This meta-analysis explores the efficacy and safety of dual antithrombotic treatment (DAT); novel oral anticoagulants (NOAC)-based triple antithrombotic therapy (TAT); vitamin K antagonist (VKA)-based TAT in patients with AF undergoing PCI. Relevant studies listed in English in PubMed, Google Scholar, the Cochrane Library, or ClinicalTrials.gov were identified. We used a random effects model to calculate odds ratios (OR) and 95% confidence intervals (CI). Endpoints included all bleeding events, intracranial hemorrhage, cardiac death, all-cause death, myocardial infarction (MI), stent thrombosis (ST), stroke, and major adverse cardiac events (MACEs). There were 6918 participants in all relevant trials, DAT showed superiority over TAT in reducing the risks of Thrombolysis in Myocardial infarction (TIMI) major bleeding (OR: 1.71, 95% CI: 1.12, 2.62), TIMI major or minor bleeding(OR: 1.75, 95%CI: 1.13, 2.71), International Society on Thrombosis and Hemostasis (ISTH) major bleeding (OR: 1.42, 95% CI: 1.03, 1.96), and Intracranial haemorrhage(OR: 2.44, 95% CI: 1.21, 4.90). In a mutual comparison of three antithrombotic regimens, NOAC-based TAT showed no statistical difference with DAT. VKA-based TAT enlarged the risk of all bleeding events relative to DAT. DAT reduced highly the risk of bleeding events. DAT and VKA-based TAT had similar efficacy outcomes. There was no statistical difference of safety and efficacy between NOAC-based TAT and DAT.
The essence of coronary artery disease (CAD)/acute coronary syndrome (ACS) involves the disequilibrium of oxygen supply and demand, with percutaneous coronary intervention (PCI) being the most effective treatment [1]. Meanwhile, thrombosis is the most important reason for antiplatelet therapy becoming the necessary treatment after PCI [2].
In addition, CAD and AF co-exist in approximately 20-30% of patients. Persistent AF causes the patient's cardiac output to decrease; increases complications such as heart failure, stroke, and thromboembolism; and has a serious impact on the patient's prognosis [3, 4]. Anticoagulant therapy is integral in addressing hemodynamic abnormalities caused by AF [5]. The European Heart Rhythm Association (EHRA) recommends that clinicians apply a single anticoagulant drug plus antiplatelet therapy to treat AF patients with coronary stents [6]. The anticoagulant drugs commonly used in clinical practice include novel oral anticoagulants (NOAC) and vitamin K antagonists (VKA). Nevertheless, it remains a global challenge to successfully combine NOAC or VKA with a P2Y12 inhibitor plus aspirin to balance the risk of bleeding and thrombosis in patients with AF who have undergone PCI [7].
The previous meta-analysis [8, 9, 10] identified that triple antithrombotic therapy (TAT) increases bleeding events relative to dual antithrombotic therapy (DAT), while NOAC decreases the risk of bleeding events as compared with VKA. However, the results of comparisons between DAT and TAT or NOAC and VKA may be more ambiguous than this. Thus, our research aimed further to evaluate the safety and efficacy of TAT vs. DAT. In our study, DAT was defined as NOAC plus a P2Y12 inhibitor and TAT was defined as either NOAC or VKA plus a P2Y12 inhibitor and aspirin. We also conducted a prespecified subgroup analysis to distinguish the safety and efficacy of different types of anticoagulant drug-based TAT vs. DAT directly and compared NOAC-based TAT with VKA-based TAT indirectly.
The methods conducted in this study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for health care interventions [11].
We searched relevant trials included in PubMed, Google Scholar, the Cochrane Library for Clinical Trials, ClinicalTrials.gov, and ClinicalTrialsRegister.eu. We searched for results without limitations on publication time, while the language was limited in English. The keywords deployed in each database included the following: “dual antithrombotic therapy”, “triple antithrombotic therapy”, “novel oral anticoagulants”, “vitamin K antagonists”, “percutaneous coronary intervention”, “atrial fibrillation” and “randomized controlled trial”.
In our study, all of the selected randomized controlled clinical trials adhered to the following criteria: participants were 18 years of age or older, were diagnosed with AF and being treated with antithrombotic therapy, and had been implanted with coronary stents for ACS or CAD, and the interventions in randomized controlled trials were confined to the comparison of DAT and TAT. Studies with different categories of intervention as well as those that were nonrandomized controlled clinical trials, observational trials, or ongoing trials with inadequate data were excluded.
