- Academic Editor
Background: Uterine arteriovenous malformations (UAVM) are especially relevant vascular abnormalities due to the vital risk that may stem from severe genital bleeding. The objective of this systematic review was to evaluate the ultrasound criteria for the diagnosis of UAVM and determine which ones are the consistently relevant for the diagnosis of UAVM. Methods: For this systemic review, we followed PRISMA 2020 guidelines. The systematic search was carried out in PubMed and Embase databases up to January 31st, 2023. The total amount of articles compiled with the search strategy was 3191. Results: 21 records met the inclusion criteria and were included in the review. The results revealed the heterogeneity in the studies on UAVM, as not all studies use the same ultrasound (US) features. Most of the included articles described data on US findings in the grayscale mode, which were variable and not specific for the diagnosis. In terms of color Doppler mapping and spectral Doppler analysis, the findings were consistent in all articles included, showing abnormal strong hypervascular lesions corresponding with a tangle of irregular vessels with multidirectional and strongly turbulent intraluminal flow. Conclusions: Ultrasound diagnosis of uterine arteriovenous malformations might be initially suspected in the grayscale mode, although the color and spectral Doppler assessment seems to be the key to achieving a consistent diagnosis with the visualization of a tangle of vessels in a ‘mosaic’ pattern with multidirectional turbulent flow in an arteriovenous shunting, with high-velocity and low-impedance values in spectral flow analysis.
Arteriovenous malformations are rare vascular abnormalities that, although they mostly affect the central nervous system, they may also appear in other peripheric locations such as the uterus [1, 2, 3]. Uterine arteriovenous malformations (UAVM) are especially relevant given that vital risk that may stem from severe genital bleeding [1, 2, 3, 4], causing between 1–2% of all intraperitoneal and genital hemorrhage [2, 5]. Its incidence is difficult to determine as it is quite uncommon and it often goes unnoticed due to either the lack of symptoms or its spontaneous resolution [1]. The first case report was published in 1926, and less than 150 cases have been reported since [1, 3], with an increase in recent years due to the introduction of common diagnostic techniques such as ultrasound among others, as well as the increase of uterine interventions [1, 6, 7]. UAVM are high-flow and low-resistance vascular abnormalities with a dilation and increased pressure gradient between the arterial and venous system that allows the blood flow through the nidus. On occasion, the central nidus is absent, with a direct link between arteries and veins, which strictly speaking makes them an arteriovenous fistula. This latter structure is more frequent in acquired UAVM, while congenital UAVM have a more complex vascular structure [1, 2, 3].
For its diagnosis it is important to make a differential diagnosis with conditions with similar clinical presentation such as the retainment of products of conception or trophoblastic gestational disease, among others [2, 6, 8]. Although histologic examination allows for the definitive diagnosis in cases which required surgical treatment, currently, angiography is considered as the gold standard as it allows for a detailed visualization of the angioarchitecture of the lesion as well as enables for the therapeutic embolization. However, many experts agree that given its invasive nature, it should be reserved only for those who would benefit from embolization treatment [1, 3, 7]. Although some authors have proposed the use of different imaging techniques such as the magnetic resonance imaging (MRI) or the computerized tomography (CT), their lengthy image-acquiring period, especially relevant in urgent cases, right along with their high costs and inaccessibility in some centers, may be a disadvantage [1]. In contrast, ultrasound (US) is a cheaper, non-ionizing and highly accessible imaging technique that comes as a useful alternative. Moreover, the addition of color Doppler allows for a real-time assessment of blood flow which is especially relevant in cases of UAVM [1, 2, 3, 4, 6, 7, 9, 10, 11]. However, the published literature consists of studies evaluating a variety of parameters, being yet to be determined which ones are useful for the diagnosis of UAVM.
The objective of this systematic review was to evaluate the ultrasound criteria for the diagnosis of UAVM and determine which ones are the consistently relevant for the diagnosis of UAVM.
