1 Department of R&D Center, The Korea Institute for Public Sperm Bank, 48242 Busan, Republic of Korea
2 Department of Technical Research, Genoheal Co., Ltd., 06120 Seoul, Republic of Korea
3 Department of Korean Medical Science, School of Korean Medicine and Korean Medicine Research Center for Healthy Aging, Pusan National University, 50612 Yangsan, Republic of Korea
4 Department of Obstetrics and Gynecology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, 51353 Changwon, Republic of Korea
†These authors contributed equally.
§These authors contributed equally.
Abstract
Background: Metabolic bone disease, associated with estrogen
deficiency, is common condition in postmenopausal women. Paeonia
lactiflora Pall. (PL) and Astragalus membranaceus (AM) have been known
to have estrogenic activity and to improve postmenopausal osteoporosis symptoms
when used as a mixture with other herbs. However, there have been no comparative
studies on the effects of PL and AM on the bone metabolic profile. Thus, the
present study aimed to investigate the effects of the single extracts of PL and
AM on bone and metabolic profile, and further to compare the effects of the two
herbs. Method: A total of 70 mice were randomly divided into seven
groups (n = 10): six groups were bilaterally ovariectomized (OVX) and one group
served as a sham-operated control (Sham). Two OVX groups received PL at 23.5
(OVX-PL-L) and 47 (OVX-PL-H) mg/kg bw/day. Another two OVX groups received AM at
38.5 (OVX-AM-L) and 77 (OVX-AM-H) mg/kg bw/day. The remaining two groups served
as positive and negative controls and received estradiol valerate (OVX-E
Keywords
- menopause
- symptoms
- Paeonia lactiflora Pall. (PL)
- Astragalus membranaceus (AM)
Menopause refers to the cessation of menstruation and the decrease in estrogen production due to a decline in ovarian function in older women. This is a natural transition in life that is experienced by all women. Menopause is accompanied by a variety of symptoms that occur due to estrogen deficiency, including hot flushes, anxiety, difficult in sleeping, and vaginal dryness. The prevalence of menopause is on the rise due to the rapid increase in the life expectancy of women. According to some reports, the number of postmenopausal women worldwide is estimated to increase to 1.2 billion by 2030, and the proportion of postmenopausal women in the total female population is expected to increase to 59.8% by 2060 [1, 2]. As a result, various healthcare systems are focusing on improving the quality of life (QoL) of postmenopausal women by addressing the physiological changes associated with obesity and lowered bone mineral density (BMD) [3].
Estrogen has many biological functions, and estrogen deficiency is known to have
adverse effects on several physiological functions. Estrogen deficiency results
in an increase in visceral fat, and imbalance in lipid metabolism, and rapid bone
loss [4]. Hormone replacement therapy (HRT) has been widely used as an the
effective treatment of menopausal-related symptoms in postmenopausal women [5].
However, the long-term use of HRT is contraindicated in women with breast cancer,
coronary heart disease, and stroke [4, 5, 6, 7]. Phytoestrogens are estrogen-like
compounds derived from plants. Their structure and/or functions are similar to
mammalian estradiol (17-
Paeonia lactiflora Pall. (PL) and Astragalus membranaceus (AM) are representative oriental herbs, which have been traditionally used in the treatment of female reproductive disorders including menstrual pain, infertility, and dysmenorrhea [9, 10]. The root of PL, also referred to as Bai Shao in Chinese, is one of the most frequently prescribed herbs for the management of menopausal hot flushes and has shown estrogen-like activity in in vitro studies in Michigan Cancer Foundation-7 (MCF-7) cells [10, 11]. In addition, PL is a component of the TiaoGeng decoction (TG-decoction), which is a Chinese herb mixture that has been used in the treatment of menopausal symptoms with significant benefits in China for more than 30 years [12]. Paeoniflorin, extracted from Paeonia lactiflora Pall., has been reported to affect bone remodeling by improving bone formation, thereby reducing osteoporosis in ovariectomized mice [13]. AM is known for its estrogenic activity and has been reported to improve postmenopausal osteoporosis symptoms when used as a mixture with Rubus coreanus Miquel (R. coreanus Miq) in ovariectomized (OVX) mice [14]. In a clinical trial in postmenopausal women, the ingestion of a mixture of AM and R. coreanus Miq significantly improved menopausal symptoms [15]. In addition, AM has been widely used in mitigating menopausal symptoms as a component of DangguiBuxue Tang (DBT), a traditional Chinese herbal, decoction also containing Angelicae Sinensis Radix [16].
Most in vitro and in vivo studies have used a mixture of extracts rather than a single extract based on the assumption that mixed extracts will have synergistic estrogen-like effects in treating menopausal symptoms compared to single extracts [10, 11, 12, 14, 15, 16]. However, there are no comparative studies on the ameliorative effects of single extracts of PL and AM on menopause-associated estrogen decline, obesity, and bone metabolism. Therefore, in this study, we first investigated the estrogen-like activity of single extracts of PL and AM on estrogen receptor (ER)-positive MCF-7 human breast cancer cells. Subsequently, a comparative study of the effects of PL and AM single extracts was performed in improving the lipid profile, body weight loss, and bone metabolic profile in OVX mice.
This was a controlled experiment study using OVX mice.
The PL and AM extracts were provided by Daehan Cell Pharm INC. (http://www.dhcp.kr). The PL and AM roots were purchased from Omni herb Co. Ltd. (Daegu, Korea). The plants were cultivated in field in the Gyeongsangbuk-do Province, Korea. The plants were identified by two botanical experts. The PL and AM roots were washed, dried naturally, and pulverized. Subsequently, the ethanol extracts of PL and AM were prepared using eight times the quantity of 30% (v/v) ethanol at 90 °C for 6 hours, and then subsequently vacuum dried at 60 °C and 50° Brix. The extracts were concentrated and dried under reduced pressure at 65 °C to obtain the PL extract (yield: 17.9%) and AM extract (yield: 38.5%). These steps were performed personnel from Daehan Bio Pharm Co., Ltd.
Estrogen receptor (ER)-positive MCF-7 human breast cancer cells were purchased fromthe American Type Culture Collection (ATCC, Manassas, VA, USA) on September 8, 2021. We have contracted a Material Transfer Agreement (MTA) with the ATCC. We also obtained a certificate from Mycoplasma Testing Services (Hoechst DNA stain method, Agar culture method, and PCR-based assay) indicating the absence of mycoplasma contamination. No cell line infections were observed within 3 to 5 weeks of purchase. The MCF-7 cell lines purchased from ATCC were tested for short tandem repeat (STR) profiles and validated for human cross-species determination. The MCF-7 cell line is a human cell line, and as a result of analyzing the alleles present in each STR locus within the cell line, the specification and the STR result of the purchased MCF-7 cell line were identical.
This cell line was maintained and cultured in Dulbecco’s modified eagle medium
(DMEM; WelGENE, Daegu, Korea) supplemented with 10% heat-inactivated fetal
bovine serum (FBS; WelGENE, Daegu, Korea), and 1% penicillin-streptomycin (P/S;
Gibco, Brooklyn, NY, USA) at 37 °C in a humidified atmosphere with 5%
CO
To investigate the downstream signaling mechanisms of the estrogenic activity of
PL and AM, the protein activation of extracellular signal-regulated kinase (ERK)
and protein kinase B (Akt) was examined in the MCF-7 cell by western blot. The
MCF-7 cells were cultured on 100 mm dishes until 70% confluency was reached.
Then, the cells were replaced with serum and phenol red-free medium, and treated
with PL or AM for 24 hours. Protein was extracted from the cultured cells using
the CETi lysis buffer (#TLP-121CETi, TransLab, Daejeon, Korea) with a phosphatase inhibitor
cocktail (#539131, Merck Millipore, MA, USA) according to the manufacturer’s
protocol. The protein content of the cell lysate was determined with a
Bicinchoninic acid (BCA) Protein Assay Kit (Thermo Fisher Scientific Inc.,
Waltham, MA, USA). Cell lysates were separated via 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a
polyvinylidene difluoride (PVDF) membrane (Millipore, Burlington, MA, USA). The membranes were blocked
with a PhosphoBLOCK® solution (TransLab) for one hour at room
temperature and washed using 1
Inbred C57BL/6 mice aged 8weeks were used in all the animal experiments. The
mice were purchased from Koatech Inc. (Pyeongtaek, Korea) and bred under 12-hour
light/dark cycle with free access to water and food in a specific pathogen-free
(SPF) animal facility at a temperature of 21
A total of 70 mice were randomly divided into seven groups (n = 10). Six groups
were performed bilaterally OVX using the dorsal approach under an intraperitoneal
administration of Zoletil® (0.5 mg/kg; Virbac Laboratories,
Carroscedex, France). Briefly, after the ovarian fat pad was lifted through the
dorsal incision, the first ovary was visualized, and the ovary was exteriorized
before distal uterine ligation and carefully removed. Then, the uterus was
returned to the abdomen. The second ovary was removed in the same manner. One
group served as a sham-operated control (Sham) and underwent the same procedure
as the OVX mice but without resection of the ovaries. After two-week recovery
period, two OVX groups received PL at two different doses (23.5 and 47 mg/kg bw/day,
OVX-PL-L and OVX-PL-H, respectively). Another two OVX groups received
AM at two different doses (38.5 and 77 mg/kg bw/day, OVX-AM-L and
OVX-AM-H, respectively). The remaining two groups served as positive and negative
controls and received estradiol valerate (OVX-E
Individual body weights were recorded on the first and last days of the
experiment. Body weights were collected prior to the withdrawal of food for
overnight fasting. Mice were sacrificed by CO
The serum concentrations of total cholesterol (TC), triglycerides
(TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein
cholesterol (LDL-C) were measured using an auto microplate chemiluminescence
analyzer (Hitachi Chemical Diagnostics Systems Co., Ltd. Tokyo, Japan).The serum
concentrations of osteocalcin and 17-
The left femur from the mice of each group was fixed in 4% paraformaldehyde (PFA) for 24 hours, washed with phosphate-buffered saline (PBS) and then scanned using micro-computed tomography (CT) (Suantum FX, PerkinElmer, Hopkinton, MA, USA) with a source voltage of 90 kV and a source current 180 µA. For the 3-dimensional histomorphometric analysis of the trabecular and cortical bone, cross-sectional images of the distal femur were used. The region of interest (ROI) of the distal femur was 5% of the femoral length starting from 0.05 mm below the growth plate and was used to determine the trabecular and cortical bone mineral density (BMD), bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp).