The prespecified efficacy endpoint events included major adverse cardiac events (MACEs), cardiac death, all-cause death, myocardial infarction (MI), definite or probable stent thrombosis (ST), and stroke. The safety endpoint events were intracranial hemorrhage and all bleeding events including major bleeding [according to the Thrombolysis in Myocardial Infarction (TIMI) criteria] [12], major and minor bleeding (according to the TIMI criteria), and International Society of Thrombosis and Hemostasis (ISTH) major bleeding [13].
Two reviewers (S. C. Shen and C. Gong) screened each trial and extracted the useful data independently; any discrepancies that arose were resolved by consensus with a third reviewer (Y. T. Sun). The key characteristics (e.g., year, the composition and duration of TAT and DAT, included criteria, outcomes, NCT number) of each randomized controlled trial included in our study are presented in Table 1. A total of 6918 participants recruited included four randomized controlled trials provided data in this meta-analysis. The mean age of the participants ranged from 69.5 to 70.8 years, 74.4% to 79.6% were male, all the participants underwent PCI and followed up at least 12 months. The baseline characteristics of participants included in our study are displayed in Table 2.
| study | WOEST [20] | PIONEER AF-PCI [21] | ENTRUST-AF PCI [22] | RE-DUAL PCI [23, 24] |
| Year | 2013 | 2016 | 2019 | 2017 |
| DAT | NOAC + P2Y12 inhibitor | NOAC + p2y12 inhibitor | NOAC + P2Y12 inhibitor | NOAC + P2Y12 inhibitor |
| DAT regimen (months) | NA | 12 | 12 | 12 |
| TAT | NOAC+P2Y12 inhibitor + ASA | (NOAC/VKA) + P2Y12 inhibitor + ASA | VKA + P2Y12 inhibitor + ASA | VKA + P2Y12 inhibitor + ASA |
| TAT regimen (months) | NA | 1,6,12 | 12 | 1,3,12 |
| Time to randomization | before or up to 4 h after PCI. | 3 days | 5 days | 5 days |
| Inclusion criteria | indication for OAC treatment; PCI; and age 18-80 years. | ≥ 18 years old; nonvalvular atrial fibrillation; PCI with stent placement were enrolled. | Atrial fibrillation requiring oral anticoagulation, ≥ 18 years old, PCI | ≥ 18 years old; nonvalvular atrial fibrillation; PCI with stent within previous 120 hours. |
| Blinding | Open-label | Open-label | Open-label | Open-label |
| Safety outcomes | TIMI & GUSTO major bleeding at 12months | Composite of TIMI major or minor bleeding or BRMA | ISTH major or CRNM bleeding at 12 months | ISTH major or CRNM bleeding at 12 months |
| Efficacy outcomes | Death, MI, stroke, TLR, and ST | MACE (composite of cardiovascular death, MI, or stroke) | Stroke, SE, MI, stent thrombosis, Composite of Any-cause death, | Composite of TE events (MI, stroke, or SE), death, or unplanned revascularization |
| Follow-up | 12 months | 12 months | 12 months | 14 months |
| NCT | NCT00769938 | NCT01830543 | NCT02866175 | NCT02164864 |
NA: not applicable; DAT: dual antithrombotic therapy; TAT: triple antithrombotic therapy; NOAC: novel oral anticoagulants; VKA: vitamin K antagonists; ASA: aspirin; PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction; GUSTO: Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; MACE: major adverse cardiovascular event; CRNM: clinically relevant non-major bleeding; MI: myocardial infarction; TLR: target lesion revascularization; ST: stent thrombosis; TE, thromboembolic event; SE: systemic embolism.