For this systemic review, we followed PRISMA 2020 guidelines and previously registered with PROSPERO (CRD420699). The systematic search was carried out in PubMed and Embase databases up to January 31st, 2023. Our search was conducted using the following medical subject heading (MeSH) terms: (“Uterus” OR “Uterine artery”) AND (“Arteriovenous Malformations” OR “Ultrasonography, Doppler, Color”). No language or date restrictions were applied. As this study was based on previously published literature, no ethical approval was needed.
For the selection of studies, we included those meeting the following criteria:
- They described the ultrasound findings in different modes (grey scale, color and spectral Doppler, etc.) for the diagnosis of UAVM.
- The design was cross-sectional, prospective or retrospective.
- They involved human subjects.
As for the exclusion criteria, they were as follows:
- Conference summaries, letters to the editor, revisions.
- Case series reports including less than 5 patients.
- Studies focused on other vascular or uterine abnormalities.
- Studies exclusively focused on diagnostic techniques other than ultrasound, such as a MRI, CT or angiography.
- The objectives were focused exclusively on other aspects, such as efficacy of treatment options, fertility sequels or pregnancy outcomes after treatments.
- They involved animals subjects.
The total amount of articles compiled with the search strategy was 3191, dating from January 1st, 1952, to January 31st, 2023. Abstracts were read and, after applying both inclusion and exclusion criteria, we obtained a total of 125 articles. Afterwards, a complete reading of the selected articles was carried out, excluding the following:
- Case reports including only one case (n = 55).
- Case series reports including less than 5 patients (n = 23).
- Revisions, conference summaries or letters to the editor (n = 14).
- Studies including other vascular and uterine abnormalities (n = 5).
- Studies that did not report ultrasound findings useful for diagnosis (n = 3).
- Studies with a final sample of UAVM cases with less than 5 patients (n = 2).
- Complete article not available (n = 2).
Therefore, a total of 21 articles meeting the inclusion and exclusion criteria were included.
The search included a total of 3191 studies that met the MeSH criteria, out of which 21 met the inclusion criteria and were included in the review (Fig. 1). Out of the excluded records, it is worth mentioning the study by Timmerman et al. [12] which, despite including quantitative data for Doppler findings, they are extracted from only 3 confirmed cases of UAVM out of the initial 30 suspected cases that composited the initial sample. A summary of the findings of the included articles is displayed in Table 1 (Ref. [3, 11, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31]). Most articles [10, 13, 14, 15, 16, 17, 19, 20, 21, 22, 25, 26, 27, 28, 31] used angiography as the technique for confirmation of the diagnosis, while others also included histology examination [10, 20, 27, 29, 30], CT [17] or MRI [20, 25]. In 4 articles there was no confirmation technique used [3, 18, 23, 24].