Results were presented as mean
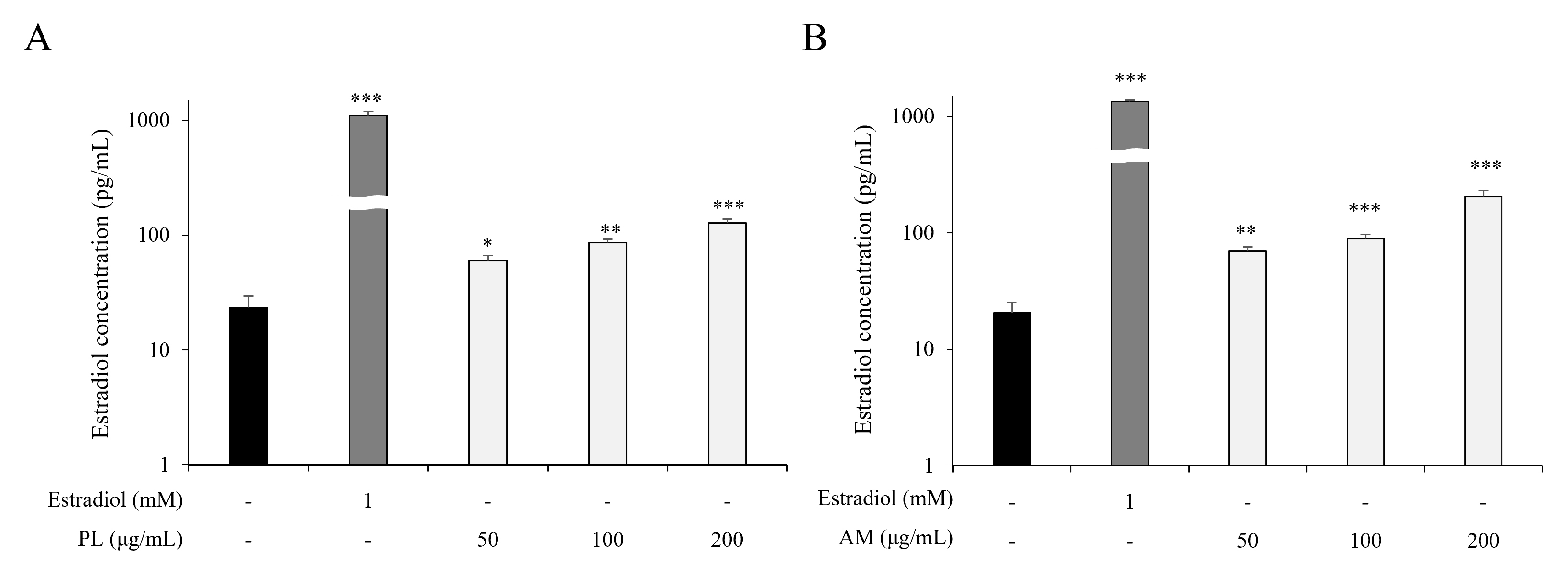
To evaluate the estrogen-like activity of the single extracts of PL and AM, the estrogen-producing ability of PL and AM was measured in the MCF-7 cells. PL and AM increased estrogen production at all concentrations compared with the control group (Fig. 1A,B). This result implied that both PL and AM single extracts increase estrogen production in MCF-7 cells.
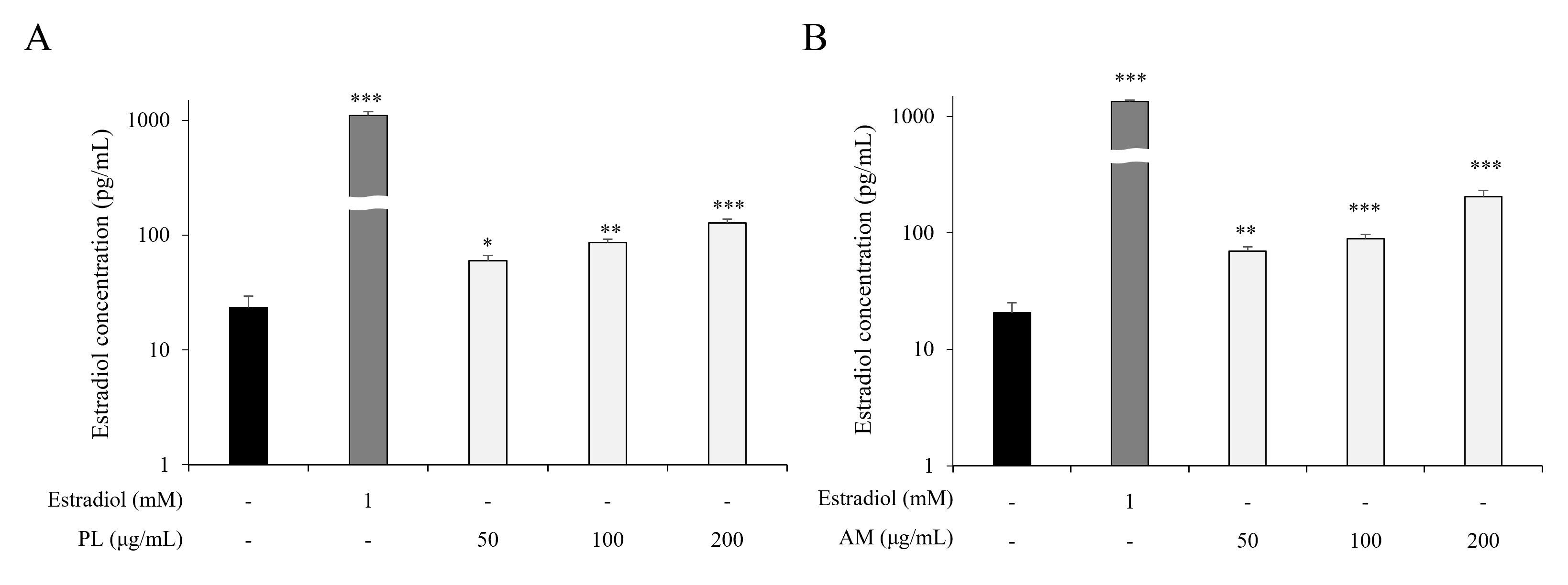 Fig. 1.