| Study | WOEST [20] | PIONEER AF PCI [21] | ENTRUST-AF PCI [22] | RE-DUAL PCI [23, 24] |
| participants | 563 | 2124 | 1506 | 2725 |
| Age (years) | mean 69.9 | mean 70.1 | mean 69.5 | mean 70.8 |
| Diabetes | 140 (24.8%) | NA | 517 (34.3%) | 993 (36.4%) |
| Male sex | 448 (79.6%) | 1581 (74.4%) | 1120 (74.4%) | 2070 (76.0%) |
| Previous MI | 196 (34.8%) | NA | 365 (24.2%) | 699 (25.7%) |
| Previous stroke | 99 (17.6%) | NA | 189 (12.5) | 226 (8.3%) |
| Previous PCI | 187 (33.2%) | NA | 394 (26.2%) | 912 (33.5%) |
| Previous CABG | 130 (23.1%) | NA | 95 (6.3%) | 287 (10.5%) |
| Type of stent | ||||
| DES | 364 (64.7%) | 1403/2118 (66.2%) | NA | 2251/2717 (82.8%) |
| BMS | 175 (31.1%) | 675/2118 (31.9%) | NA | 404/2717 (14.9%) |
| DES+BMS | 14 (2.5%) | 40/2118 (1.9%) | NA | 41/2717 (1.5%) |
| Other | 9 (1.6%) | NA | NA | 21/2717 (0.8%) |
| P2Y12 inhibitor | ||||
| Clopidogrel | NA | 2004 (94.4%) | 1391 (92.4%) | 2398 (88.0%) |
| Ticagrelor | NA | 92 (4.3%) | 106 (7.0%) | 327 (12.0%) |
| Prasugrel | NA | 28 (1.3%) | 8 (0.5%) | NA |
| Type of atrial fibrillation | ||||
| Persistent | NA | 441 (20.8%) | 286 (19.0%) | 484 (17.8%) |
| Permanent | NA | 743 (35.0%) | 459 (30.5%) | 888 (32.6%) |
| Paroxysmal | NA | 938 (44.2%) | 760 (50.5%) | 1351 (49.6%) |
| CHA2DS2-VASc score | NA | mean 3.8 | mean 4.0 | mean 3.6 |
| HAS-BLED score | NA | mean 3.0 | mean 4.0 | mean 2.7 |
NA: not applicable; MI: myocardial infarction; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; DES: drug-eluting stent; BMS: ‘kiiibare-metal stent
The risks of bias of included trials were estimated using Cochrane collaboration’s tool, which contains seven items as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias [14].
Data were analyzed according to the intention to treat, and Revman 5.3 (Cochrane, London, UK) and Stata version 12.0 (StataCorp LLC, College Station, TX, USA) were used to conduct statistical analysis. Odds ratios (ORs) were employed to monitor the statistics and quantify the efficacy and safety of different types of antithrombotic therapy. The value of OR of greater than 1 signified that the efficacy or safety was inclined toward DAT rather than TAT, while that of less than 1 indicated the outcome was opposite. Pooled ORs were calculated using a random-effects model with the Mantel-Haenszel method given the existence of heterogeneity.
Separately, 95% confidence intervals (CIs) were used to represent the rationality and credibility of the results in the study we performed. Also, the I2 value was used to represent heterogeneity between each trial. For I2 values of less than 25%, between 25% and 50%, and between 50% and 75%, the heterogeneity was regarded as low, moderate, and high, respectively [15]. A p-value of less than 0.05 was considered to indicate statistical significance.
We used a forest plot to display the effectiveness directly. We conducted Egger’s test using Stata version 12.0 and estimated visually the inverted symmetric funnel plot by way of Revman version 5.3 to assess the bias. Prespecified subgroup analyses, which included NOAC-based TAT and VKA-based TAT, assessed the efficacy and safety of comparing DAT and TAT of the two different combinations.
By searching MeSH terms on the database, 16,010 citations were identified that met the search criteria. Then, 9,624 records were left after excluding duplicate citations. After excluding other trials that met the exclusion criteria, the full texts of 12 potentially eligible trials were scrutinized. Eventually, four trials were included in this study. The process of screening study is displayed in Fig. 1.
 Figure 1.
Figure 1.Flow diagram. From Moher D, Liberati A, Tetzlaff J, Altman DG, Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med 6 (7): e1000097. doi: 10.1371/journal.pmed.1000097.
We evaluated the quality of these four studies that met the included criteria using the Cochrane risk-of-bias tool. The risk-of-bias graph is shown in Fig. 2A. Fig. 2B indicates there was no publication bias in the funnel plots. Fig. 2C reveals that Egger’s test presented p > 0.05, respectively.
 Figure 2.
Figure 2.A: risk of bias graph; B: funnel plot of all the outcome; C: Egger’s test plot.
3.2.1 Safety outcome (Fig. 3)
A total of 6918 participants were found among all trials that reported data of bleeding events. DAT was superior to TAT in reducing the risks of TIMI major bleeding (OR: 1.71, 95% CI: 1.12-2.62; p < 0.01; I2 = 27%) (Fig. 3A), TIMI major or minor bleeding (OR: 1.75, 95% CI: 1.13-2.71; p = 0.01; I2 = 81%) (Fig. 3B), ISTH major bleeding (OR: 1.42, 95% CI: 1.03-1.96; p = 0.03; I2 = 53%) (Fig. 3C), and intracranial hemorrhage (OR: 2.44, 95% CI: 1.21-4.90; p = 0.51; I2 = 0%) (Fig. 3D).
