- Academic Editor
Background: Ovarian clear cell carcinoma (OCCC) is the most common
pathological type of ovarian cancer associated with endometriosis. The effect of
endometriosis on the prognosis of ovarian clear cell carcinoma remains
controversial. This study aimed to investigate the clinical features and
prognostic factors of pure OCCC. Methods: This single-center
retrospective study analyzed 136 cases of pure OCCC after surgical treatment
between 2010 and 2019. Patients were divided into two groups according to whether
the pathologically relevant background lesion was ovarian endometriosis. Clinical
data were compared between the groups. The Kaplan–Meier test and Cox regression
analysis determined prognostic factors for survival. The primary outcome
measure of the study was the duration of survival. Results: 83 (61%)
participants had ovarian endometriosis of pure OCCC. Patients with ovarian
endometriosis were significantly younger (50.55
Ovarian clear cell carcinoma (OCCC) is a rare ovarian malignancy, accounting for
Endometriosis is a prevalent benign gynecopathy that affects approximately 11% of reproductive-aged patients [6]. Two meta-analyses of studies have shown an increased risk of ovarian cancer in patients with endometriosis (risk ratio [RR], 1.964; 95% confidence interval [CI], 1.685–2.29 and summary relative risks [SRR], 1.93; 95% CI, 1.28–2.22, respectively) that was strongest for clear cell subtype (SRR 3.44; 95% CI, 2.82–4.42) [7, 8]. Subsequently, researchers have identified ovarian clear cell carcinoma as the most common pathological type of endometriosis-associated ovarian cancer [9]. Early menarche, infertility, low parity, and late menopause affect endometriosis malignancy. Genetic factors such as PTEN, p53, PI3KCA and ARID1A mutations may promote the malignant transformation of endometriosis to ovarian cancer [10].
It is widely acknowledged that endometriosis plays a crucial role in the occurrence of OCCC, but opinions differ on whether endometriosis affects the prognosis. Orezzoli et al. [11] concluded that the median overall survival of patients with OCCC with endometriosis was 196 months versus 34 months in patients with OCCC without endometriosis (p = 0.001). It has also been suggested that ovarian cancer patients with endometriosis have progression-free survival (PFS) and overall survival (OS). Nevertheless, endometriosis was not an independent prognostic factor for OCCC [12]. A meta-analysis reported that the presence of endometriosis did not affect the prognosis of ovarian cancer [13]. However, the sample sizes in the previous studies were small and mainly included mixed ovarian clear cell carcinoma. This study included a relatively large number of patients, excluded mixed tumors, and aimed to investigate whether endometriosis was a prognostic factor for pure OCCC.
We retrospectively reviewed the data of 136 consecutive patients with OCCC treated at the Department of Gynecology, Tianjin Central Hospital of Gynecology and Obstetrics, China, between January 1, 2010, and December 1, 2019. Approval for this retrospective study was obtained from the Ethics Committee of the Tianjin Central Obstetrics and Gynecology Hospital, and all procedures were performed by the ethical standards of the institutional and national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
The inclusion criteria were as follows: patients diagnosed with OCCC and those who received treatment by initial staging or debulking surgery and underwent follow-up. Pure OCCC was histologic considered relative to mixed OCCC. The mixed component was less than 10% from the pathological point of view. The exclusion criteria were as follows: patients with mixed OCCC or metastatic ovarian carcinoma and those who received preoperative chemotherapy. Pathologic tissue sections were reviewed by two pathologists at our institution.
Clinicopathological and follow-up data were collected from medical records, including age at diagnosis, menopausal status, gravidity, parity, dysmenorrhea, family history of cancer, initial symptom, preoperative serum cancer antigen 125 (CA-125) level, tumor size, ovarian involvement, ascites, lymphatic metastasis, International Federation of Gynecology and Obstetrics (FIGO) stage, phlebothrombosis, chemotherapy and chemotherapy periods, chemoresistance and survival state. Patients were divided into OCCC with endometriosis group and OCCC without endometriosis group according to whether the pathologically associated background lesion was ovarian endometriosis. The median follow-up was 61 months (12–119 months). OS refers to the time from treatment to death, and PFS refers to the time from diagnosis/treatment to disease progression or death. The interval between the recurrence and the last chemotherapy was less than six months and was defined as chemotherapeutic resistance.
