of Lactobacillus rhamnosus supplementation have shown promise concerning the mediation of some of the negative consequences associated with gut dysbiosis. Specifically, supplementation with Lactobacillus rhamnosus has been shown to reduce visceral adiposity accumulation and diet-induced obesity while improving the integrity of the gastrointestinal microbial lining in animal models (Le Barz et al., 2019). Since the composition of the GIT microbiota has proven to be an important agent in TMA and subsequently TMAO accumulation, supplementation with Lactobacillus rhamnosus may help restore the eubiotic microbiotic environment and mitigate the enhanced production and accumulation of atherosclerotic plaque-promoting factors, in part, by a high-fat diet (Fig. 1 and 2).
While some studies have shown a positive correlation between increased plasma concentrations of TMAO and cardiovascular disease risk, others have evidenced an inverse relationship. In a study exploring the relationship between TMAO levels, gut dysbiosis, and atherosclerosis, Yin et al. (2015) did not find a clear association between increased plasma TMAO levels and the development of atherosclerosis. In fact, it was found that patients who had sustained strokes and transient ischemic attacks had significantly lower concentrations of plasma TMAO levels than asymptomatic control patients. Furthermore, no significant change in plasma TMAO levels or the gut microbiota of patients with asymptomatic atherosclerosis was found. However, patients who had sustained strokes and transient ischemic attacks did exhibit a significant dysbiotic gut microbial environment (Yin et al., 2015).
Factors beyond dietary habits influencing gut dysbiosis have also been proposed. In a study by Shafi et al. (2017) , it was found that, compared to black hemodialysis patients, white patients displayed a nearly two-fold increase in plasma TMAO (Shafi et al., 2017). These findings support the idea that racial variation should be considered in such studies. Furthermore, in a study of inter- and intra-individual differences in TMAO concentration over time, Kuhn et al. (2017) found that TMAO varied more greatly within individuals than between individuals, placing its usefulness as a biomarker in longitudinal studies in question (Velasquez et al., 2016). It has also been proposed that gender could impact dietary preferences and potentially alter plasma TMAO levels. Women were more likely to adhere to the Mediterranean diet (rich with fish, poultry, beans, and eggs) than men in a study by Barrea et al (2019) , with men displaying higher concentrations of plasma TMAO. This is significant, because the Mediterranean diet has been associated with promoting overall cardiovascular health. Despite this, it is important to note that the utility of the Mediterranean diet in reducing cardiovascular risk remains under debate. Indeed, TMAO is abundant in Mediterranean staples such as fish and eggs (Tomova et al., 2019).
Finally, some animal experimental evidence supports the notion that TMAO may provide a benefit. In a hypertensive rat model, a moderate increase in TMAO did not promote negative physiological consequences. In fact, increased TMAO reduced diastolic dysfunction (Huc et al., 2018). Furthermore, it has been proposed that increased plasma TMAO levels may protect against hypertension by mitigating the negative effects of osmotic and hydrostatic stress in at-risk individuals (Ufnal and Nowinski, 2019).
The relationship between diet, gut dysbiosis, TMAO concentration, and cardiovascular disease risk is still being explored. While many studies have proposed a possible relationship between TMAO concentration and cardiovascular disease risk, contradictory evidence is an impetus for further investigation (Fukami et al., 2015; Meyer et al., 2016; Shafi et al., 2017; Yin et al., 2015). To date, no interventional studies have demonstrated a causal relationship between plasma TMAO and cardiovascular risk.
A probiotic is broadly defined as a supplement of beneficial bacteria that naturally occurs in the gut flora. These supplements can aid the recolonization of depleted microbial strains or enhance the functioning of strains that are present in the GIT. Recent studies have explored the possible connection between supplementation with probiotics and cardiovascular health. We propose that supplementation with probiotics may alleviate the deleterious consequences associated with dysbiosis due to a high fat diet, as well as possibly mitigating the adverse effects related to the accumulation of TMAO (Fig. 2). There is continued research interest in the relationship between diet, the microbial composition of the GIT, and the functioning of various organ systems. In this article, we focused on the possible connections between these parameters and the cardiovascular system. There is a strong rationale that the relative composition of the GIT microbiota can be altered by an individual’s diet. Since adverse consequences of extended probiotic use have not yet surfaced, further research could address whether limited or prolonged use of select probiotics or synthetic versions of their metabolites would provide a benefit in metabolic and cardiovascular diseases.
This work was supported by NIH grants, HL74185, HL139047, and AR-71789. The authors would like to thank all members of the laboratory for their continued help and support.
The authors declare that there is no conflict of interest regarding the publication of this article.