 Figure 3.
Figure 3.A: Forest plot of TIMI major bleeding; B: forest plot of TIMI major or minor bleeding; C: forest plot of ISTH major bleeding; D: forest plot of intracranial hemorrhage; TAT: triple antithrombotic therapy; DAT: dual antithrombotic therapy.
3.2.2 Efficacy outcome (Fig. 4)
Regarding the efficacy outcome, both TAT and DAT showed no statistical significance in addressing MACEs (OR: 1.04, 95% CI: 0.81-1.32; p = 0.78; I2 = 45%) (Fig. 4A), cardiovascular death (OR: 0.96, 95% CI: 0.70-1.31; p = 0.80; I2 = 0%) (Fig. 4B), all-cause death (OR: 1.11, 95% CI: 0.67-1.83; p = 0.68; I2 = 64%) (Fig. 4C), myocardial infarction (OR: 0.86, 95% CI: 0.65-1.13; p = 0.28; I2 = 0%) (Fig. 4D), stent thrombosis (OR: 0.92, 95% CI: 0.55-1.53; p = 0.73; I2 = 3%) (Fig. 4E), and stroke (OR: 1.12, 95% CI: 0.73-1.70; p = 0.61; I2 = 0%) (Fig. 4F).
 Figure 4.
Figure 4.A: Forest plot of MACE; B: forest plot of cardiovascular death; C: forest plot of all-cause death; D: forest plot of myocardial infarction; E: forest plot of stent thrombosis; F: forest plot of stroke; MACE: major adverse cardiovascular event; TAT: triple antithrombotic therapy; DAT: dual antithrombotic therapy.
3.2.3 Subgroup analysis based on different types of TAT and patient health (Fig. 5)
Supplementary Material Fig. 5 shows that, relative to DAT, NOAC-based TAT did not achieve any statistical significance in decreasing the risks of adverse safety or efficacy outcomes. Similarly, VKA-based TAT showed no superiority in reducing the risks of TIMI major bleeding or all efficacy outcomes. However, as compared with DAT, VKA-based TAT increased the risks of TIMI major bleeding (OR: 1.93, 95% CI: 1.28-2.90; p = 0.002; I2 = 5%) (Fig. 5A), TIMI major or minor bleeding (OR: 1.61, 95% CI: 1.04-2.50; p = 0.03; I2 = 75%) (Fig. 5B), ISTH major bleeding (OR: 1.54, 95% CI: 1.09-2.17; p = 0.01; I2 = 56%) Fig. 5C), and intracranial hemorrhage (OR: 3.17, 95% CI: 1.46-6.91; p = 0.004; I2 = 0%) (Fig. 5D).
 Figure 5.
Figure 5.A: Subgroup forest plot of TIMI major bleeding; B: subgroup forest plot of TIMI major or minor bleeding; C: subgroup forest plot of ISTH major bleeding; D: subgroup forest plot of intracranial hemorrhage; TAT: triple antithrombotic therapy; DAT: dual antithrombotic therapy.
3.2.3 Sensitivity analyses (Fig. 6)
Given the existence of high heterogeneity, one-study-omitted sensitivity analysis was performed to explore the leading cause. Fig. 6 shows individual data did not play an important role in influencing our results; in other words, the results of this study were stable and robust.