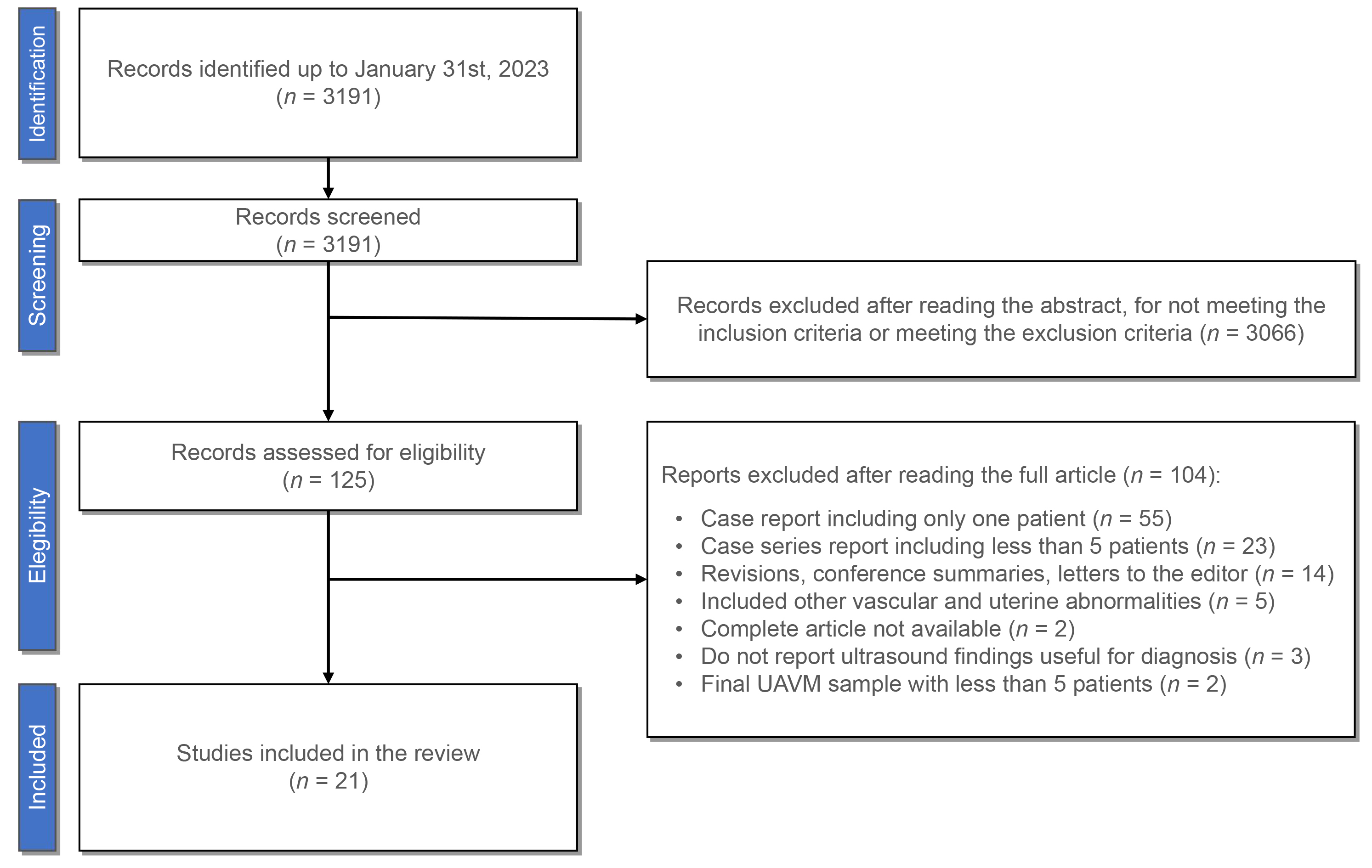 Fig. 1.
Fig. 1.PRISMA flow diagram. UAVM, uterine arteriovenous malformations.
| Article | Type of study | n | Diagnosis confirmation | Size | Uterine location | Color/Spectral Doppler | Grayscale mode | PSV | RI | PI | TAMVX | n of vessels | Diagnostic capacity |
| Hong et al. [11] | Retrospective | N = 13 | Angiography (12) | + | + | + | + | ||||||
| Histology (1) | |||||||||||||
| Abd ElGawad et al. [13] | Prospective | N = 20 | Angiography (20) | + | + | + | + | + | |||||
| Timor-Tritsch et al. [3] | Retrospective | N = 27 | + | + | + | ||||||||
| Hugues et al. [14] | Retrospective | N = 38 | Angiography (24) | + | + | + | + | ||||||
| Aslan et al. [15] | Case series | N = 6 | Angiography (5) | + | + | ||||||||
| Lalitha et al. [16] | Retrospective | N = 5 | Angiography (4) | + | + | + | + | ||||||
| Narang et al. [17] | Case series | N = 7 (Total) | CT (3) | + | + | + | + | + | + | ||||
| N = 5 (UAVM) | Angiography (2) | ||||||||||||
| Lee et al. [18] | Prospective | N = 85 (UAVM) | + | + | + | + | + | + | + | + | |||
| Yazawa et al. [19] | Prospective | N = 6 (UAVM) | Angiography (6) | + | + | + | + | + | |||||
| Machado et al. [20] | Prospective | N = 8 | Angiography (5) | + | + | ||||||||
| Histology (1) | |||||||||||||
| MRI (1) | |||||||||||||
| O’Brien et al. [21] | Retrospective | N = 21 | Angiography (14) | + | + | + | + | + | |||||
| Kwon and Kim [22] | Retrospective | N = 24 (Total) | Angiography (9) | + | + | + | + | ||||||
| N = 9 (UAVM) | |||||||||||||
| Wiebe and Switzer [23] | Case series | N = 7 | + | + | |||||||||
| Timmerman et al. [24] | Prospective | N = 265 (Total) | + | + | + | + | + | + | |||||
| N = 9 (UAVM) | |||||||||||||
| Huang et al. [25] | Retrospective | N = 10 | MRI (4) | + | + | + | + | + | |||||
| Angiography (9) | |||||||||||||
| Dar et al. [26] | Prospective | N = 8 | Angiography (2) | + | + | ||||||||
| Yang et al. [27] | Retrospective | N = 15 | Angiography (14) | + | + | ||||||||
| Histology (1) | |||||||||||||
| Vaknin et al. [28] | Retrospective | N = 16 | Angiography (9) | + | + | + | + | + | |||||
| Calzolari et al. [29] | Retrospective | N = 11 | Histology (11) | + | + | + | + | + | |||||
| Degani et al. [30] | Prospective | N = 12 | Histology (3) | + | + | + | + | + | + | ||||
| Gilbert et al. [31] | Retrospective | N = 31 (Total) | Angiography (6) | + | + | + | + | + | + | + | |||
| N = 6 (UAVM) |
UAVM, uterine arteriovenous malformation; PSV, peak systolic velocity; RI, resistance index; PI, pulsatile index; TAMXV, time-averaged maximum velocity; CT, computerized tomography; MRI, magnetic resonance imaging.
The uterine location of UAVM was described only 6 articles [11, 13, 16, 17, 28, 29] without any predominance of one over the others. It varied between anterior and posterior uterine walls, without any clear preference, and sometimes in the right or left cornual myometrium and even in the posterior lip of the cervix in one case [11]. Abd ElGawad et al. [13] described that 50% of the cases presented unilaterally, while the other 50% presented bilaterally or extending across the uterine midline or in the fundus, whereas in the study by Vaknin et al. [28] all cases were unilateral.