Fig. 1.Effects of PL and AM on estrogenic activity. MCF-7 cells were
treated with different concentrations of PL (A) and AM (B) for 24 hours,
respectively. The E
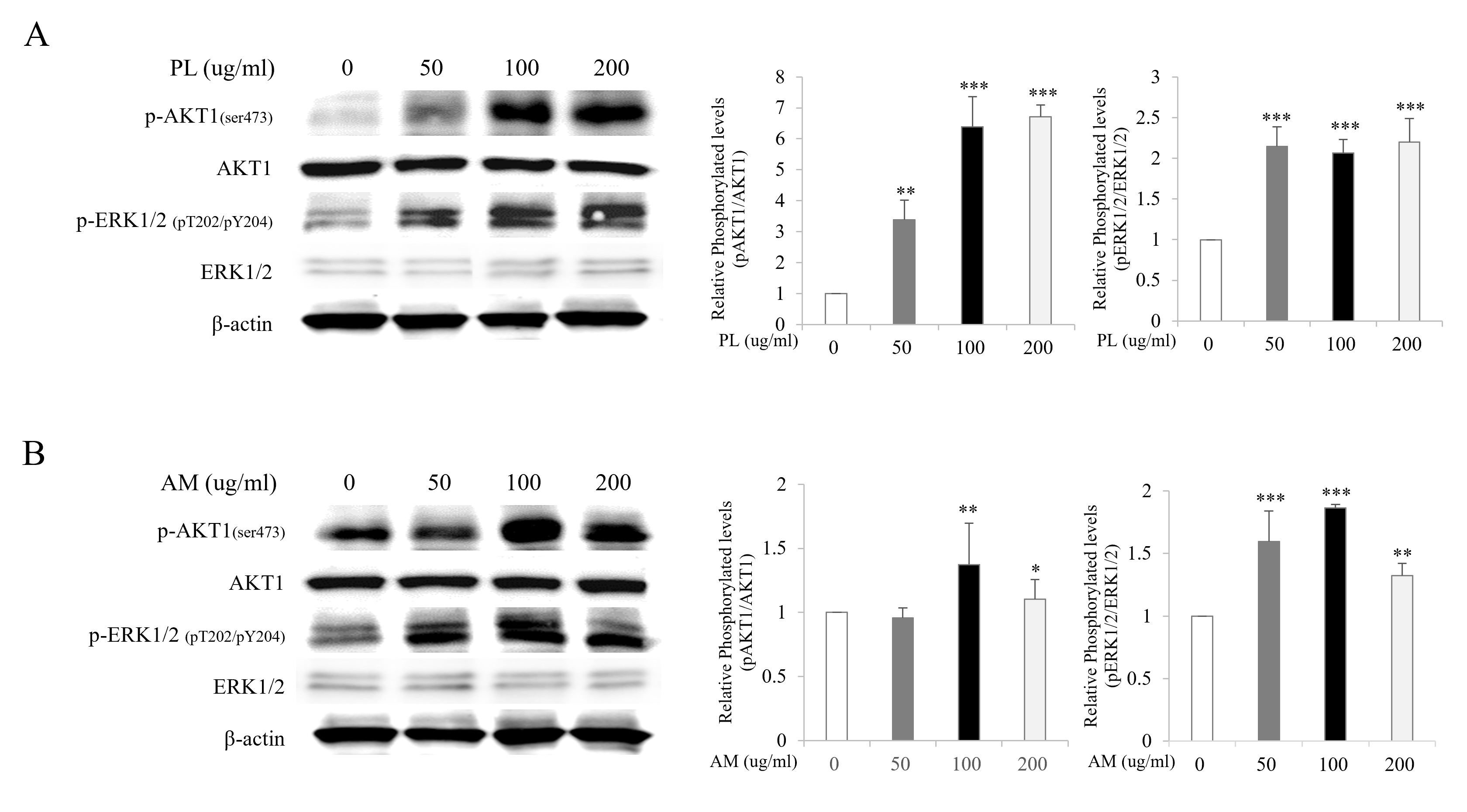
To elucidate the estrogen-like activity of PL and AM via the ER-dependent downstream signaling pathway, the protein expressions of ERK and Akt were examined using the western blot on MCF-7 cells. The expression of phosphorylated ERK increased at all concentrations of PL and AM compared with the control. The expression of phosphorylated Akt increased at concentrations of 50, 100, and 200 µg/mL of PL and at concentrations of 100 and 200 µg/mL of AM compared with the control group (Fig. 2A,B). These results suggest that all the single extracts of PL and AM had estrogen-like activities and their effects are mediated through the activation of the ER-dependent signaling pathway.
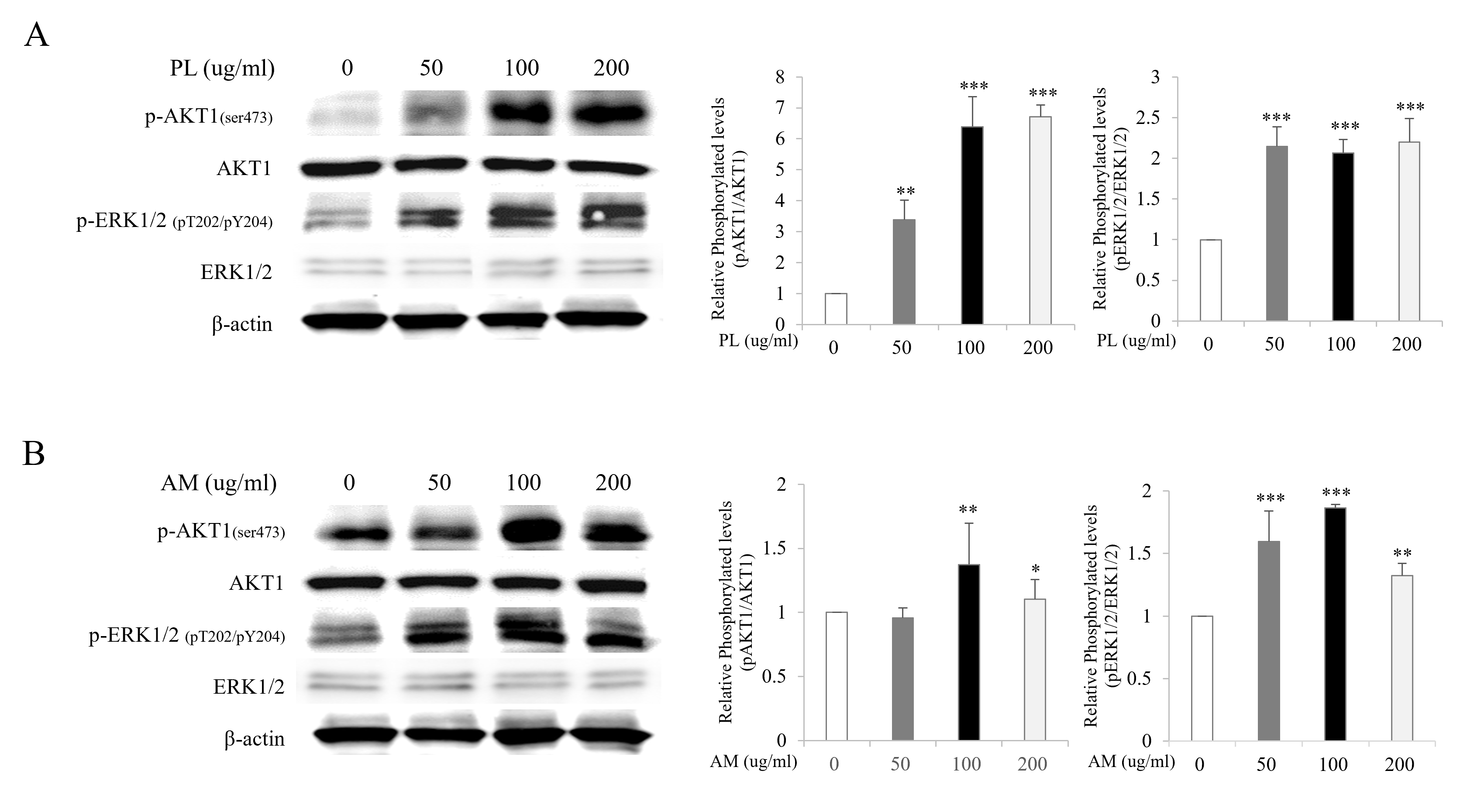 Fig. 2.
Fig. 2.Effects of PL and AM on the activation of ERK and Akt in the
MCF-7 cells. MCF-7 cells were treated with PL (A) and AM (B) at concentrations of
50, 100, and 200 µg/mL, respectively, for 24 hours. The whole cell lysates
were immunoblotted with anti-Akt1 (Ser473)/ERK1/2 (pT202/pY204) or Akt1/ERK1/2
antibodies. *p
The changes in the mean body weight, visceral fat mass, and organ weights at the experimental end point are presented in Tables 1,2. To elucidate the effects of the single extracts of PL and AM in OVX-induced body weight gain, visceral fat was collected from each mouse at necropsy and weighed. The body weight and visceral fat mass were significantly increased in the OVX-vehicle group compared to the Sham group. However, the oral administration of PL, AM and estrogen to OVX mice for 6 weeks significantly inhibited body weight and visceral fat mass gain (Table 1). The uterine weight significantly decreased in the OVX-vehicle group compared to the Sham group. However, the treatment of the OVX mice with PL and AM for 6 weeks did not affect uterine weight, unlike the estrogen treatment (Table 2). The weights of the other organs such as the liver, heart, spleen, and kidney did not change after ovariectomy and the oral administration of PL, AM and estrogen (Table 2). These results demonstrated the effects of the PL and AM single extracts in lowering body weight and visceral fat mass gain in OVX mice, and also revealed that, unlike estrogen, the extracts had no effect on uterine weight.
