- Academic Editor
Background: Both objective and subjective transvaginal sonography (TVS)
methods are used to assess the degree of myometrial invasion (MI). Subjective TVS
assessment of MI (
Gynecological examination is usually completed by transvaginal sonography (TVS), which is usually the first imaging method [1].
When TVS is performed by an experienced examiner, it is not always necessary to perform additional investigations to assess the local extent of the disease when it comes to endometrial cancer, such as magnetic resonance imaging (MRI) and computed tomography (CT) [1, 2].
On TVS, myometrial invasion (MI) appears as iso-hyperechoic tissue relative to the surrounding myometrium. Although MI is often well-diagnosed, it can sometimes only be suspected based on the irregular appearance of the endomyometrial zone [1, 3].
Both objective and subjective TVS methods are used to assess the degree of MI [1]. Objective evaluation is done using the Gordon and Karlsson ratio or method [2, 3, 4, 5].
Subjective TVS assessment of MI (
Alcázar et al. [4] investigated that the sensitivity and specificity of TVS in detecting deep MI in endometrial cancer (EC) is 82% and 81%.
Currently, TVS represents one of the best possible inexpensive recording techniques. In addition to wide availability and cost-effectiveness, further advantages are that with this technique the examination can be performed quickly, reliably, safely and without additional stress for the patient. The reliability of the method depends on the quality of the ultrasound device, as well as the experience of the doctor performing the examination. The presence of adenomyosis, leiomyoma or endometrial polyps can be an aggravating circumstance in the assessment of MI [5, 6].
The sensitivity, specificity, positive and negative predictive value and diagnostic accuracy achieved in the study by Miklos et al. [6] further justify the integration of TVS into the standard preoperative protocol for EC patients. The rationale is that TVS provides information on the basis of which there is a possibility of making the correct decision about what type of surgical treatment should be performed, thus increasing the survival rates of patients and the overall therapeutic benefit [5, 6].
The aim of this study is to examine the ultrasound characteristics of endometrial cancer in two groups of patients; with myometrial invasion less and greater than 50%.
The prospective study was conducted in the Clinic for Gynecology and Obstetrics, University Clinical Center Tuzla in the period from 2019 to 2021. The research included 60 female patients hospitalized in the Clinic for Gynecology and Obstetrics for a planned surgical procedure, due to pathohistologically (PHD) proven endometrial cancer.
The gold standard for assessing the degree of myometrial invasion in endometrial carcinoma was the PHD finding of the surgical material.
The following parameters were monitored and analyzed:
-EC dimensions in three planes on TVS;
-volume of EC on TVS;
-distance of EC from serosa measured by TVS;
-degree of MI by subjective assessment method (
The inclusion criteria are patients with pathohistologically proven endometrial cancer.
The exclusion criteria for patients with PHD proven EC are those subjects with a history of another malignant disease, who were previously operated for EC, treated with chemotherapy or radiotherapy for EC or another malignant disease, who underwent preoperative CT and PHD proven EC incidentally after hysterectomy.
Patients were divided into two groups, after surgery and PHD assessment of MI degree, into those with less and more than 50% MI.
The prospective study was conducted with the approval of the Ethics Committee of the University Clinical Center Tuzla, and each patient signed an informed consent for inclusion in biomedical research before being included in this study.
All TVS examinations were performed by one examiner (AC). TVS examinations were performed using a standardized protocol containing all investigated sonographic parameters defined before the beginning of the study. All TVS examinations were performed with a 5 MHz vaginal probe on a GE Voluson E8 ultrasound machine (GE HealthCare Technologies, Heller International Building, Chicago, IL, USA) the day before surgery [7].
The entire uterus was examined in the sagittal plane from one lateral to the contralateral edge, as well as in the horizontal plane from the cervix to the fundus. EC was evaluated in 2D gray scale and described using International Endometrial Tumor Analysis (IETA) terminology [7, 8].
TVS variables evaluated during the two-dimensional examination include data on: size of the uterus and EC in three planes, volume of the EC, distance of the EC from the anterior, posterior wall, fundus and cervix of the uterus, the smallest distance of the EC from the serosa or minimal tumor-free margin, corresponding to normal myometrial width, degree of MI, presence, location and number of fibroids [5, 7].
EC was measured with three orthogonal dimensions. Two dimensions of EC in the sagittal plane are anteroposterior (AP) (tumor thickness) and craniocaudal (tumor length), the third dimension is laterolateral (tumor width) measured in the transverse plane [7, 8].