In terms of size, it was specifically described in 5 articles [11, 15, 16, 17, 21]. In most cases the size was approximately 2–3 cm, with the biggest reported diameters being 5 cm [11] and 7 cm [16]. O’Brien et al. [21] reported the sizes of UAVM divided in two groups: one group of UAVM requiring embolization (median 17.0 mm, range 10–35 mm), and another group with conservatively managed cases (median 16.4 mm, range 4–30 mm), without statistically significant differences between groups (p = 0.218).
Only 3 articles [18, 24, 30] reported data regarding the number of vessels. Lee et al. [18] reported that a 71% (n = 32) of the severe cases requiring embolization had a single vessel, while 29% (n = 13) of them had multiple vessels, and similar percentages were found in cases conservatively managed, with a 60% (n = 18) and a 40% (n = 12) of them having single and multiple vessels, respectively. The reports made by Timmerman et al. [24] are similar, with 4 cases (44.4%) displaying multiple vessels, and 5 cases (55.6%) having one single vessel, while Degani et al. [30] reported 4 cases (33.3%) with a single vessel and 8 cases (66.7%) with multiple vessels.
Most of the included articles [3, 11, 13, 14, 15, 16, 17, 18, 20, 21, 22, 23, 24, 25, 26, 27, 29] described data on US findings in the grayscale mode, which were variable and nonspecific, not being the key finding in which the diagnosis was based [25]. Most of them described an “heterogeneous echo occupancy in the myometrium with anechoic spaces of tortuous dilated vessels, often protruding to the uterine cavity” [11, 20]. Most of them described the findings as unspecific, with subtle myometrial heterogeneity [13, 21, 22, 27] and sometimes with the presence of small anechoic spaces [13, 17, 21, 22, 23, 27, 29], that in some cases presented as unusual multiple tubular tortuous anechoic structures often protruding [3, 14, 15, 16, 20, 24, 29] without mass effect, with some branching giving it the appearance of blood vessels [16]. The majority of cases in the study by Huang et al. [25] were diffusely uniform, which gave the myometrium a ‘spongey’ texture, while in other cases presented as an intramural mass mimicking a polyp or a cervical fibroid or carcinoma. One case in the study by Aslan et al. [15] presented as an hyperechogenic mass surrounded by an anechoic zone. In the study by Lee et al. [18], which divided patients depending on the strategy followed, the mass was hypoechogenic in 40% of conservatively managed cases and 60% of therapeutically managed cases, while it was isoechogenic in 60% and 40% of cases, respectively. Machado et al. [20] described that in some cases presented as characteristic lacunar images while Dar et al. [26] found them as a uterine intramyometrial mass with fluid motion. In addition, Kwon and Kim mentioned that an adjacent anechoic sac can also be observed when there is a pseudoaneurysm present [22].