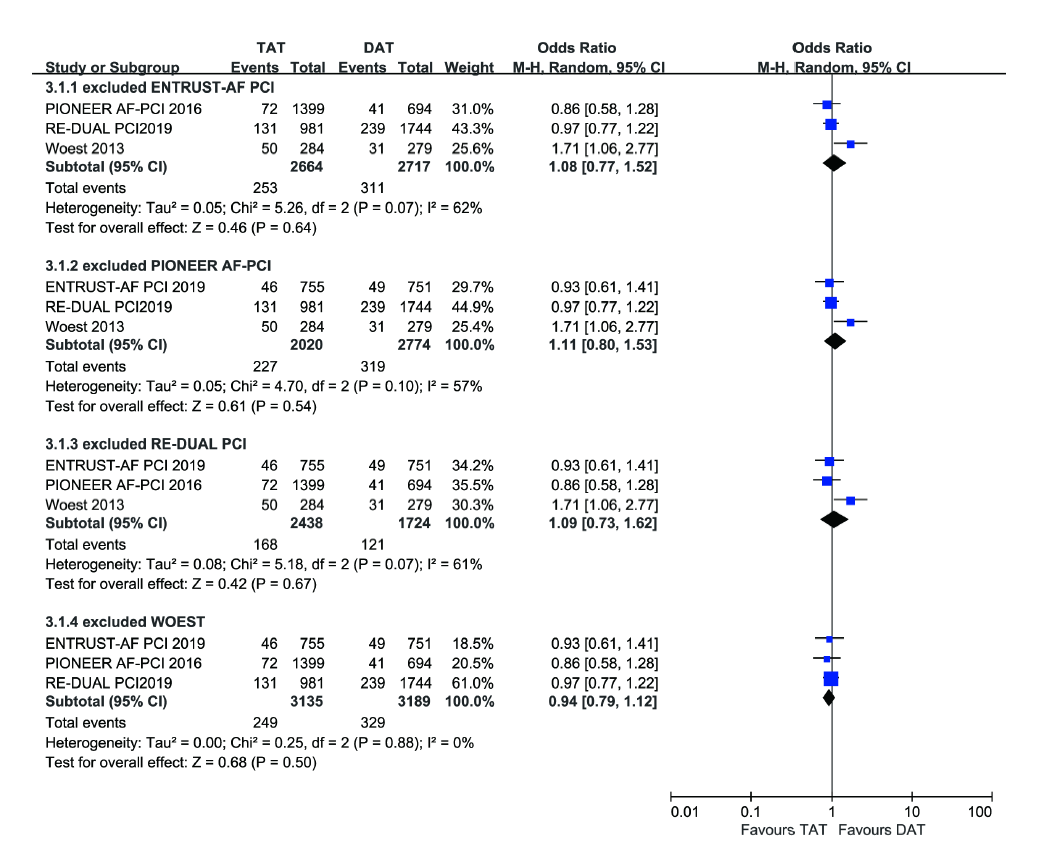 Figure 6.
Figure 6.Sensitivity analysis of a forest plot of major adverse cardiovascular event.
AF patients need to be treated with antiplatelet therapy and anticoagulant therapy simultaneously after coronary artery stent implantation, which can increase the incidence rate of bleeding. Patients are vulnerable to ischemic stroke when taking antiplatelet drugs without anticoagulant medication, while stopping using antiplatelet drugs increases the risk of MACEs like ST and MI. Thus, we performed this study, including all available randomized controlled trials that met the inclusion criteria, to elucidate which was the best composition of antithrombotic therapy.
Our research confirmed that it was necessary to apply antithrombotic therapy in patients diagnosed with AF undergoing PCI with coronary stents for ACS or CAD. On the one hand, In terms of reducing the risk of cardiac death, MI, ST, and other efficacy endpoints, there was no statistical difference between DAT consisting of NOAC plus a P2Y12 inhibitor and TAT. On the other hand, as compared with TAT, which prompted a high incidence rate of bleeding events, DAT reduced the risk of TIMI major bleeding by 59% and that of ISTH major bleeding by 39%. This finding was similar to conclusion in previous meta-analysis [8, 9, 10].
Agasthi et al. [8] conducted a meta-analysis to explore the safety and efficacy of NOAC and VKA, but the randomized controlled trials included in their study were designed to compare NOAC plus a P2Y12 inhibitor and VKA plus a P2Y12 inhibitor and aspirin; that omitting the effect of aspirin made the study conclusions less clinically relevant. Potpara, et al. [9] performed a meta-analysis to assess the different effects when applying antithrombotic therapy consisting of aspirin or not in patients with AF and coronary stents. VKA and NOAC constituted the different regimens of DAT and TAT. However, given multiple confounding factors, the conclusions of comparisons between DAT and TAT may be regarded as hasty and undemanding. Golwala’s study [10] included four randomized controlled clinical trials aiming to elucidate the safety and efficacy of DAT and TAT, one of which was ISAR TRIPLE [16]. Patients who received concomitant aspirin and NOAC in ISAR TRIPLE were randomly divided to take either six-week clopidogrel therapy or six-month clopidogrel therapy. However, whether the treatment of the six weeks of TAT would affect the occurrence of endpoint events during subsequent DAT was unknown; therefore, the conclusion of this meta-analysis should be considered cautiously. Lopes, et al. [17] made a network meta-analysis by collecting data of five RCT, even the use of a network meta-analysis allows for simultaneous comparisons and evidence-based grading to draw overall conclusions, the differences in methodology design and data of results among every trial makes the credibility of the conclusion greatly reduced.
