- Academic Editor
Objective: Crohn’s disease (CD) is an immune-mediated inflammatory bowel disease (IBD), which comprises an idiopathic aberrant systemic and local inflammatory response. This response is a result of unknown interactions between the luminal content and the intestinal wall. This article is a review of the current state of knowledge providing information to help obstetricians to manage patients with CD, and to understand the particularities of these patients, with emphasis during pregnancy and postpartum, including recommendation for the birthing methods. It is important to explain the usefulness of the pursue of treatment during pregnancy, taking into consideration the drugs allowed during pregnancy, and addressing the challenges that CD may pose in addition to the physiological adaptations of pregnancy. Mechanism: As both an obstetrician and a gastroenterologist, this topic can be approach from two distinct perspectives. Firstly, how CD influences fertility and pregnancy, and secondly, an exploration on how hormonal changes and immune system tolerance during pregnancy probably influences CD. Findings in Brief: Data shows that pregnancy outcomes are influenced by the clinical course of CD at the time of conception. Latent disease prior to conception is associated with uneventful pregnancies and favorable neonatal outcomes, comparable to general population. Conversely, an active disease during pregnancy and ileal localization can be associated with prematurity, stillbirth, and small-for-gestational age (SGA) infants. A high risk of preeclampsia was reported in pregnancy with severe CD and oral or systemic corticosteroids administration. Optimal management approach involves a multidisciplinary team consisting of an obstetrician, gastroenterologist, and surgeon. Thiopurines and biologic agents are considered safe during pregnancy and breastfeeding. In infants with CD, alteration in the composition of the maternal microbiome may contribute to the systemic inflammation and to influence the transmission of an altered microbiota to the infants. This suggests that modulating the early microbiome can be an effective strategy to reduce cases of CD. Conclusions: Healthcare practitioners and patients must be aware that CD patients can have a successful pregnancy and a healthy infant. A multidisciplinary team can provide supportive care and help address significant information to adapt the treatment plan, and to monitor pregnancy.
Crohn’s disease (CD) is an immune-mediated inflammatory bowel disease (IBD) characterized by an idiopathic aberrant systemic and local inflammatory response, due to and including insufficiently understood interactions between the luminal content and the intestinal wall. CD is a chronic, acquired immune digestive disease, with the onset generally between 15 and 35 years old, coinciding with the reproductive period [1], being thus understood all the concerns regarding the unwanted effects of pregnancy on the underlying disease, but also of CD on the fetus. This condition is characterized by periods of flare/inflammatory activity and quiescent periods.
CD can affect any segment of the gastrointestinal tract, predominantly the
terminal ileum and adjacent colon, characterized by a segmental, asymmetric
distribution of immune determined granulomatous inflammation [2]. Common clinical
manifestation includes abdominal pain and tenderness, fatigue, fever, weight
loss, malnutrition, chronic diarrhea, as well as perineal and perianal lesions
such as hemorrhoids, skin tags, fissures, abscesses, ulcers, fistulae,
lymphoedema, strictures. Extradigestive symptoms are represented by anemia,
specific granulomatous skin lesions, and spondyloarthritis [3, 4, 5]. It is
characterized by periods of remission alternating with relapses, with surgical
intervention sometimes necessary [6]. A population-based review has demonstrated
that, for a majority of patients, CD is a progressive and destructive disease,
particularly when located in the ileum and ileocolonic regions. Risk factors for
progression include onset at age
In the active phase of CD, pregnancy poses a higher risk of miscarriage, preterm delivery, poor maternal weight gain and small-for-gestational age (SGA) babies, risk of need for Cesarean section (CS) [8, 9, 10, 11, 12, 13]. A high risk of preeclampsia has been reported in pregnancies with severe CD and the administration of oral or systemic corticosteroids administration [14]. In the past, due to the necessity for control of CD activity and mitigating pregnancy complications, concerns about the risk of surgery during pregnancy and to the medications prescribed for IBD, it was believed that pregnancy was contraindicated for women suffering from CD [15].