In terms of color Doppler mapping and spectral doppler analysis, the findings were consistent in all articles included. The activation of color Doppler showed abnormal strong hypervascular lesions [3, 16, 17, 24, 26, 31] corresponding with a tangle of irregular vessels with multidirectional and strongly turbulent intraluminal flow [15, 19, 20, 24, 26, 30, 31]. This defined a characteristic ‘color mosaic’ pattern [11, 13, 15, 19, 21, 27, 28, 29], also described as ‘fireball’ pattern [11] that could manifest in two ways: apparent flow reversals of juxtaposed red and blue components with different flow directions; or red and blue components separated by yellow and white components, manifesting color aliasing with different velocities [22, 25]. The spectral Doppler analysis showed that this hypervascularity was composed of high-velocity and low-impedance flow vessels [13, 14, 15, 16, 20, 21, 22, 24, 29]. Although in some cases the flow was predominantly arterial, there was venous flow present, consistent with an arteriovenous mix shunting pattern [11, 15, 17, 21, 22, 23]. The waveform was usually broad and irregular, resulting from the troubled flow of the multidirectional arteriovenous connections [28]. The arterial spectral waveform had a high diastolic component, while the venous waveform had a pulsatile high velocity with little variation in systolic-diastolic velocities [22], with continuous high blood flow throughout both components of the cardiac cycle [28].
In 10 of the included articles there was data reporting the quantitative analysis of the spectral Doppler [3, 13, 17, 18, 21, 22, 24, 25, 29, 30]. The most used parameter among the reporting data was the PSV (peak systolic velocity), followed by the RI (resistance index), while the PI (pulsatile index) and the time-averaged maximum velocity (TAMXV) was reported in only 3 articles. A summary of the reported data is displayed in Table 2 (Ref. [3, 13, 17, 18, 21, 22, 24, 25, 29, 30]). UAVM had high-velocity flow with high average PSV values ranging from 35.8 cm/s [18] to 138 cm/s [25]. Similar results were found for PI, with high average PI values ranging from 0.43 to 0.51 [18, 24, 25], as well as for TAMXV values, which had mean values between 34.2 cm/s and 50.8 cm/s [9]. The low impedance flow manifested in the form of low resistance index (RI), with average values between 0.30 [22] and 0.42 [13]. In the study conducted by Kwon and Kim [22] PSV and RI values of UAVM cases were compared to those of a control group of healthy premenopausal patients. The latter had lower PSV (43.6 cm/s vs 11.6 cm/s) and higher RI (0.30 vs 0.72). Additionally, Lee et al. [18] compared the data between patients with UAVM that received conservative management with those who required therapeutic management, with the latter group displaying higher PSV (35.8 cm/s vs 76.2 cm/s), PI (0.43 vs 0.53) and TAMXV (34.2 vs 50.8), and a lower RI (0.40 vs 0.28). Similarly, in the study by Timor-Tritsch et al. [3], they found that patients requiring embolization and those with intractable bleeding had a mean PSV of 85.2 cm/s and 72.1 cm/s, respectively, while the rest of patients had a lower mean PSV (59.5 cm/s).