The minimal tumor-free margin was measured in any plane in which the distance
from the tumor to the serosa appeared to be the smallest. EC volume was
calculated from the three dimensions of the tumor, according to the formula for
the ellipsoid (V = 4/3
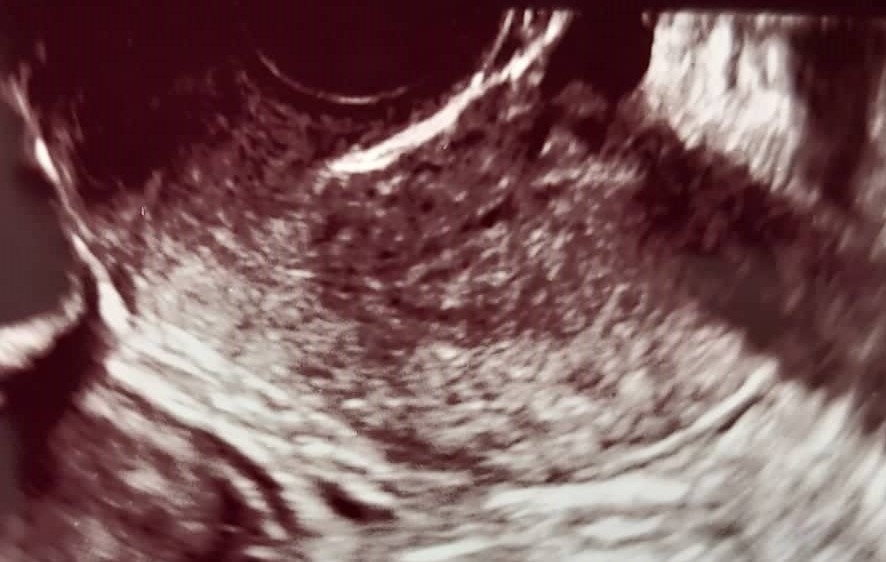 Fig. 1.
Fig. 1.Assessment of myometrial invasion in patient with subjective method of transvaginal ultrasonography (TVUS). Stage IA endometrial cancer in a 65-year-old postmenopausal woman, correctly diagnosed by TVUS.
 Fig. 2.
Fig. 2.Assessment of myometrial invasion in patient with subjective method of transvaginal ultrasonography (TVUS). Stage IB endometrial cancer in a 62-year-old postmenopausal woman, correctly diagnosed by TVUS.
The selected cut off limit of the degree of MI (50%) follows the latest International Federation of Gynecology and Obstetrics (FIGO) classification from 2009.
Statistical processing was done in the SPSS 24.0 software package (IBM SPSS, Chicago, IL, USA). Basic tests of descriptive statistics were performed, with the display of measures of central tendency and dispersion.
Each variable was tested for belonging to a normal distribution using the
Kolmogorov-Smirnov test and a histogram display. Quantitative variables were
compared by t-test with correction for unequal variances where they were
normally distributed. Where they did not, the Mann-Whitney test was used. All
statistical tests were performed with a statistical probability level of 95%
(p
The basic demographic and clinical characteristics of study participants are shown in Table 1.
| Characteristics | Value (%) | |
| Age at menopause (years) | 50.1 (40‒61) * | |
| Premenopausal | 8 (13.3) * | |
| Perimenopausal | 7 (11.7) | |
| Postmenopausal | 45 (75) | |
| Abnormal uterine bleeding as first sign | 58 (96.7) | |
| Myometrial invasion | ||
| Superficial ( |
34 (56.7) | |
| Deep ( |
26 (43.3) | |
*Data are given as median (5th percentile; 95th percentile) for continuous variables; n (%) for categorical variables.
The average degrees of MI in EC for the gold standard - pathohistological method, are shown in Table 2.
| Method | PHD |
PHD |
Total % | p (student t test) |
| PHD %, SD ( |
22 (0.144) | 69 (0.144) | 42.71 (27.47) | 0.00001 |
| Rank | 0–45 | 51–98 | 0–98 |
PHD, pathohistologically; SD, standard deviation.
The three EC dimensions obtained by TVS (expressed in centimeters) are
statistically significantly higher in patients with MI
| Dimensions (cm) | PHD |
PHD |
Total | p (student t test) |
| Anteroposterior, SD ( |
1.57 (0.71) | 2.86 (1.06) | 2.13 (1.08) | 0.00001 |
| Rank | 0.51–3.12 | 0.7–5.93 | 0.51–5.93 | |
| Craniocaudal, SD ( |
2.97 (0.91) | 3.64 (1.03) | 3.26 (1.08) | 0.0049 |
| Rank | 1.34–4.59 | 1.96–6.33 | 1.34–6.33 | |
| Laterolateral, SD ( |
2.26 (0.86) | 2.73 (1.02) | 2.47 (0.95) | 0.0029 |
| Rank | 1–4.45 | 0.89–4.34 | 0.89–4.45 |
TVS, transvaginal sonography.