Descriptive statistics were used to describe the outcomes. Continuous variables
that followed a normal distribution pattern and had homogenous variance were
expressed as means
Among the 136 patients diagnosed with pure OCCC between 2010 and 2019, 83 (61%) were rendered as OCCC with endometriosis (Fig. 1), and 53 (39%) were assigned to OCCC without endometriosis (Fig. 2).
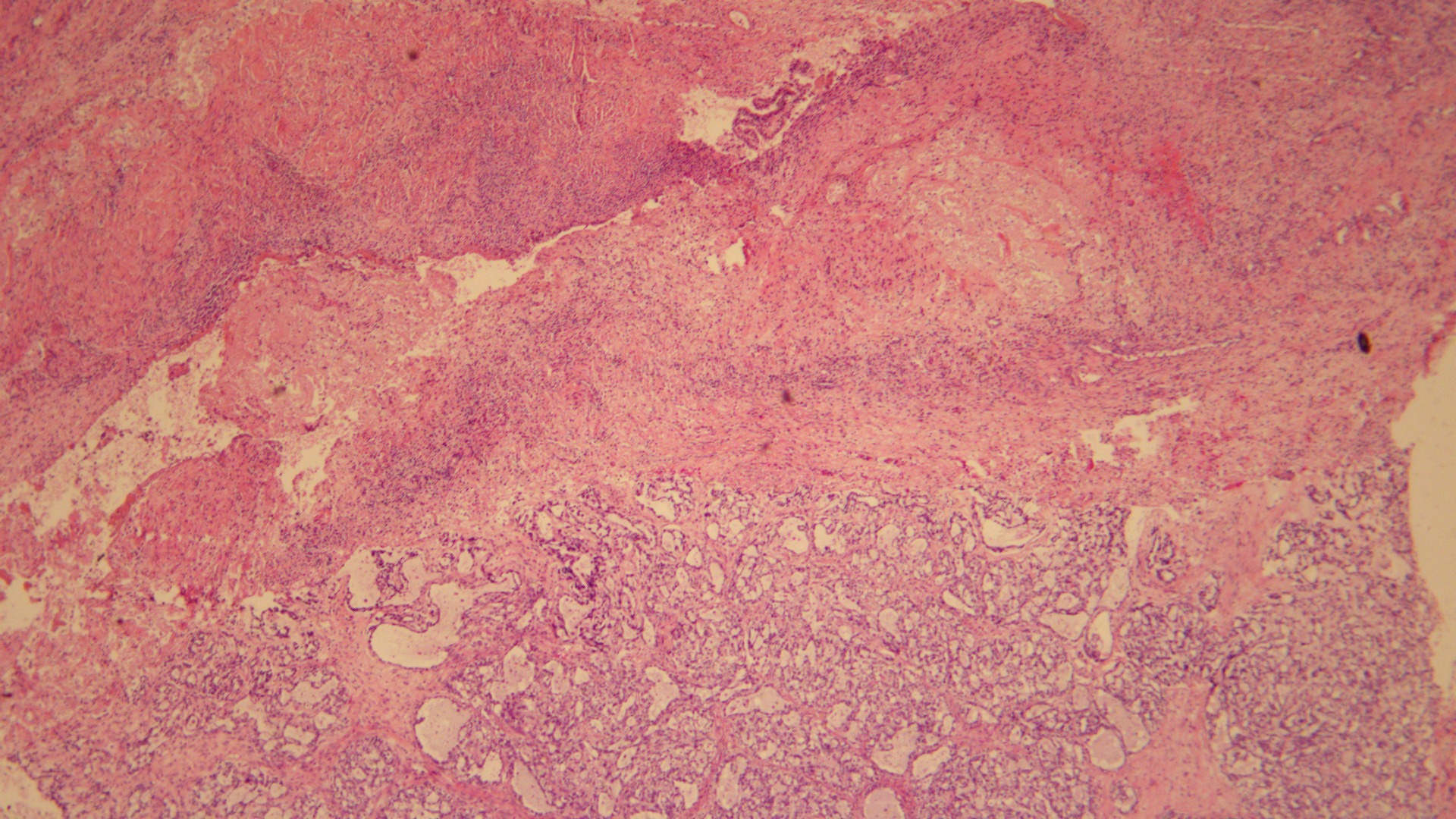 Fig. 1.