Nevertheless, contrary to the previous meta-analysis, the composition of DAT in the four trials included in our study was limited to NOAC plus a P2Y12 inhibitor, and the compositions of TAT were NOAC plus a P2Y12 inhibitor and aspirin or VKA plus a P2Y12 inhibitor and aspirin, respectively. And the AUGUSTUS trial, did not distinguish NOAC-based from VKA-based DAT, was excluded. Further, we not only conducted a comparison between DAT and TAT but also completed a predefined subgroup analysis to compare the safety and efficacy between different types of TAT and DAT and displayed the different effects of two types of TAT indirectly. This was one of the aspects that distinguished our study from previous meta-analyses.
According to the subgroup analysis, it was apparent that, regardless of whether aspirin was added or not, NOAC-based TAT had the same effects as DAT. However, VKA-based TAT showed obvious different effects relative to DAT, even though the risk of efficacy outcome was similar between VKA-based TAT and DAT, VKA-based TAT roughly increased the risks of TIMI major bleeding, TIMI major or minor bleeding, ISTH major bleeding, and intracranial hemorrhage when compared with DAT alone. The effects were similar to those in the blanket analysis of DAT and TAT. The results also revealed that VKA caused higher risk of bleeding events compared to NOAC under the same antiplatelet drug compatibility.
The 2018 European Society of Cardiology/European Association for Cardiothoracic Surgery statement recommended patients with a high risk of bleeding should take an anticoagulant drug plus P2Y12 to replace TAT [18]. Meanwhile, the 2019 American College of Cardiology /American Heart Association/Heart Rhythm Society AF management guide suggested the rationality of applying dual therapy (clopidogrel + rivaroxaban 15 mg once daily or dabigatran 150 mg twice daily) after PCI in patients with AF at increased risk of stroke to reduce the risk of bleeding [19]. These recommendations are consistent with our results; even given the existence of high heterogeneity among bleeding events, one-study-omitted sensitivity analysis suggested that the results of our study were consistent and robust.
This study had some limitations other than the general limitations of meta-analysis. First, the sample size was small even though it was larger than that of any meta-analysis conducted previously. Also, the ability to perform subgroup analyses was limited by the lack of individual patient data. Further, the duration of antithrombotic therapy and follow-up time differed among the studies, so we need more randomized controlled trials of high quality to improve and perfect the comparison between different types of TAT and DAT.
Our results have significant clinical implications. First, our findings confirmed that DAT was superior over TAT for use in AF patients undergoing PCI due to both showing similar efficacy outcomes but the latter having a high risk of bleeding events. Second, our study found that TAT has a high risk of bleeding events only when the composition of TAT includes VKA, not NOAC. In other words, NOAC-based antithrombotic therapy is more preferable over VKA-based antithrombotic therapy in clinical practice. Further studies are needed to explore the vintage NOAC-based antithrombotic regimen composition of NOAC (e.g., apixaban, rivaroxaban, edoxaban) plus a P2Y12 inhibitor (e.g., ticagrelor, clopidogrel, prasugrel), and elucidate the optimal duration of antithrombotic therapy to achieve a balance between bleeding and ischemia after PCI, maximize effectiveness, and minimize the risks of bleeding and ischemia.
In conclusion, our findings support that VKA-based TAT increases the risk of bleeding events relative to DAT, but with a similar efficacy outcome. The comparison about safety and efficacy of DAT and NOAC-based TAT was no statistical difference.
This work was supported by Youth Elites Support Plan in universities of Anhui Province (Grant No. gxyq2019013), Excellent Young Talents Fund Program of Higher Education Institutions of Anhui Province (Grant No. gxgwfx2019010). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. Thanks to all the peer reviewers and editors for their opinions and suggestions.
The authors declare no conflict of interest.
PCI: percutaneous coronary intervention; AF: atrial fibrillation; DAT: dual antithrombotic therapy; NOAC: novel oral anticoagulants; TAT: triple antithrombotic therapy; VKA: Vitamin K antagonist; ACS: Acute coronary syndrome; CAD: Coronary artery disease; MI: myocardial infarction; MACE: Major adverse cardiovascular events; ST: Definite or probable stent thrombosis; TIMI: Thrombolysis in Myocardial Infarction; ISTH: International Society of Thrombosis and Hemostasis; PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses; ESC: European Society of Cardiology; EACTS: European Association for Cardio-Thoracic Surgery.