The frequency of AP diameter of EC, measured by TVS, of less and greater than 2
centimeters in the two examined groups is shown in Table 4. The frequency of AP
diameter of EC greater than 2 cm was statistically significantly higher in the
group of subjects with MI
| Anteroposterior diameter | PHD |
PHD |
Total % | p (Z test) |
| 26 (76.4) | 5 (19.2) | 31 (51.6) | 0.00001 | |
| 8 (23.5) | 21 (80.7) | 29 (48.3) | 0.00001 | |
| Total N (%) | 34 (100) | 26 (100) | 60 (100) | - |
The volume of EC on TVS expressed in milliliters in the two examined groups is
shown in Table 5. The volume of EC in the group of patients with MI
| Volume | PHD |
PHD |
Total | p |
| Volume (mL), SD ( |
6.10 (5.46) | 16.58 (12.72) | 10.6 (10.61) | 0.00014 |
| Rank | 0.81–21.09 | 1.02–54.24 | 0.81–54.24 |
The distance of the EC from the serosa—the minimum tumor-free zone expressed in centimeters, measured by TVS, is shown in Table 6. The difference in the average distance of the EC from the serosa is 1.1 cm between the two examined groups and is statistically significant (Mann Whitney; Z = 2.05; p = 0.0394) (Table 6).
| Distance from serosa | PHD |
PHD |
Total | p |
| Distance (cm), SD ( |
1.15 (0.56) | 1.04 (1.29) | 1.10 (0.94) | 0.0394 |
| Rank | 0.31–2.6 | 0.1–7.0 | 0.1–7.0 |
The concordance of the diagnosis of MI over 50% by means of TVS method was then evaluated against the gold standard - the finding obtained by PHD diagnostics.
When it came to the subjective TVS method, a tabular representation of the
concordance of the findings is given in Table 7. The kappa concordance
coefficient for the subjective TVS method versus the PHD finding was
statistically significant (p
| Subjective method TVS |
PHD |
PHD |
Total | p (Z test) |
| N (%) | N (%) | N (%) | ||
| Yes | 2 (5.9) | 20 (76.9) | 22 (36.7) | 0.00001 |
| No | 32 (94.1) | 6 (23.1) | 38 (63.3) | 0.00001 |
| Total N (%) | 34 (100) | 26 (100) | 60 (100) | - |
Accuracy = 0.87 (95% CI: 0.74–0.92);
Sensitivity = 0.77 (95% CI: 0.63–0.83); Specificity = 0.94 (95% CI:
0.83–0.99);
PPV = 0.91 (95% CI: 0.74–0.98); NPV = 0.84 (95% CI: 0.75–0.89);
+LR = 13.08 (95% CI: 3.74–74.46); –LR = 0.25 (95% CI: 0.17–0.45);
+LR, positive likelihood ratio; –LR, negative likelihood ratio; 95% CI, 95% confidence interval; PPV, positive predictive value; NPV, negative
predictive value.
Our prospective cohort study, in which we analyzed the ultrasound
characteristics of endometrial cancer and the degree of myometrial invasion,
showed that there was no statistically significant difference in the assessment
of the degree of MI between the subjective TVS method and histopathology as gold
standard. Analysis of the diagnostic accuracy showed that the subjective TVS
method compared to the gold standard in the diagnosis of MI
Ultrasound parameters; three dimensions of EC obtained by TVS, AP diameter of EC
greater than 2 cm as well as volume of EC are statistically significantly higher
in subjects with MI
The average degree of MI measured by the PHD method in our study was 42.71%, the difference between the two groups was significant. There are studies with higher and lower average degrees of MI. The average rate of MI in our study is similar to the study by Prueksaritanond et al. [9], 48.4%. In the study by Oge et al. [10], the average degree of MI was 70%, higher than in our study, given that it was only about patients with stage IB EC, while in the study by Kim et al. [11], the average degree of MI was 18.4%, lower than in our study considering the larger number of patients with IA stage EC.