The study on pregnant women with uncontrolled CD is very limited, given such cases are not very common [16]. Moreover, small series focusing on pregnancy with ileostomy have reported uneventful pregnancies, with very rare stoma complications, including bleeding, retraction, stenosis, laceration, prolapse, hernia, or intestinal obstructions [17].
This article is a review on the current knowledge about women with CD who wish to conceive or who are already pregnant. It is also important to mention that many of the studies discussed in the literature were not exclusively focused on CD, but on IBD in general. Medical management, including all the therapies for CD during pregnancy and breastfeeding, will not be discussed here because these aspects are well covered by recent guidelines [18, 19, 20].
These multifactorial pathologies arise from the interplay between an altered response of the immune system and an abnormal intestinal microflora, culminating in chronic inflammation of the digestive tract. Medical experts are still trying to discover the exact etiopathogenesis of CD, but so far this has not been successful. However, it was found that genetic susceptibility plays an extremely important role, and up to 200 genetic loci associated with an increased risk of developing these pathologies have been identified [21, 22]. Studying these loci highlighted the role of innate immunity and bacterial implication in IBD etiology through numerous genes that are involved in innate immune cell function, adaptive immunity, or epithelial barrier function [22].
From a structural point of view, the contact area is represented by the
following components: bacteria, mucous layer, antimicrobial peptides, and barrier
cells. Inflammatory pathologies involve alterations in the protective mucus layer
[23]. Through these openings that form in the mucous layer, bacteria can more
readily reach the epithelial layer, resulting in inflammatory responses that lead
to the release of cytokines, such as interferon-
In pregnancy, there are immune shifts to facilitate alloimmune fetal tolerance, the maternal immune response changes from the inflammatory Th1 cytokine pattern to the Th2 pattern. The maintenance of pregnancy and feto-maternal tolerance is associated with Th2 and Th17/Th2 cells, and CD4+CD25+Foxp3+ Treg cells. Th1-type and Th17-type cytokines that promote fetal semi-allograft rejection may compromise pregnancy, whereas Th2-type cytokines may improve pregnancy outcomes. Treg cells play an essential role in the induction of paternal antigen tolerance early in pregnancy, by suppressing immune responses caused by paternal and fetal antigen recognition. Late in pregnancy, the occurrence of preeclampsia may be explained by the disturbance of paternal antigen-specific tolerance, marked by a decrease in clonal populations of decidual effector Treg cells [27]. The immunology of pregnancy and feto-maternal immune tolerance is related to CD4+ T cell cytokines [28, 29]. T cell responses in immune and autoimmune disease are influenced by the state of pregnancy.
During pregnancy, in the first-trimester, there is a marked and rapid increase
in the levels of estrogen and progesterone. The maximum levels of estrogens are
reached in the third-trimester to ensure the necessary support for the
development of the placenta and the vascularization of the uterus, facilitating
normal fetal development. A study shows that low levels of 17
Given the immune mechanisms described earlier, Th1- and Th17-type autoimmune disorders during pregnancy are associated with a lower risk of progression and a reduced rate of exacerbation when there is a rise in Th2-type cytokines in pregnancy (e.g., CD, RA, Graves disease, and multiple sclerosis). Conversely, Th2-type autoimmune disorders could worsen in pregnancy with an increase in Th2-type cytokines (e.g., psoriasis, lupus nephritis, systemic lupus erythematosus (SLE)). Furthermore, in the postpartum period, inflammatory and autoimmune disorders may worsen due to the loss of the immunosuppressive state of pregnancy and the decrease in estrogen levels. This decrease can lead to a reduction in Th2-type cytokines along with the decrease of Th1 and Th17-type.