Fig. 1.Pure OCCC with endometriosis A typical OCCC arising from the
wall of an endometriotic cyst. Endometrial stroma and hemosiderin cells were
found in the ovarian sac wall (Hematoxylin and eosin stain; original
magnification,
 Fig. 2.
Fig. 2.Pure OCCC without endometriosis. A carcinoma composed of clear
cells, eosinophils, and shoenail-like cells, without an endometriotic background
(Hematoxylin and eosin stain; original magnification,
The patients’ clinical and pathological characteristics are summarized in Table 1. Patients in the OCCC with endometriosis group were younger than those in the
OCCC without endometriosis group (50.55
| OCCC with endometriosis (n = 83) | OCCC without endometriosis (n = 53) | p-value | ||
|---|---|---|---|---|
| Age, years (mean |
50.55 |
54.57 |
0.004 | |
| Menopausal status | 0.186 | |||
| Premenopausal | 31 (37.3%) | 14 (26.4%) | ||
| Postmenopausal | 52 (62.7%) | 39 (73.6%) | ||
| Gravidity | 0.420 | |||
| 29 (34.9%) | 15 (28.3%) | |||
| 54 (65.1%) | 38 (71.7%) | |||
| Parity | 0.296 | |||
| 0 | 13 (15.7%) | 5 (9.4%) | ||
| 70 (84.3%) | 48 (90.6%) | |||
| Dysmenorrhea | 28 (33.7%) | 17 (32.1%) | 0.841 | |
| Family history of cancer | 12 (14.5%) | 11 (20.8%) | 0.339 | |
| Initial symptom | ||||
| Adnexal mass | 59 (71.1%) | 32 (60.4%) | 0.196 | |
| Pelvic pain | 18 (21.7%) | 17 (32.1%) | 0.177 | |
| Postmenopausal bleeding | 2 (2.4%) | 3 (5.7%) | 0.326 | |
| Abnormal uterine bleeding | 4 (4.8%) | 2 (3.8%) | 0.772 | |
| Serum CA-125 (U/mL) | 0.063 | |||
| 40 (48.2%) | 17 (32.1%) | |||
| 43 (51.8%) | 36 (67.9%) | |||
| Tumor size (Max diameter) | 0.166 | |||
| 28 (33.7%) | 12 (22.6%) | |||
| 55 (66.3%) | 41 (77.4%) | |||
| Ovarian involvement | 0.561 | |||
| Unilateral | 80 (96.4%) | 52 (98.1%) | ||
| Bilateral | 3 (3.6%) | 1 (1.9%) | ||
| Ascites | 16 (19.3%) | 16 (30.2%) | 0.143 | |
| Lymphatic metastasis | 6 (7.2%) | 5 (9.4%) | 0.646 | |
| FIGO stage | 0.089 | |||
| I | 67 (80.7%) | 34 (64.2%) | ||
| II | 7 (8.4%) | 7 (13.2%) | ||
| III | 9 (10.8%) | 12 (22.6%) | ||
| IV | 0 | 0 | ||
| Phlebothrombosis | 5 (6.0%) | 11 (20.8%) | 0.009 | |
| Chemotherapy | 78 (94.0%) | 51 (96.2%) | 0.562 | |
| Chemotherapy periods | 0.526 | |||
| 18 (21.7%) | 14 (26.4%) | |||
| 65 (78.3%) | 39 (73.6%) | |||
| Chemoresistance | 3 (3.6%) | 6 (11.3%) | 0.078 | |
OCCC, ovarian clear cell carcinoma; CA-125,cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics.