| Groups | Body weight (g) | Visceral Fat mass (g) |
| Sham | 20.57 |
0.34 |
| OVX-Vehicle | 26.88 |
1.11 |
| OVX-E |
22.15 |
0.70 |
| OVX-PL-L | 23.00 |
0.79 |
| OVX-PL-H | 24.60 |
0.94 |
| OVX-AM-L | 23.32 |
0.93 |
| OVX-AM-H | 22.14 |
0.81 |
*p
| Groups | Organ weight (g) | ||||
| Liver | Heart | Spleen | Kidney | Uterus | |
| Sham | 1.38 |
0.12 |
0.09 |
0.18 |
0.11 |
| OVX-Vehicle | 1.44 |
0.11 |
0.09 |
0.19 |
0.05 |
| OVX-E |
1.42 |
0.12 |
0.12 |
0.19 |
0.12 |
| OVX-PL-L | 1.36 |
0.11 |
0.11 |
0.18 |
0.06 |
| OVX-PL-H | 1.43 |
0.11 |
0.10 |
0.17 |
0.06 |
| OVX-AM-L | 1.49 |
0.12 |
0.10 |
0.18 |
0.07 |
| OVX-AM-H | 1.36 |
0.12 |
0.09 |
0.18 |
0.07 |
*p
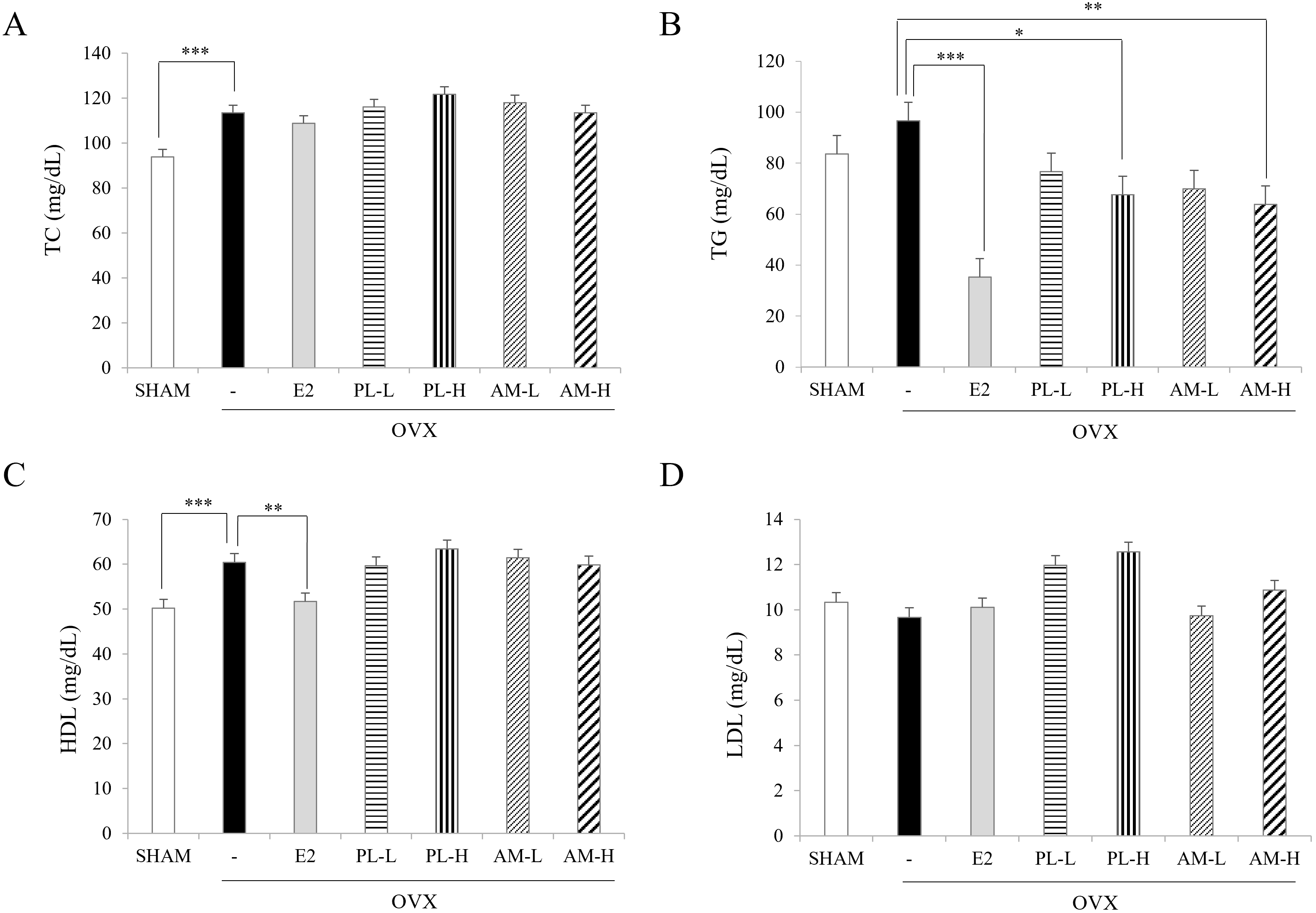
A significant increase in TC, TG, and HDL-C levels was found in the OVX-vehicle
group compared with the Sham group. After oral administration of PL and AM to the
OVX for 6 weeks, there was no change in the TC levels (Fig. 3A), but the TG levels
were significantly reduced by estrogen (OVX-E
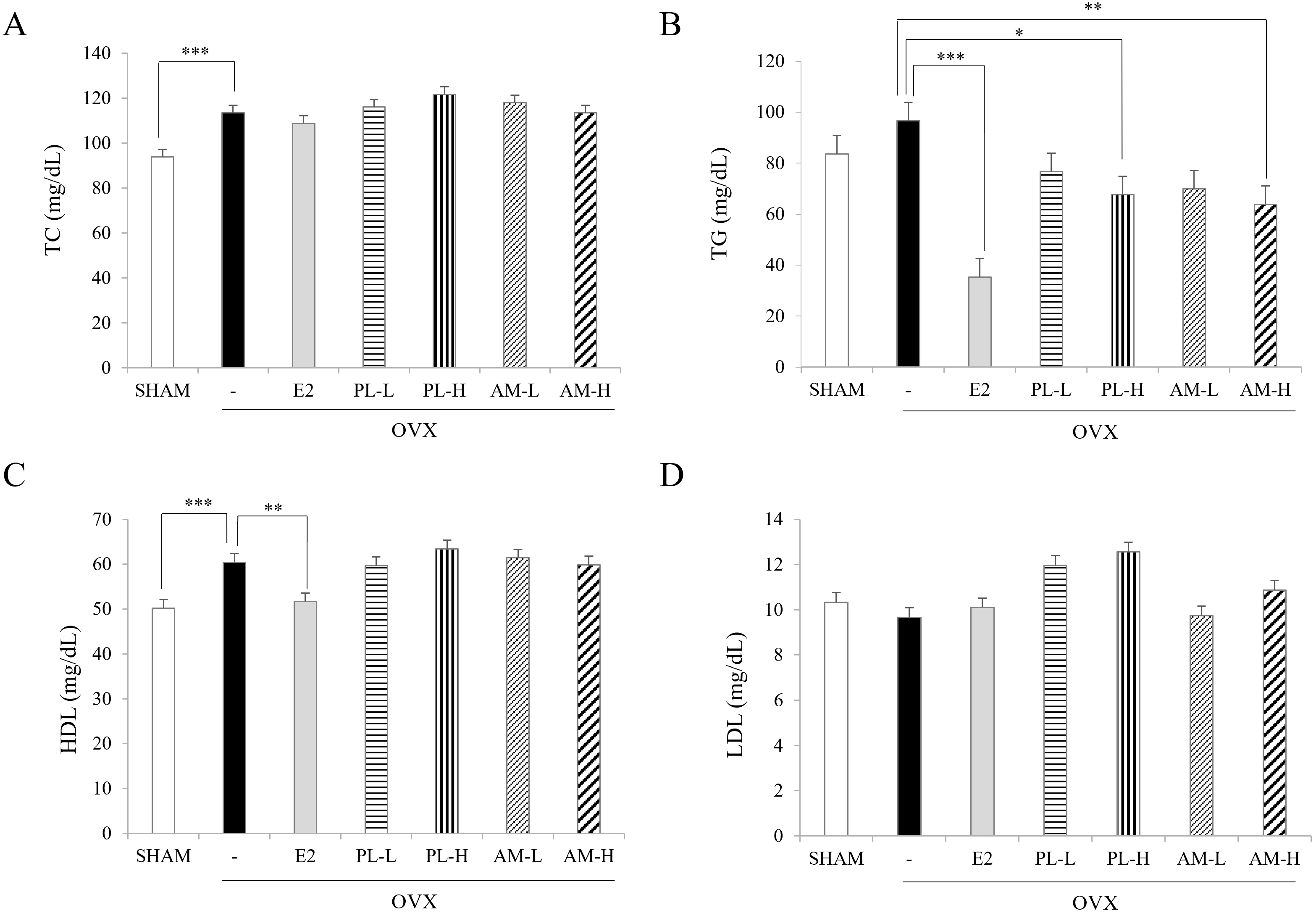 Fig. 3.