Autoimmune diseases have been associated with a high frequency of flares and exacerbation of autoimmune disease symptomatology due to the changes in immune hormones modulated by the chronic disease, which modifies Th1/Th2 immunity [37]. In IBD populational studies, menstrual period is associated with dysmenorrhea, especially one year before the diagnosis of CD [38, 39].
The reason for sexual dysfunction among CD patients is considered to be multifactorial, including psychological factors related to CD manifestations, such as an altered perception of body image, followed by disease-specific factors, surgical interventions, osteotomy, medical therapies, pelvic floor disorders and hypogonadism, and social and cultural traits of personality [40]. Due to these reasons along with the higher incidence of depressive symptoms in these patients, a significantly lower rate of sexual contact is reported among them compared to a control group [41]. A study shows that in general this is not a matter of structural impotence, reinforced by an objectified defect, but rather a greater difficulty in achieving orgasm, deep and increased dyspareunia [42]. Considering this significant impact on sexual life, not only will the quality of life be affected, but also the conception rate. The causes for increased sexual dysfunction should be discussed together with the patient, and efforts should be made to find a solution through treatment, surgery, and psychotherapy.
Women with CD, especially those who underwent surgical treatment, have shown lower fertility rates compared to the control group, potentially due to inflammation and scarring of the fallopian tubes [10, 43, 44]. In cases of women with CD in remission, who have never undergone surgical treatment, fertility rates are age-dependent and almost equal the general population. Optimizing disease management may help conception [43]. However, there are a multitude of variables that can affect fertility, which include the status of the disease, prior surgical interventions, and the medical treatment approached. In terms of the type of intervention, there are no studies that demonstrate a better fertility rate for the laparoscopic approach than the classic one, except in the case of ileal pouch-anal anastomosis [45, 46, 47].
The studies about assisted reproduction techniques (ART) in CD show a similar success rate of in vitro fertilization (IVF) procedures among patients with CD and those without this pathology [48, 49]. IBD patients over 30, who have undergone surgical interventions and have been unsuccessful in conceiving for 6 months, should be recommended a fertility evaluation and consider ART [50, 51, 52]. It is important to note that there is a study that shows that medications for inflammatory digestive pathologies do not impact the effectiveness of ART or egg freezing, nor do the hormones used for increasing the success of ART do not lead to a destabilization of the underlying disease [19].
Nonetheless, a period of remission of 3 to 6 months until conception is recommended to reduce the risk of relapse or flare-up during pregnancy and the postpartum period [53]. For this purpose, oral contraceptives, hormonal or non-hormonal intrauterine devices, or contraceptive implants can be recommended. It should also be considered that oral contraceptives are not effective in the case of active small bowel inflammation or extensive small bowel resection [54]. Caution must be taken in patients with personal or family history of deep venous thrombosis or pulmonary embolism.
Table 1 (Ref. [1, 19]) summarize the main idea about fertility and contraception in women with CD.
| Fertility | Active disease decrease fertility. |
| Decreased fertility in patients with ileal pouch anal anastomosis. | |
| ART are recommended if, after 6 months of unprotected sexual contact, the couple does not get pregnant. | |
| Contraception | Long-acting, non-estrogen-containing contraception in patients with active disease due to the risk of venous thromboembolism [19]. |
| Absorption of oral contraceptives is almost normal in patients with mild CD or short ileal resections. | |
| In cases of extensive small bowel resection or active disease, non-oral contraceptives are recommended [1]. |
CD, Crohn’s disease; ART, assisted reproduction techniques.