| Article | PSV | RI | PI | TAMVX |
| Abd ElGawad et al. [13] | 60.36 |
0.42 |
||
| 19–132 cm/s (Min–Max) | 0.26–0.74 (Min–Max) | |||
| Timor-Tritsch et al. [3] | UAE: 85.2 cm/s (Mean) | |||
| No UAE: mean 59.5 cm/s (Mean) | ||||
| Intractable bleeding: 72.1 cm/s (Mean) | ||||
| Persistent UAVM: 72.0 cm/s (Mean) | ||||
| Narang et al. [17] | Case 1. 22.4 cm/s | Case 4. Low resistance flow. RI 0.38 | ||
| Lee et al. [18] | CM: | CM: | CM: | CM: |
| 35.8 |
0.40 |
0.43 |
34.2 | |
| 18–68 cm/s (Min–Max) | 0.30–0.55 (Min–Max) | 0.27–0.52 (Min–Max) | 19–52 cm/s (Min–Max) | |
| TM: | TM: | TM: | TM: | |
| 76.2 |
0.28 |
0.53 |
50.8 | |
| 53–103 cm/s (Min–Max) | 0.22–0.42 (Min–Max) | 0.43–0.60 (Min–Max) | 41–91 cm/s (Min–Max) | |
| O’Brien et al. [21] | 60.37 |
0.41 |
||
| 25–110 cm/s (Min–Max) | 0.27–0.75 (Min–Max) | |||
| Kwon and Kim [22] | UAVM: | UAVM: | ||
| 43.6 |
0.30 |
|||
| 20–67 cm/s (Min–Max) | 0.17–0.52 (Min–Max) | |||
| Control group: | Control group: | |||
| 11.6 cm/s (Mean) | 0.72 (Mean) | |||
| 4–38 cm/s (Min–Max) | 0.53–0.98 (Min–Max) | |||
| Timmerman et al. [24] | 44 cm/s (Mean) | 0.56 (Mean) | 0.37 (Mean) | 0.45 cm/s (Mean) |
| 16–68 cm/s (Min–Max) | 21–96 (Min–Max) | 0.29–0.50 (Min–Max) | 0.38–0.71 cm/s (Min–Max) | |
| Huang et al. [25] | 138 |
0.38 |
0.51 |
|
| 96–201 cm/s (Min–Max) | 0.25–0.55 (Min–Max) | 0.40–0.59 (Min–Max) | ||
| Calzolari et al. [29] | 0.97, 0.5–1.2 cm/s (Median, IQR) | |||
| 0.50–1.20 (Min–Max) | ||||
| Degani et al. [30] | 54, 21–102 cm/s (Median, IQR) | 0.41, 0.22–0.60 (Median, IQR) | 43, 18–86 cm/s (Median, IQR) |
PSV, peak systolic velocity; RI, resistance index; PI, pulsatile index; TAMVX, time-averaged maximum velocity; UAVM, uterine arteriovenous malformation; UAE, uterine arterial embolization; CM, conservative management; TM, therapeutic management; IQR, interquartile range, SD, Standard deviation.
Only 2 articles reported data regarding the diagnostic capacity of US [28, 31]. In the study by Vaknin et al. [28], they performed an angiography on 11 out of 16 patients who had an ultrasound diagnosis of UAVM, confirming the diagnosis in all 11 (100%), although the diagnosis was not confirmed in the other 5 patients as they did not require an angiography for clinical reasons. On the other hand, Gilbert et al. [31] evaluated the accuracy of US performing an angiography in all 31 patients of the sample. Out of them, only 6 had a confirmed true UAVM (19.4%).