Fig. 3.Effects of PL and AM on serum lipids in OVX mice. (A) Total
cholesterol. (B) Triacylglycerols. (C) HDL-cholesterol. (D) LDL-cholesterol. All
the single extracts were administered by oral gavage. Data are shown as mean
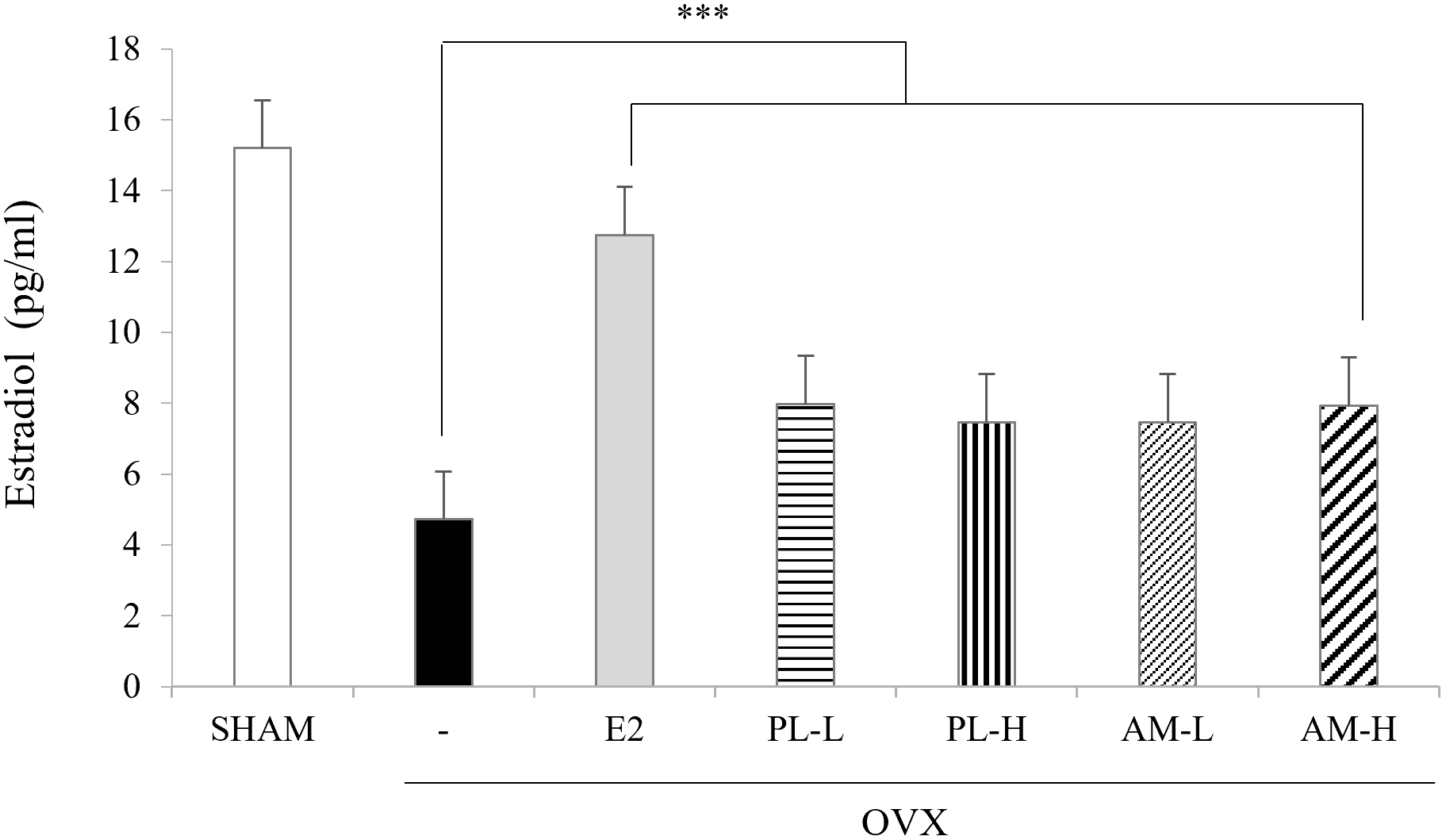
Serum estradiol levels were significantly decreased in the OVX-vehicle group
(4.8
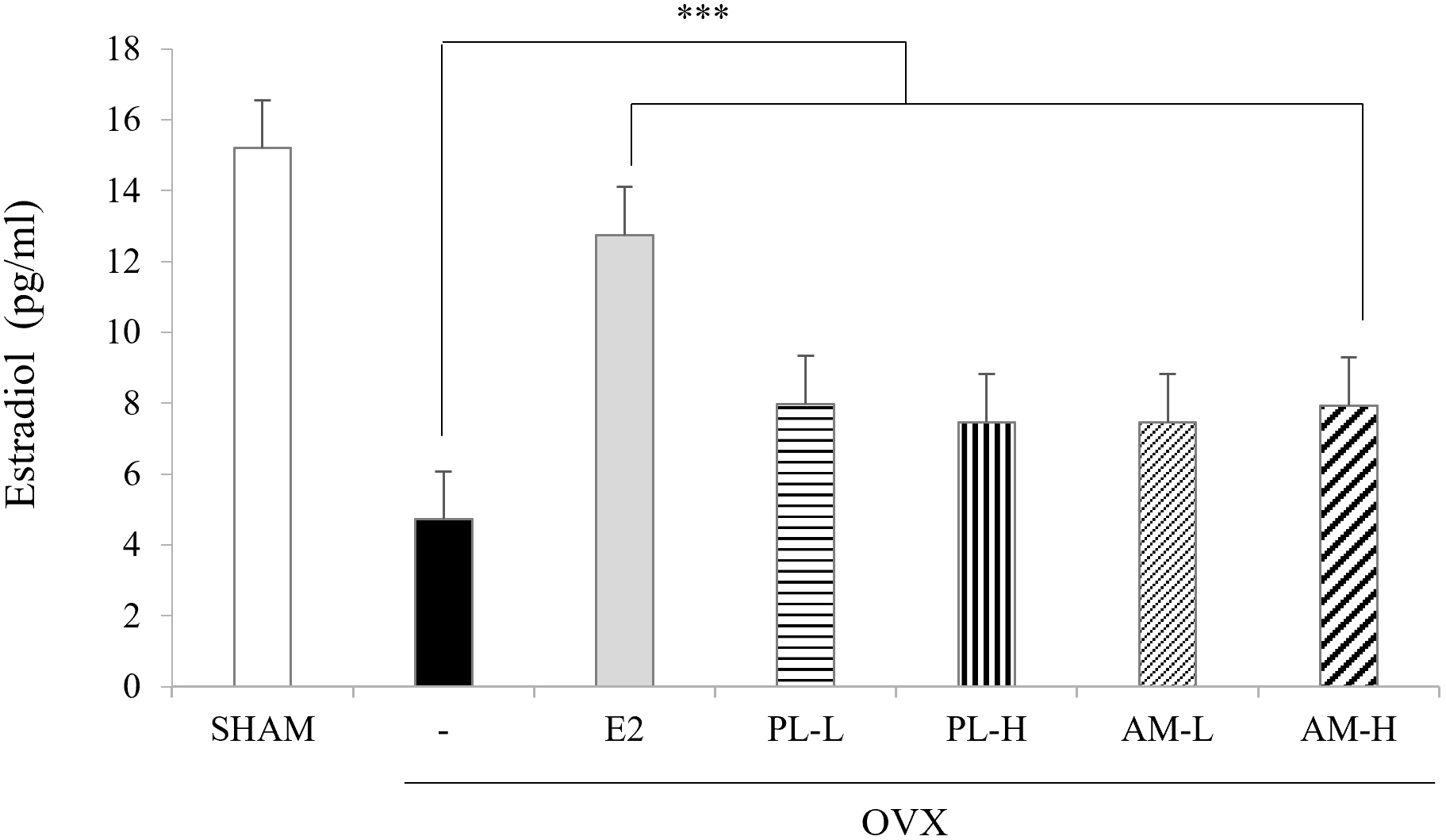 Fig. 4.
Fig. 4.Effects of PL and AM on the levels of serum estradiol in OVX
mice. Data are shown as mean
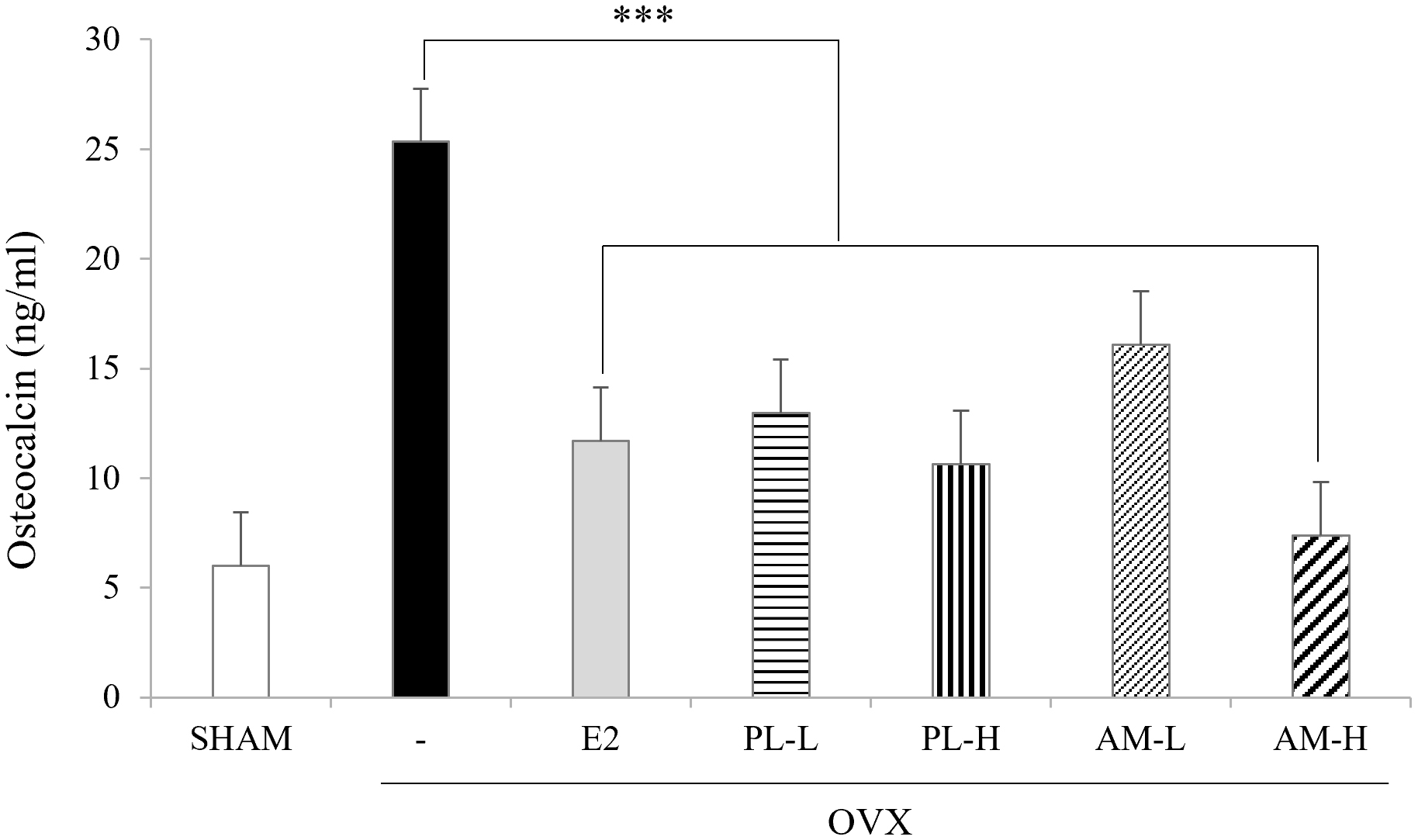
To investigate the effect of each extract of PL and AM on bone formation, serum
osteocalcin levels, bone mineral densities (BMD) and trabecular structures of the
right femur were measured. The OVX-vehicle group showed significant elevations of