The survival information for patients with and without endometriosis is summarized in Table 2. The death and recurrence rates in OCCC with endometriosis and OCCC without endometriosis groups were 8.4% vs. 24.5% and 12.1% vs. 28.9%, respectively. The 5-year and 3-year OS rates between the two groups were 89.8% vs. 57.9% and 95.4% vs. 68.8%, respectively. The 5-year and 3-year PFS rates between the two groups were 87.8% vs. 52.6% and 93.85% vs. 62.5%, respectively.
| OCCC with endometriosis (n = 83) | OCCC without endometriosis (n = 53) | p-value | ||
|---|---|---|---|---|
| Living condition | ||||
| Death | 7 (8.4%) | 13 (24.5%) | 0.014 | |
| Recurrence | 10 (12.1%) | 15 (28.9%) | 0.021 | |
| OS analysis | ||||
| Median survival time (months) | 56 | 26 | ||
| Range | 6–119 | 4–114 | ||
| 5-year OS rate | 89.9% | 57.9% | 0.003 | |
| 3-year OS rate | 95.4% | 68.8% | ||
| PFS analysis | ||||
| Median survival time (months) | 54 | 23 | ||
| Range | 3–119 | 4–114 | ||
| 5-year PFS rate | 87.7% | 52.6% | ||
| 3-year PFS rate | 93.85% | 62.5% | ||
OS, overall survival; PFS, progression-free survival; OCCC, ovarian clear cell carcinoma.
Kaplan–Meier univariate analysis using survival time as the dependent variable
was performed for various factors that might affect patient prognosis (Fig. 3).
The results showed that CA-125 level, endometriosis, tumor size, ascites, FIGO
stage, and chemotherapy resistance were significant prognostic factors for OS
(p
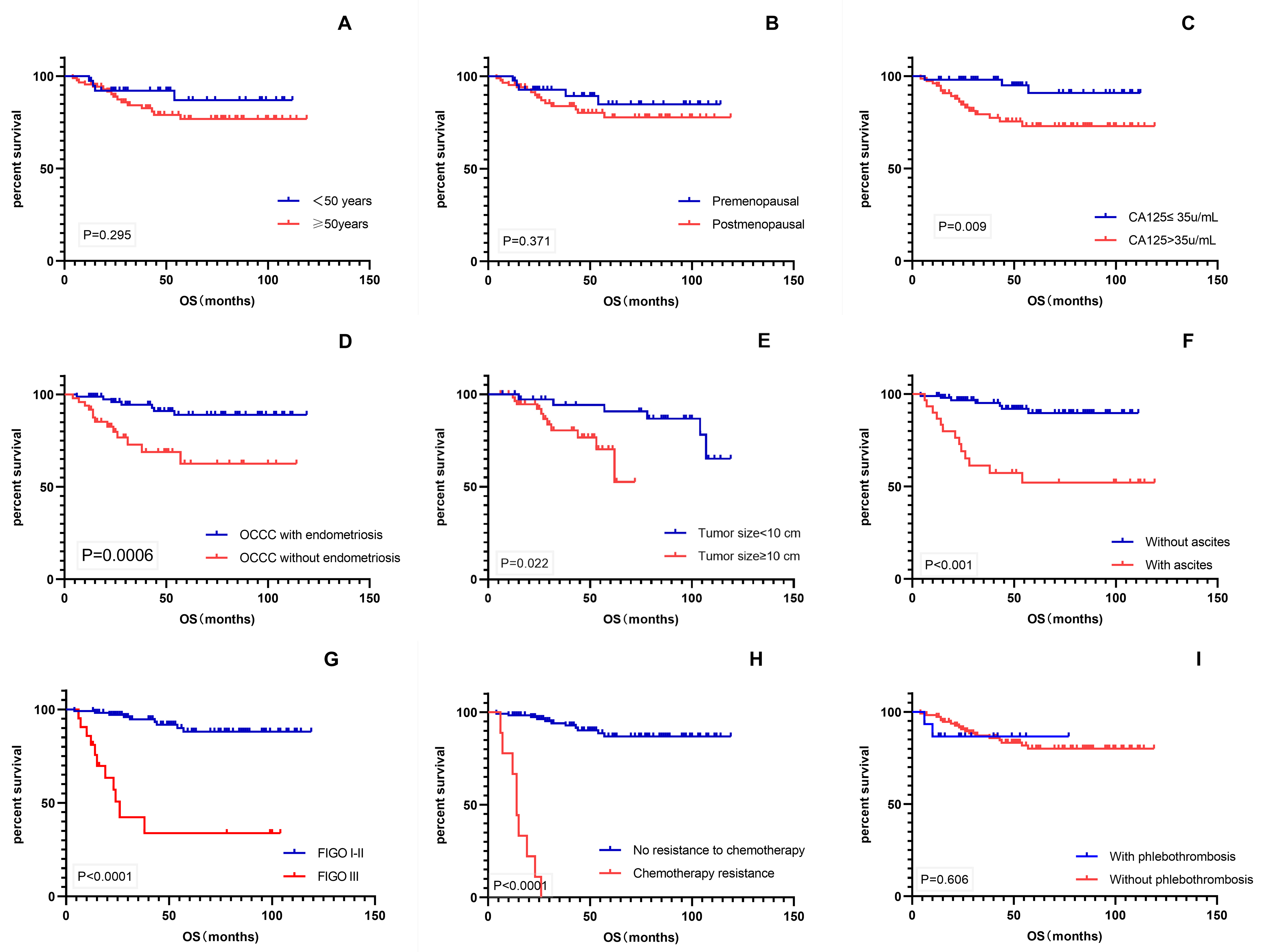 Fig. 3.