The results extracted from this review reveal the heterogeneity in the studies on UAVM, as not all studies use the same US features. Nonetheless some ideas come clear as a result. While some variables such as the location, size or number of vessels do not seem significantly relevant, there are others which can enable the diagnosis of UAVM. For instance, initial evaluation in the grayscale mode might indicate the initial suspicion of UAVM, although the changes are often subtle and unspecific. While most studies reported the presence of anechoic tortuous spaces [3, 14, 15, 16, 20, 24, 29], others also reported the presence of small anechoic spaces [13, 17, 21, 22, 23, 27, 29], and even in some cases the only sign was the presence of a heterogeneous isoechoic area in the myometrium [18, 25]. This heterogeneity suggests that, although US assessment in the grayscale mode might be helpful, the diagnosis of UAVM should not be based on these findings alone, highlighting the potential role of the Doppler assessment which, unlike the grayscale findings, are generally consistent among all studies. All included records agree that UAVM seems to show a ‘mosaic’ pattern in color Doppler mapping [11, 13, 15, 19, 21, 27, 28, 29], due to the tangle of vessels with a multidirectional highly turbulent flow [3, 15, 16, 17, 19, 20, 24, 26, 30, 31]. Specifically, two types of patterns have been described, one composed of juxtaposed red and blue components as multidirectional flow, and another which also displays signs of aliasing with yellow and white components [22, 25].
In addition, spectral Doppler analysis adds more information, revealing the
high-velocity and low-impedance blood flow of the vessels forming the
arteriovenous shunting. PSV is the most widely used measurement in the included
studies and higher values are more likely to confirm the presence of an UAVM. In
studies like the one by Tritsch et al. [3], a higher PSV was present in
patients with intractable bleeding and requiring uterine arterial embolization.
However, it is worth mentioning that there is no evidence regarding specific
cut-off values for the diagnosis, or even for the severity, of UAVM. For example,
Timmerman et al. [12] performed a prospective study including 30
patients with an US diagnosis of UAVM, 8 of whom were submitted to a follow-up
angiography. According to their angiographic findings, patients were classified
in either two categories: those with a true UAVM (n = 3), and those with a
uterine non-arteriovenous vascular malformation, with no early venous contrast
filling (n = 5). They estimated cut-off values to establish three risk
categories: potentially dangerous UAVM (PSV
Although there is still a lack of consensus and evidence regarding cut-off values for Doppler spectral flow index, they seem to be the key that should be explored in future studies. For instance, the only 2 articles that explored the diagnostic capacity of US had very different results, one with a 100% agreement rate between US and angiography [28], while only a 19.4% agreement rate was found in the other one [31]. Vaknin et al. [28] specifically applied spectral Doppler assessment, performing the angiography only in those who clinically required it, and they had higher velocity and lower resistance values. Meanwhile, the study by Gilbert et al. [31] did not evaluated Doppler values and had a large amount of false positives with retained products of conception. This seems to highlight the potential role of measuring spectral Doppler flow values. Likewise, this is supported by the fact that a high PSV was defined as 4–6 times higher than normal myometrial vessels [28].
The main strength of our study is its novelty, as there is no other systematic review specifically focused on the US diagnosis of UAVM. Its restricted inclusion criteria support this strength, excluding articles focused on other aspects such as efficacy of treatment or reproductive repercussions. Nonetheless, our study also has its limitation, with the lack of homogeneity among studies being the main one as the evaluated parameter reported in the studies differ from one record to another. In addition, some articles did not confirm the presence of the UAVM with histological or angiographic evaluation, which might cause some bias as the image evaluated might be related to another cause. This hinders the true assessment of the diagnostic capacity of US for UAVM, which manifests in the lack of studies evaluating this aspect. All of this highlights the need for homogenization of inclusion criteria and measurement methods that should be taken into consideration in future studies evaluating US diagnosis of UAVM.
Ultrasound diagnosis of uterine arteriovenous malformations might be initially suspected in the grayscale mode, although the color and spectral Doppler assessment seems to be the key to achieving a consistent diagnosis with the visualization of a tangle of vessels in a ‘mosaic’ pattern with multidirectional turbulent flow in an arteriovenous shunting, with high-velocity and low-impedance values in spectral flow analysis.
All data generated or analyzed during this study are included in this published article.
JAGM designed the research study. JAGM and LRC performed the research. RGJ provided help and advice on review. JAGM, RGJ, LRC, RNM, CB and JASB analyzed the data. JAGM, RGJ, LRC, RNM, CB and JASB wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. José Antonio García-Mejido and José Antonio Sainz-Bueno are serving as one of the Guest editors of this journal. We declare that José Antonio García-Mejido and José Antonio Sainz-Bueno had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Andrea Tinelli.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