osteocalcin (26.1
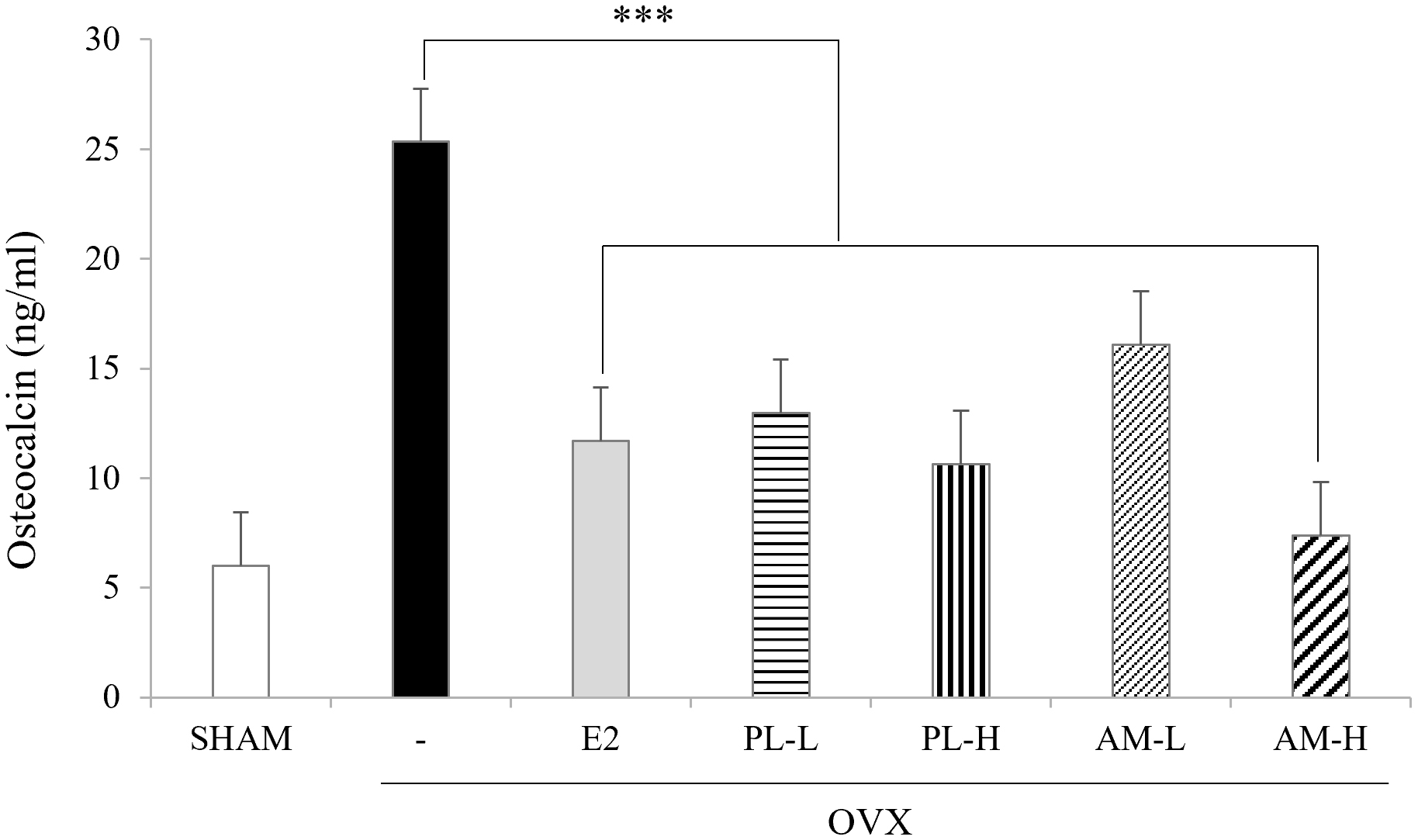 Fig. 5.
Fig. 5.Effects of PL and AM on the levels of serum osteocalcin in OVX
mice. Data are shown as mean
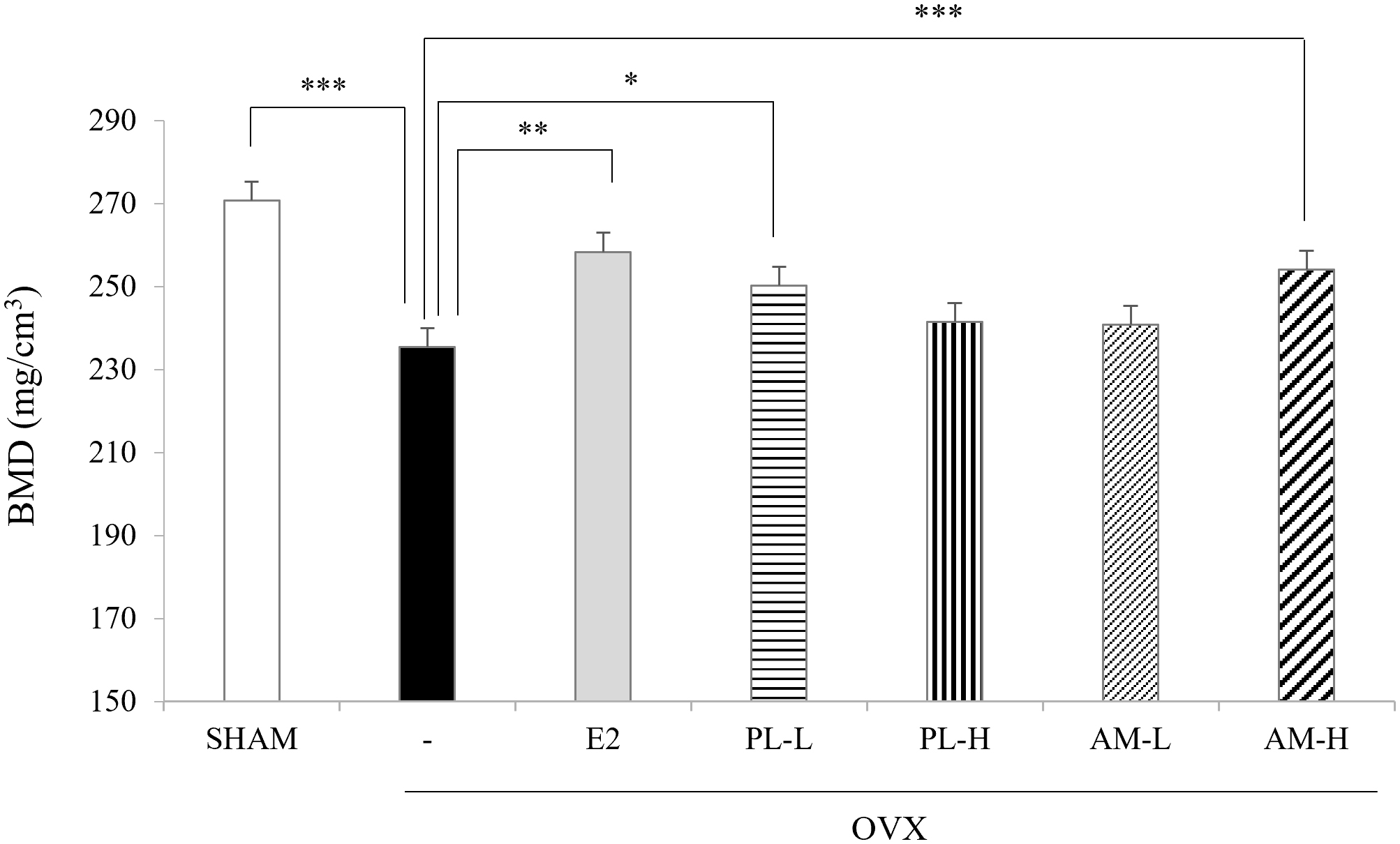
BMD also significantly decreased in the OVX-vehicle group compared with the Sham
group, but was significantly elevated in the OVX-E
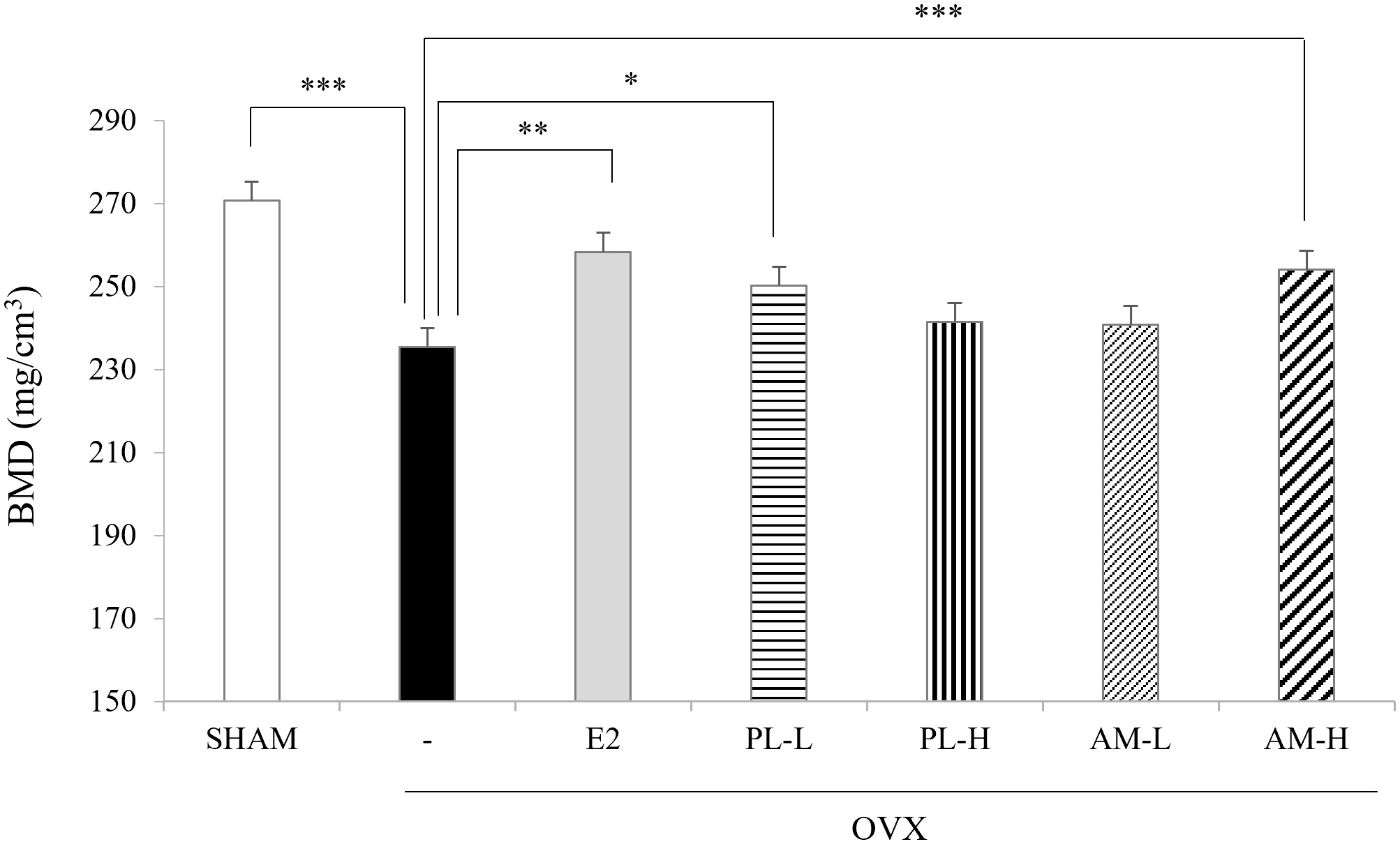 Fig. 6.
Fig. 6.Effects of PL and AM on bone mineral density (BMD) in OVX
mice. *p
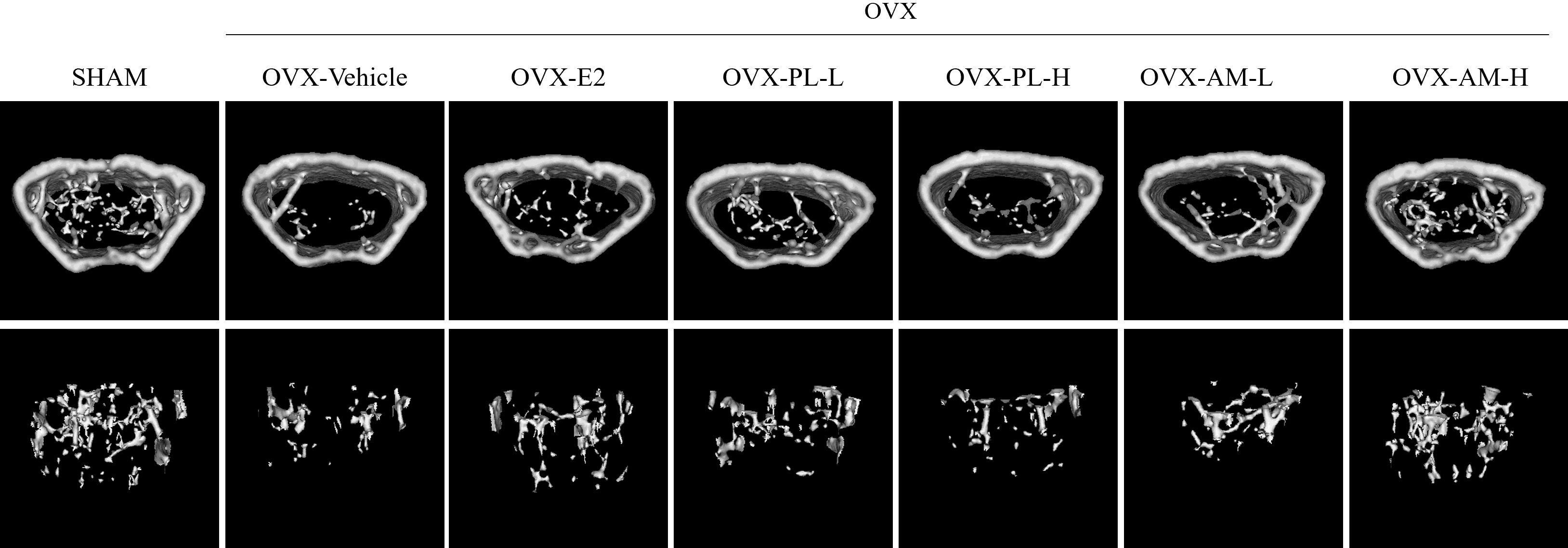
Fig. 7 showed the micro-CT image of the trabecular bone structure. Spongy (cancellous) bones were observed to be dense in the Sham group, but a decrease in density was observed in the OVX-vehicle group. The OVX-induced loss in spongy bone showed a significant recovery with the oral administration