Fig. 3.Kaplan–Meier survival curves. (A–I) OS of patients with age, menopausal status, CA-125 level, endometriosis, tumor size, ascites, FIGO stage, chemotherapy resistance, and phlebothrombosis. OS, overall survival; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics.
Kaplan–Meier univariate analysis using survival time as the dependent variable
was performed for various factors that might affect patient prognosis (Fig. 4).
The results showed that CA-125 level, endometriosis, ascites, FIGO stage, and
chemotherapy resistance were significant prognostic factors for PFS (p
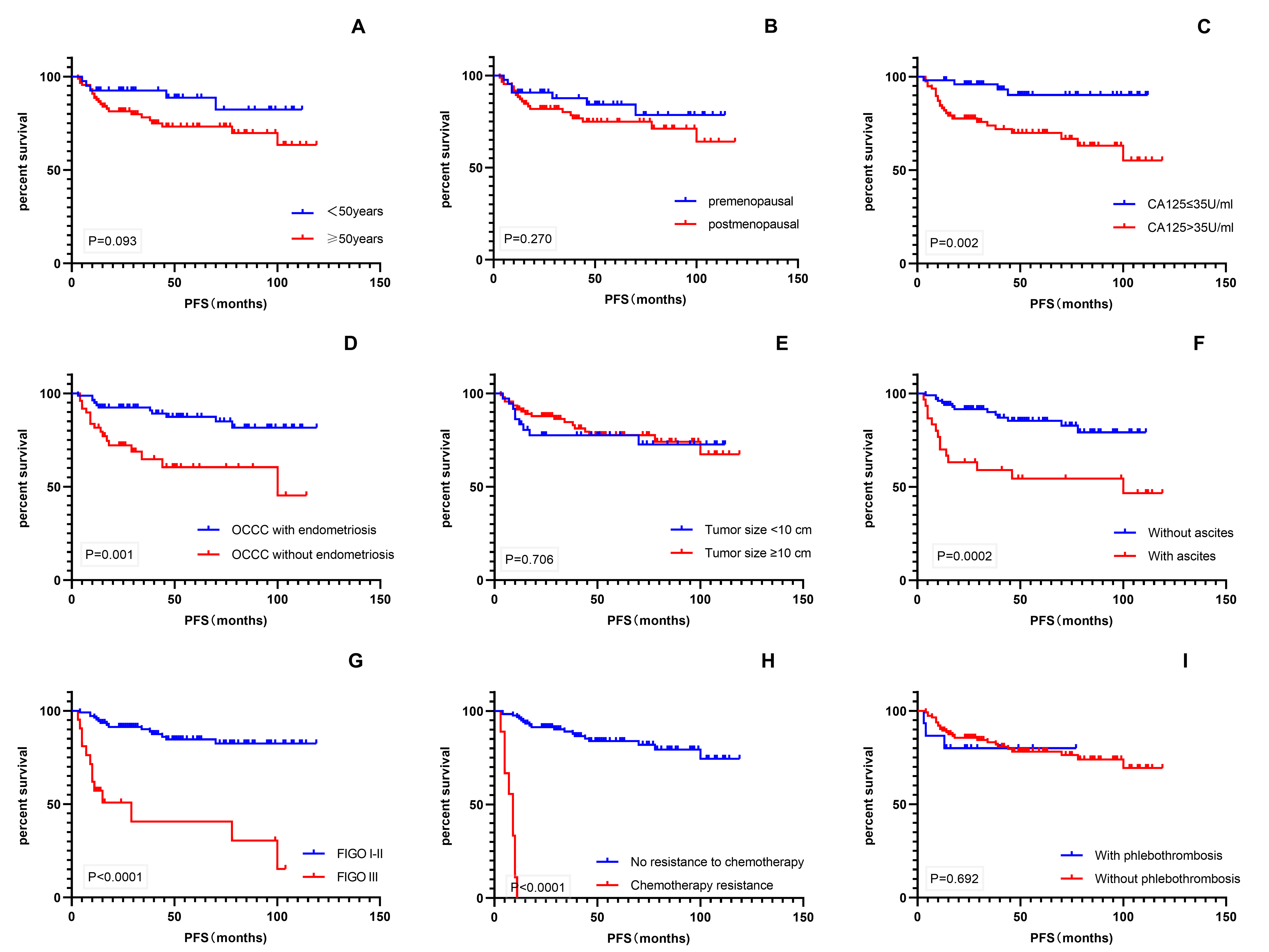 Fig. 4.