The greatest priority during pregnancy is to control the underlying disease as best as possible, so that no symptoms or relapse appear. This is generally dictated by the status of the disease at the time of conception. A disease in remission at that time not only facilitates conception but also helps in maintaining control during all trimesters of pregnancy, especially in cases of perineal pain or discharge. Table 2 (Ref. [18, 19, 55]) present the main recommendations about pregnancy planning and treatment during pregnancy, including education and nutritional status in case of CD during pregnancy. The follow-up of pregnancy in cases of CD in remission is identical as in high-risk pregnancies, with gestational weight and blood pressure careful monitoring.
| Screening before conceiving | Papanicolaou smear. |
| Vaccination with all necessary non-live vaccines prior to pregnancy: diphtheria, tetanus, pertussis (dTpa), and influenza. | |
| Live vaccination is contraindicated if the patient is receiving immunosuppressive treatment. | |
| Alcohol, smoking, and illicit drugs cessation. | |
| Education | Heredity of IBD. |
| Mode of delivery. | |
| Safety of ART when needed. | |
| Adequate gestational weight gain. | |
| Disease flare and advice when is important to make an appointment to the doctor. | |
| Safety of breastfeeding. | |
| Disease management | Three months of corticosteroid-free remission. |
| Confirm remission based on clinically, biochemical, intestinal ultrasonography, magnetic resonance imaging, and radiological assessment. | |
| Recognition of ileostomy complications if present. | |
| Medication during pregnancy | Low risk medication: |
| Mesalazine/sulphasalazine (but these are not currently recommended for CD management, only in ulcerative colitis) | |
| Corticosteroids (only if needed for a flare, as remission free corticosteroid should be achieved at least 3 months before conception) | |
| Azathioprine (may be stopped only if prescribed in association with infliximab and already in long term remission, together with assessing infliximab through levels) | |
| Anti-TNF- | |
| Limited data about medication: | |
| Vedolizumab, ustekinumab (should be probably continued, as antiTNF- | |
| Ciclosporin (only used for induction of remission, in active flare, not suited for conception time) | |
| Contraindicated: | |
| Metotrexate | |
| Tofacitinib | |
| Thalidomide | |
| Filgotinib | |
| Ozanimond | |
| Nutritional status | 2–5 mg daily dose of folate for cases with fiber-restricted diet, extensive ileal disease or those taking sulfasalazine for three months prior to conception, or in the first 12 weeks of pregnancy. |
| Normal range for iron, vitamin B12, and folate before conception. |
CD, Crohn’s disease; IBD, inflammatory bowel disease; TNF-
The pregnancy follow-up in case of CD in remission is identical as in high-risk pregnancies, with careful monitoring of gestational weight and blood pressure. Clinical examination of the perineum and checking for clinical symptoms may help confirm or exclude CD relapse. Fig. 1 shows two perianal fistulas diagnosed at 30 weeks of pregnancy in a patient with CD.
 Fig. 1.
Fig. 1.Perianal fistula in a primipara primigravida with CD at 30 weeks of gestation after surgical treatment: Fistulectomy associated with slow total sphincterotomy, using loose setons (Silvestri). CD, Crohn’s disease.
More than half of CD patients with perianal lesions have multiple lesions [56]. Fig. 2 shows typical lesions at colonoscopy in a patient with CD.