of estrogen, PL-L, and AM-H for 6 weeks. In addition, the OVX-vehicle group also showed a significant decrease in BV/TV, Tb.N, and Tb.Th compared to the Sham group (Table 3). However, when the OVX mice were treated with PL and AM for 6 weeks, the OVX-PL-L group showed a significant increase in BV/TV, and the OVX-AM-H group showed significant increases in BV/TV, Tb.N, and Tb.Th. Conversely, trabecular separation (Tb.Sp) significantly increased in the OVX-vehicle group compared with the Sham group, but showed a significant decrease with all doses of PL and AM. These results suggested that PL and AM can effectively prevent OVX-induced bone loss, but AM is more effective than PL in the formation of trabecular bone structures.
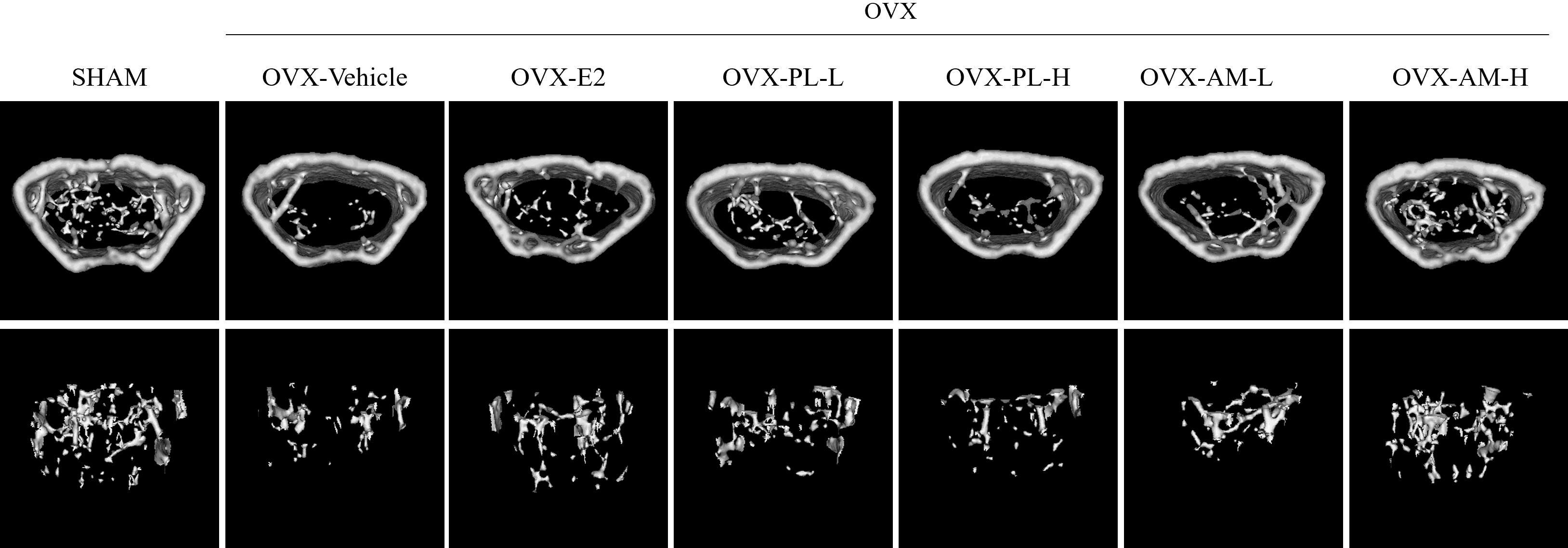 Fig. 7.