Fig. 4.Kaplan–Meier survival curves. (A–I) PFS of patients with age, menopausal status, CA-125 level, endometriosis, tumor size, ascites, FIGO stage, chemotherapy resistance, and phlebothrombosis. PFS, progression-free survival; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics.
The Cox proportional hazards model of pure OCCC survival is shown in Table 3. Multivariate analysis demonstrated that chemotherapy resistance and FIGO stage were independent prognostic factors, whereas endometriosis was not an independent predictor of survival.
| OS | PFS | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Endometriosis (yes vs. no) | 0.36 (0.12–0.18) | 0.068 | 1.1 (0.93–5.15) | 0.073 |
| Serum CA-125 (>35 vs. |
0.61 (0.15–2.43) | 0.610 | 1.13 (0.77–4.40) | 0.131 |
| Tumor size ( |
1.63 (0.55–4.82) | 0.377 | ||
| Ascites (yes vs. no) | 0.43 (0.13–1.44) | 0.172 | 1.31 (0.52–3.34) | 0.568 |
| FIGO stage (III vs. I–II) | 2.79 (1.37–10.60) | 2.28 (1.78–6.66) | ||
| Chemoresistance (yes vs. no) | 3.25 (1.81–14.25) | 10.58 (4.39–27.58) | ||
OS, overall survival; PFS, progression-free survival; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics.
In this study, OCCC with endometriosis occurred more frequently in younger women
and had lower deep venous thrombosis incidence. In univariate analysis, the
presence of endometriosis was related to improved PFS and OS rates in OCCC
patients (p
Endometriosis-associated ovarian cancer has distinctive clinical features. Most patients are 7–10 years younger than those without endometriosis and are diagnosed at an earlier stage [11, 14]. Our study showed that the OCCC with endometriosis group was four years younger than the OCCC without endometriosis group. Our data on the stage at diagnosis agree with the data that 80.7% of patients with endometriosis were in FIGO stage I versus 64.2% in patients without endometriosis, suggesting that the younger age is due to a higher number of stage I patients and early detection. Furthermore, early diagnosis is credited to the unique symptoms of endometriosis and the standardized long-term management of the disease [15].
It is well known that endometriosis is closely related to the occurrence and
development of OCCC. Ogawa et al. [16] reported that the incidences of
ovarian serous adenocarcinoma, mucinous adenocarcinoma, OCCC, and endometrioid
carcinoma complicated with endometriosis were 6.7%, 0%, 69.7%, and 42.9%,
respectively. In our study, 61% of OCCC cases were associated with
endometriosis, consistent with the published reports. Furthermore, studies have
shown that endometriosis increases the risk of developing OCCC three-fold [17].
Although OCCC and ovarian endometrioid carcinoma are widely recognized as
associated with endometriosis, the differentiation mechanism of these two
morphologically distinct tumors remains unclear. Studies investigating the
potential origins of OCCC concluded that it might arise from the endometrium,
endometriotic cyst epithelium, fallopian tube epithelial cells, or ovarian
surface epithelium [18, 19, 20]. Recent opinions favor an extra ovarian origin,
suggesting that the ovary provides fuel for the growing cancer cells [21].