In our study, according to the gold standard, PHD diagnosis, the superficial
degree of MI (
Tumor size is the strongest single TVS predictor of high-risk EC. The three
dimensions of EC obtained by TVS (expressed in centimeters) are statistically
significantly higher in subjects with MI
There is a significant difference in prognosis and treatment between patients
with EC smaller than 2 cm or larger than 2 cm [23]. The diagnostic performance of
AP tumor diameter
In the study by Epstein et al. [8], EC volume was shown in different
histological grades G1 (5.1 mL), G2 (7.7 mL) and G3 (14.9 mL). The most
significant TVS difference between high-grade and low-grade EC is the size of the
tumor (diameter and volume), color score, shape of blood vessels, and damage to
the endomyometrial zone [8]. In our study, the average EC volume for the entire
sample was 10.6 mL. In the MI
The volume of EC successfully reflects the stage of the disease, the prognosis and the type of surgery required. The percentage of patients with high-risk pathohistological findings (grade 3 and non-endometrioid EC type), deep MI, lymph node invasion, and extrauterine spread of the disease increases with increasing EC volume [23]. The volume of the EC is important for making a decision on the type of surgery required. Preoperatively measured EC volume by MRI can provide useful information about EC stage, lymph node invasion, prognosis, and required surgical treatment. However, the volume of EC is not superior than assessment of the degree of MI in predicting the presence of metastases in the lymph nodes [23].
The distance of EC from the serosa measured by TVS is a promising diagnostic and prognostic marker. Liro et al. [26] compared the usefulness of the distance of EC from the serosa and the degree of MI in assessing the locoregional extension of the disease and lymph node invasion. They came to the conclusion that both methods, alone or in combination, are a significant aid in obtaining preoperative information about the status of lymph nodes. They believe that the use of the distance of EC from the serosa is easier compared to measuring the degree of MI as a marker of EC invasion, given that both methods have similar predictive value in the assessment of invasion and lymph node status. The cut-off value of the distance from the serosa is 5.2 mm in the prediction of lymph node metastases [26].
The distance of the EC from the serosa—the minimal tumor-free zone measured by
TVS, is greater in the group of subjects with MI
The kappa concordance coefficient for the subjective method of TVS versus PHD
findings was significant, and was kappa = 0.72, significantly higher than in the
study by Eriksson et al. [13], in which it was 0.52 and 0.48, while in
the study by Savelli et al. [27] it was 0.67. The subjective method of
TVS against the gold standard in the diagnosis of MI
For the subjective TVS method, the reviewed studies reported sensitivity from 56% to 85%, specificity from 69% to 91.3%, PPV from 61% to 79%, NPV from 79% to 92% and overall accuracy from 75.7% to 84% [4, 5, 13, 14, 15, 16, 21, 24, 27, 28, 29].
In a large prospective study, the performance of subjective and objective TVS methods in the detection of deep MI in grade 1 and 2 EC was compared. A subjective prediction model, which included the histological grade of EC and a subjective assessment of the degree of MI, and an objective prediction model, which included the histological grade and an objective measurement of the distance of the EC from the serosa, were also used. In the three analyzed studies, they came to the conclusion that subjective TVS assessment is superior to ultrasound measurements, objective TVS methods in assessing the degree of MI [5, 14, 24].
Based on our experience during the conducted study, we can list several
differences between endometrial hyperplasia, endometrial polyps and endometrial
cancer which may be of practical importance. Endometrial hyperplasia gives an
ultrasound image of inhomogeneous, hyperechoic and thickened endometrium with
small cysts. In the case of an endometrial polyp, there is a clearly limited
hyperechoic formation inside the uterine cavity, usually with a scant amount of
fluid in the uterine cavity. Ultrasound characteristics of endometrial cancer are
thickened endometrium,
Our study has limitations, primarily a small sample of study participants and a short period of sample collection. The advantage of the study refers to the prospective, and to the fact that one person performed ultrasound examinations and analyzed all study participants.
The three dimensions, as well as the volume of endometrial cancer, obtained by
TVS, are significantly higher in subjects with
The subjective method of TVS has a good diagnostic accuracy in the diagnosis of
myometrial invasion
All data points generated or analyzed during this study are included in this article and there are no further underlying data necessary to reproduce the results.
AC designed the research study. AC performed the research. DH and ZH provided help and advice on the experiments and analyzed the data. All authors contributed to editorial changes in the manuscript. All authors have read and approved the final manuscript.
All subjects gave informed consent to be included before participating in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the University Clinical Center Tuzla (approval number No 02-09/2-2/20).
Thanks to all the peer reviewers for their opinions and suggestions which improved the quality of our manuscript.
This research received no external funding.
The authors declare no conflict of interest. Dubravko Habek is serving as one of the Editorial Board members/Guest editors of this journal. We declare that Dubravko Habek had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Michael H. Dahan.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