Fig. 7.Effects of PL and AM on bone formation in OVX mice. Micro-computed tomography (CT) images of trabecular bone. Upper: cortical bone + trabecular bone; Lower: trabecular bone.
| Groups | Parameters | |||
| BV/TV (%) | Tb.N (1/mm) | Tb.Th (mm) | Tb.Sp (mm) | |
| Sham | 3.8 |
0.8 |
0.081 |
0.23 |
| OVX-Vehicle | 2.0 |
0.3 |
0.069 |
0.55 |
| OVX-E |
2.7 |
0.5 |
0.074 |
0.33 |
| OVX-PL-L | 2.6 |
0.4 |
0.071 |
0.44 |
| OVX-PL-H | 2.1 |
0.3 |
0.072 |
0.43 |
| OVX-AM-L | 2.3 |
0.4 |
0.068 |
0.39 |
| OVX-AM-H | 2.7 |
0.5 |
0.076 |
0.33 |
Data are expressed as means
Several studies have shown that PL and AM have estrogenic activity and improve symptoms associated with postmenopause when used as a mixture with other herbs [10, 11, 12, 13, 14, 15, 16]. However, the present study revealed that single extracts of PL and AM had anti-menopausal effects by improving estradiol production and preventing bone loss in OVX mice, and that the effect was more pronounced with AM than with PL. To the best of our knowledge, this is the first study comparing the effects of single extracts of PL and AM with respect to their beneficial effects on bone loss due to estrogen deficiency.
Earlier in vitro studies have shown that MCF-7 cells are the best-characterized ER-positive cell line for investigating estrogen-like activity [17]. Our study also first examined the estrogenic potency of single extracts of PL and AM in MCF-7 cells, and then confirmed the estrogenic effect in OVX mice in vivo. The OVX mice model has been widely used as a representative model to study the anti-menopausal effects of various agents as the changes specific to menopause in human such as weight gain, increased serum osteocalcin levels, increased bone turnover, and decreased bone formation can be understood and extrapolated to menopause in human subjects [15, 16, 17, 18, 19, 20]. In this respect, the present study also used OVX mice as a menopausal animal model.
The present study also showed that the OVX mice had a significant increase in body weight and visceral fat mass. The OVX mice treated with PL and AM showed significant recovery from the changes in body weight and visceral fat mass, which occurred due to ovariectomy. This effect was more remarkable in low-dose PL (OVX-PL-L group) and high-dose AM (OVX-AM-H group), and there was no significant difference observed between the PL and AM groups.
Various studies have reported the measurement of visceral fat mass. However, there has been no consensus regarding which fat deposit among retroperitoneal, perigonadal, perirenal and mesenteric adipose tissue is most appropriate for representing visceral fat mass [21, 22]. Some studies have measured visceral fat mass by measuring only the perirenal fat pad [21]. For this reason, our present study measured visceral fat mass as the accumulation of intra-abdominal fat, including perirenal, mesenteric, and periuterine adipose tissue.
Ovariectomy causes a decrease in uterine epithelial height, myometrial
thickness, and uterine stroma expansion. As a result, The OVX mice showed
significant atrophy of the uteri and a decrease in uterine weight [23]. The
17-
Another characteristic of menopause is the alterations in lipid metabolism. Estrogen deficiency affects lipid metabolism, resulting in increased abdominal obesity and an imbalance between the HDL and LDL cholesterol levels [26, 27]. Indeed, some studies have reported that estrogen treatment in postmenopausal women increases serum TG levels [19], and the administration of estrogen in menopausal women with osteoporosis reduces serum LDL-C, but increases serum HDL-C levels [28]. In several studies, treatment with herbal extracts resulted in improvements in serum lipid levels in postmenopausal women [29, 30, 31]. Some studies reported that serum TG levels were negatively associated with serum estradiol levels in postmenopausal women [19, 28]. In other studies, the gradual weight gain observed during the menopausal transition was strongly associated in an increase in the TC and LDL-C levels, with no change in the HDL-C levels [19, 32]. Overall, the associations between menopause and serum lipid profiles have been highly inconsistent, probably due to differences in the menopausal status or aging [33]. The present study showed that estrogen treatment of the OVX mice reduced TG and HDL-C levels, but did not change TC and LDL-C levels. Both PL and AM effectively decreased the TG levels alone only at high doses (PL-H and AM-H), but did not affect the TC, HDL-C, and LDL-C levels. These results imply that the association between menopause and serum lipid profiles is very complex and further investigation is needed to understand the effects of PL and AM on the serum lipid profiles.
The development of osteoporosis due to increased bone turnover and decreased BMD is also another important consequence of estrogen deficiency in menopause [14]. Estrogen plays a critical role in skeletal homeostasis and the regulation of bone turnover [34], and estrogen deficiency is associated with bone loss in menopausal women [17]. The serum osteocalcin level is the representative biomarker of bone turnover. BMD is the amount of mineral (calcium hydroxyapatite) per unit of bone and is used to evaluate fracture risk and decreases in postmenopausal women with osteoporosis [17, 35]. In the present study, the OVX mice showed decreased serum estradiol levels and BMD, but increased serum osteocalcin levels, a condition typical of menopause. The administration of PL and AM to the OVX mice increased the serum estradiol levels, while decreasing serum osteocalcin levels like that seen with estrogen. However, BMD was effectively increased only at a low dose of PL (PL-L) and a high dose of AM (AM-H). Changes in trabecular structure leading to bone loss are important factors in the progression of postmenopausal osteoporosis [36]. In the present study, the micro-CT image of the trabecular bone structure revealed that parameters such as connections to the cancellous reticulum, BV/TV, Tb.N and Tb.Th improved when the OVX-mice were treated with estrogen, PL, and AM. Specifically, a high dose of AM showed an effect similar to that of estrogen and was superior to the effect of PL. This effect of AM could be attributed to polysaccharides in AM, which have been reported, in an earlier study, to exert significant anti-osteoporotic activity by increasing the BMD in a dose-dependent manner [37]. Overall, there were no significant differences in the effects of the single extracts of PL and AM on estradiol production and BMD, but a high dose of AM was more effective in lowering the levels of osteocalcinandin the formation of trabecular bone structure than PL. These results suggest that AM could be superior to PL in preventing the risk of osteoporosis.
The present study confirmed effect of the single extracts of PL and AM in the
production of estradiol in ER-positive MCF-7 cells, and then examined
phosphorylation of ERK and Akt, ER-dependent downstream signaling pathway to
understand whether these effects of PL and AM involved ER. ERKs are signal
transducers of the mitogen-activated protein kinase (MAPK) family, which is a
downstream signal for the activation of ERs [38, 39]. The results of the present
study showed that the expressions of phosphorylated-ERK and -Akt were increased
at high concentrations (
The results of this study suggest that both PL and AM alone can be effective herbal medicines for improving estradiol production, inhibit body weight gain, and improve bone metabolism. These effects were more pronounced with AM than with PL. However, the present study has several limitations: first, it is uncertain whether 6 weeks of administration of PL and AM is sufficient. Treatment with PL and AM single extracts for 6 weeks was sufficient to improve bone metabolism. However, this period was probably insufficient to observe any significant changes in the uterine weight and lipid profile. In some studies, the duration of the herbal treatment was 8 weeks or longer. Therefore further studies are needed to examine the effect of PL and AM based on the treatment period. Second, the present study did not investigate whether treatment with a mixture of PL and AM had a synergistic effect on alleviating menopausal symptoms compared with single treatments. Therefore, if additional studies that can address these limitations could be conducted in the future, it is believed that we could have a better understanding of the beneficial effects of PL and AM on menopause-related obesity, and bone loss post-menopause.
The ownership of the data belongs to the Korea Institute for Public Sperm Bank and Genoheal Co., Ltd. Upon request, the data used to support the findings of this study may be available from the corresponding author with permission of the Korea Institute for Public Sperm Bank and Genoheal Co., Ltd.
MJP and CSK performed Conceptualization, Methodology, Writing—Original draft preparation. KTH help and advice on analysis and interpretation of data. JHB help on the experiments for acquisition of data. HC and YL analyzed the data and interpretation of data. CWK and BSJ performed the conception or design of the work, the acquisition, analysis, interpretation of data for the work, critical revision of the manuscript, and provided obtaining research funding. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All animal experiments were conducted based on the Guide for the Care and Use of Laboratory Animals, funded by the National Institutes of Health, and approved by the Pusan National University Hospital Institutional Animal Care and Use Committee. This study was approved by the Institutional Review Board of Pusan National University Hospital (PNUH-2022-209).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. In addition, we would like to thank the reviewers for taking the necessary time and effort to review the manuscript and providing valuable inputs to improve its quality.
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HF21C0131).
The authors declare no conflict of interest. Additionally, Bo Sun Joo is serving as one of the Editorial Board members of this journal. Declares that he was not involved in the peer review of this article and had no access to information regarding its peer review. Min Jung Park, Cha Soon Kim and Bo Sun Joo are from the Genoheal Co. Ltd. team. They are participating researchers and designed the research study and the acquisition of data. We have received no funding from Genoheal Co., Ltd.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.