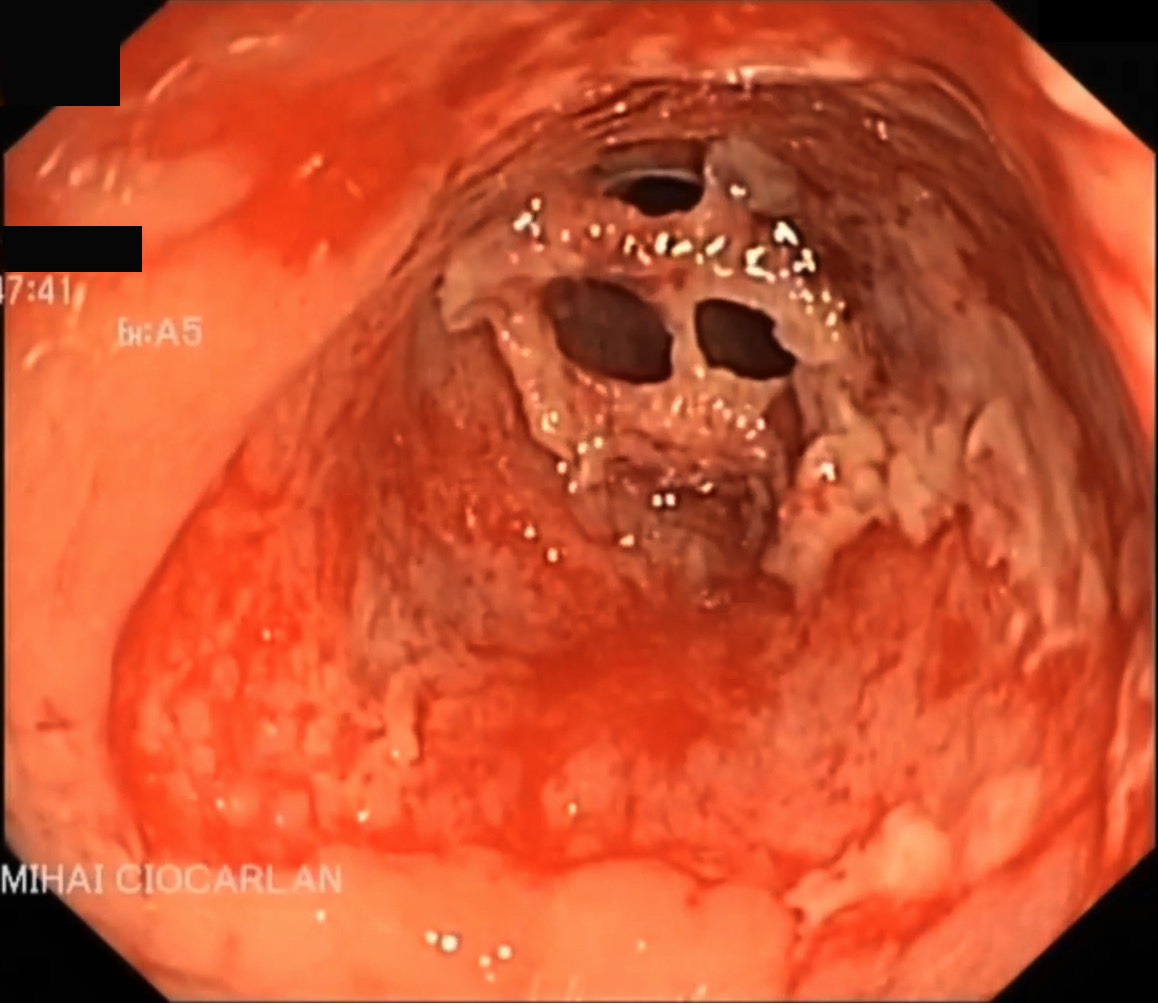 Fig. 2.
Fig. 2.Colonoscopy aspect of CD: a narrow colonic stricture with ulcerated mucosa. CD, Crohn’s disease.
Complete blood count for hemoglobin level, C-reactive protein (CRP) and fecal calprotectin (FC) for disease activity, B12 level, liver enzymes, and albumin are performed in every trimester of pregnancy. A careful evaluation of fetal growth is required during every visit because patients suffering from CD are prone to malnutrition, which can be difficult to correct during the early stages of pregnancy when placental development takes place rapidly, and in the last trimester when the nutrient requirement of the fetus increases. Serum albumin levels are an important marker of nutritional status and of CD activity [57, 58, 59, 60], but these levels may decrease during pregnancy due to physiological hemodilution [61]. Additionally, the guidelines recommend that women with CD be closely investigated regarding vitamin D level, folic acid level, micronutrient status, micronutrient deficiencies, and ferritin levels. Specific guidelines about clinical nutrition counselling and recommendations in CD have been updated [62]. A study conducted by the Epidemiology Committee (EpiCom) of the European Crohn’s and Colitis Organization (ECCO) demonstrated that there is no difference in the evolution of the disease in pregnant versus non-pregnant women with CD [53]. There are studies that reinforce this idea, demonstrating that patients with active disease at the time of conception are more likely to have an active disease during pregnancy, than those who conceive when in remission [63, 64, 65]. It was also demonstrated that pregnancies that occur during the period of active disease are more subject to obstetric complications, such as preterm birth, SGA infants, and spontaneous abortion. For this reason, the ECCO guidelines recommend achieving remission before getting pregnant [19, 66].