Tsuchiya et al. [22] demonstrated for the first time that hepatocyte
nuclear factor 1
It has been reported that approximately 20% of patients with OCCC have venous thrombosis [17]. The possible mechanism might be FVII gene activation via the sterol regulatory element-binding protein-1 and glucocorticoid-induced leucine zipper pathway in OCCC cells. This pathway is activated by cholesterol starvation and hypoxia, producing procoagulant microvesicles and resulting in thrombosis [24]. In our study, 6.0% and 20.8% of the OCCC with endometriosis group and OCCC without endometriosis group were associated with lower extremity venous thrombosis. Given these results, it is tempting to speculate the existence of two OCCC subtypes. This question will be the focus of future research efforts.
To the best of our knowledge, endometriosis is closely related to OCCC. The loss of expression following mutations in the ARID1A and PI3KCA genes plays an essential role in the early transition from endometriosis to ovarian cancer [25, 26]. PIK3CA mutation increases cell invasion and metastasis by stimulating downstream AKT. In addition, activation of this pathway is related to cell cycle regulation and chemotherapy resistance in ovarian cancer. Therefore, it plays a vital role in the development and prognosis of ovarian cancer [27]. It has been suggested that patients with OCCC with endometriosis have a good prognosis [28]. However, other researchers found no difference in stage, grade, survival, and cancer incidence between patients with or without endometriosis [12, 14]. From the survival information of this study, patients with endometriosis appeared to have a good prognosis, such as lower mortality and recurrence rates. However, multivariate analysis showed that endometriosis was not an independent prognostic factor for pure OCCC. Larger prospective studies are required to validate the prognostic role of endometriosis.
Several studies have reported that FIGO staging was the main factor affecting
the OCCC prognosis. Kajiyama et al. [29] found the 5-year OS rates with
ovarian clear cell carcinoma were as follows: stage I (90.2%), stage II
(57.9%), and stage III/IV (39.3%), respectively (p
The consensus is that the current first-line chemotherapy regimens, namely platinum-based chemotherapy, have little effect on OCCC, with sensitivity rates of 11–27%. When combined with paclitaxel, platinum-based chemotherapy regimen sensitivity rates were 22–56% [31]. Goff et al. [32] assessed 24 patients with stage III OCCC who received conventional platinum-based chemotherapy and found that 70% of the patients had progressed. Cox regression analysis in our study indicated that chemotherapy resistance was an independent prognostic predictor. Consequently, new chemotherapeutic agents and protocols are needed to improve the efficacy of OCCC treatment.
OCCC differs from other ovarian epithelial carcinomas because of its distinctive characteristics. Presently, no effective treatment is available. Therefore, it is worth investigating the difference in prognosis between the two pure ovarian clear cell carcinoma subtypes. Further studies are needed to elucidate its molecular mechanism, discover new tumor markers and develop targeted drug therapy to improve the early diagnosis rate, overcome chemotherapy resistance, and improve the prognosis.
The strengths of this study include its relatively large cohort and relatively complete data. We rule out the mixed histology to avoid possible bias. Compared to the previous studies, our cohort seems more homogenous with all pure clear cell histology. The limitation of this work lies in the single-institutional retrospective design. The high proportion of stage I patients and the limited number of advanced patients may be one of the reasons why endometriosis did not affect the prognosis. Therefore, multicenter studies are needed for further confirmation.
In conclusion, patients of pure OCCC with endometriosis showed better prognosis. However, “endometriosis” could not be identified as an independent prognostic factor in pure OCCC. The prognostic trend shown in the study is clinically meaningful. Further large-scale, long-term studies focusing on OCCC are required.
OCCC, ovarian clear cell carcinoma; CA-125, cancer antigen 125; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; SIR, standardized incidence ratio.
All data generated or analyzed in the course of this study are included in this paper. Further enquiries can be directed to the corresponding author.
PQ and YG contributed to designing the study. YG collected the data and wrote the manuscript. WD contributed to data collection and data analysis. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Approval for this retrospective study was obtained from the Ethics Committee of the Tianjin Central Obstetrics and Gynecology Hospital (2019KY198). Informed consent was obtained from all individual participants included in the study.
We would like to express our gratitude to Guichun Tan for her constant encouragement and assistance.
This work was supported by the Tianjin Municipal Health Commission (grant no. KJ20098) and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-043A).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