Additionally, recommendations for the 9 months of pregnancy are provided, from which the women with IBD must not deviate for a favorable evolution of the pregnancy. In their study, Oron et al. [67] concluded that patients with IBD who gained less than 12 kg during pregnancy frequently had complications, such as premature birth, SGA infants, and the need for neonatal intensive care. A study from 2017 also showed that women with CD had inadequate gestational weight gain (34.3%) more often than pregnant women without IBD (19.4%) [68].
Furthermore, meta-analysis has demonstrated an increased risk of gestational diabetes in the case of CD-associated pregnancies [69]. The theory that this occurs more often due to corticosteroid treatment has been proposed, but this connection is still questionable. Many authors recommend for early screening of gestational diabetes in patients undergoing corticotherapy treated during pregnancy [70]. There is no clear consensus regarding the association between steroids exposure and gestational diabetes [69, 70]. Patients with active IBD are more prone to severe forms of preeclampsia [63], as well as venous thromboembolism complication than in the general population. This is more elevated, especially antepartum and in the 12-week postpartum period, compared with women without IBD [14]. As such, there are several recommendations for thromboprophylaxis in CD in pregnancy. The British Royal College of Gynecologists and Obstetricians recommend venous thromboembolism prophylaxis for pregnant women with IBD who had one or more episodes of active disease during the third-trimester of pregnancy [71]. The Canadian Association of Gastroenterology recommends that women with IBD who have undergone a CS, at least be given anticoagulants during pregnancy and, if there is a history of venous thromboembolism, up to 6 weeks after birth [72].
Complex and multiple perianal fistulas in pregnancy can be extremely debilitating for women, requiring medical and surgical treatment. Perianal fistulas may be treated in order to avoid perianal abscess and premature delivery. During pregnancy, medical interventions such as antibiotherapy are employed, while in postpartum period, immunomodulators and biologic treatment may be used.
Joint management with specialist surgeon is required if perianal disease becomes active in pregnancy. In case of perianal fistulas, the methods of surgical treatment are as follows: fistulotomy with partial sphincterotomy, use of fibrin adhesives, anal fistula plug, endorectal advancement flap, and intersphincteric fistula tract ligation procedure. All these procedures have very low efficiency in patients with CD. If the patient has active proctitis and pelvic abscess, it is recommended to only drain the abscesses and place the free seton, given the potential risks of iatrogenic injuries and delayed wound healing [73].
The use of setons keeps the fistulas open, allowing fluid to escape and prevent abscess formation. The setons delay the healing and closure of the fistula. Its use during treatment with infliximab can prevent an abscess from forming. Currently, uncut setons are used because the ‘cutting’ setons, which, when sequentially tightened at regular intervals, caused damage to the sphincter and anal incontinence [74].
The most common perineal manifestations are perineal fistulae and abscesses, as well as anal fistulas of rectovaginal type [75, 76, 77, 78, 79]. A higher incidence of CS was founded in women with CD when compared to women without CD, but for obstetrical factors [80]. Recent data and current guidelines suggest that CS is recommended for patients with active perineal disease or rectovaginal fistulae [81, 82]. While there is no CS recommendation for patients with CD without perineal disease and without relapse, obstetrical factors must be considered [18, 19, 20, 83]. Episiotomy should be avoided if possible [84]. Cheng et al. [84] have reported on a case-control series involving 11 patients with vaginal delivery compared with 50 patients CS, showing no differences for perianal disease; the rate of perianal flare was found to be similar in pregnant and non-pregnant CD control cases. Approximately two-thirds of CS delivery cases had a symptomatic perianal flare within 2 years after delivery, compared to 45% for vaginal delivery [84]. It should also be considered that for these women, the choice regarding the type of birth has implications both for the current pregnancy and for subsequent ones. It is also important to mention that none of the birth methods are ideal and free of possible complications. In these cases, vaginal birth can be associated with perineal trauma, sphincter injury, or perineal fistulae [85], while cesarean birth should be avoided as much as possible due to the risk of adhesion formation in patients who often need subsequent surgical treatment for the underlying pathology. In approximately 50% of cases with CD after several years of evolution, the perineal region is also involved, prompting questions regarding the indicated mode of birth.
The risk of 3rd and 4th degree ruptures is not higher in patients with IBD after vaginal birth [86].
It has been recently hypothesized that the gut microbiota is a key factor in the pathogenesis of CD [87]. Newborns whose mothers have CD develop this pathology more often than the rest of the population, suggesting that the microbiota also plays an important role in this pathology. A pregnant woman with CD has an altered microbiota, transmitting this altered microbiota to the newborn, which will be the ideal environment for the baby to develop the same pathology. Besides this direct transmission, there are also indirect factors, such as maternal diet, feeding behavior, or antibiotic exposure that increase the risk of developing IBD [88, 89, 90, 91, 92]. Also, due to the mother’s pathology, girls are more often born by CS and are exclusively breastfed less often than the general population, and these two factors contribute to the development of an altered microbiota [93, 94]. A study from 2020 showed that babies born from mothers with IBD have a higher abundance of proinflammatory bacteria like Proteobacteria and a lower level than the control group of Bifidobacteria, which are generally part of the microbiome of the eutrophic newborn [95]. There are also studies that suggest a female predominance for familial CD and IBD, a female sex-specific epigenetic inheritance pattern and higher transmission rate from mothers with CD compared to fathers [96, 97].
Considering all of the above, it would appear that in the future, modulating the early microbiome could be an effective strategy to reduce cases of CD. Breastfeeding is crucial and should be encouraged.
It is extremely important that the baby is closely monitored even after birth,
through a follow-up with clinical, biochemical, and psychological assessment.
Within the first 12 months postpartum, 30% of patients have a flare, most
frequently for those who reduced IBD therapy during or immediately following
pregnancy, or for those with active disease in the third-trimester [98, 99].
During breastfeeding, all IBD medications taken during pregnancy should be
continued. The levels of these medications in breastmilk are very low, so they
are safe during lactation, with negligible effect on the infant [18]. Live
vaccines (Bacille Calmette-Guérin (BCG), rotavirus, measles, mumps, and
rubella) are postponed for infants
Healthcare practitioners and patients must be aware that CD patients can have a successful pregnancy and a healthy infant. During pre-conception, prenatal, and postpartum periods, a multidisciplinary team can provide supportive care and help address significant information to adapt the treatment plan, and balance benefits to outweigh risks in preventing flares. Biologic therapies are demonstrated to be safe during pregnancy, labor, and breastfeeding and it must be continued throughout. Vaginal birth and breastfeeding must be encouraged, since CS is recommended to patients with active perineal disease. Attention must be paid to live vaccination after biologic therapies in the third-trimester of pregnancy, live vaccines are contraindicated in infants at least 6 months postpartum due to the risk of placental transfer. Lastly, live vaccination is contraindicated in mothers receiving immunosuppressive treatment.
AAS and BMD designed the research study. AAS, BMD, AMM and MC performed the research. AAS, BMD, AMM and MC analyzed the data. BMD and AAS wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Written informed consent has been obtained from the patients from Figs. 1,2 to publish these pictures.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
