- Academic Editor
Background: Corifollitropin alfa (CFA) is a long-acting recombinant follicle-stimulating hormone (rFSH) used for controlled ovarian stimulation (COS). Several studies analyzing the clinical efficacy and safety of CFA compared to daily rFSH during COS have been carried out. The present study offers a meta-analysis of the randomized controlled trials (RCTs) on this topic. Methods: A computerized search of the published literature was carried out using PubMed, MEDLINE, Science direct and Google Scholar databases. The comparison between CFA and daily rFSH treatments during COS were investigated only in RCTs. The primary endpoint of the study is represented by the number of total oocytes retrieved at ovum pick-up. The studies included in the analysis were pooled together in order to estimate the log odds ratio (OR) or the mean difference (MD) along with the corresponding 95% confidence intervals (CI) by using a random effects model. The heterogeneity between the studies was evaluated with the Higgins and Chi-square tests. Results: The study examined a total of twelve RCTs published from 2004 to date and included a total of 4980 patients, with 2664 receiving CFA and 2316 patients receiving daily rFSH for COS. Women treated with CFA had higher number of total oocytes retrieved at ovum pick-up (MD 0.91, 95% CI [0.34, 1.49], p = 0.001), and higher number of metaphase II (MII) oocytes (MD 1.00, 95% CI [0.37, 1.62], p = 0.002) compared to those receiving daily rFSH. There were no significant differences between the two study groups regarding the other outcomes analyzed. The subgroup analysis performed comparing “normal” versus “poor” responders revealed that normal responders receiving CFA showed an higher cancellation rate, with respect to those receiving rFSH. Conclusions: This study shows that COS with CFA results in a higher number of oocytes retrieved at ovum pick-up in comparison with daily rFSH.
Corifollitropin alfa (CFA) is a long-acting recombinant follicle stimulating hormone (rFSH) used for the controlled ovarian stimulation (COS) during in vitro fertilization (IVF) programs [1].
The molecular structure of CFA is a heterodimer composed by the FSH
CFA is administered as a single injection from day 2 or 3 of menstrual cycle
and, if needed, daily injections of rFSH are given from day 8 of stimulation [7].
Age and weight of patients are factors to consider when determining the optimal
CFA dose. The optimal doses are 100
The clinical effectiveness and safety of CFA compared to daily rFSH during COS represent the topic of several randomized controlled trials (RCTs). The aim of this study is to provide an updated meta-analysis pooling the data of the RCTs published to date on this matter.
The results of this study are reported according to the guidelines outlined in the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [8].
The target population was represented by infertile couples undergoing IVF/intracytoplasmic sperm injection (ICSI) or eggs donation. Only RCTs comparing CFA and daily rFSH treatments during COS and RCTs assessing the clinical effectiveness and safety of CFA were analyzed.
The primary endpoint of the present meta-analysis is represented by the number of oocytes retrieved at ovum pick-up, as suggested by the European medicines agency (EMA) for the comparison between gonadotropins [9].
The additional considered outcomes were: total duration of stimulation, cycle cancellation rate, number of metaphase II (MII) oocytes retrieved, fertilization rate, number of embryos obtained, implantation rate, clinical pregnancy rate, ongoing pregnancy rate, live birth rate, miscarriage rate, and incidence of ovarian hyperstimulation syndrome (OHSS). Randomized controlled trials with primary endpoints different from IVF outcomes but likewise exploring the clinical parameters considered in our study were also included in the present meta-analysis.
Articles not written in English and studies different from RCTs together with abstracts, editorials, letters to the editor, comments and studies with no control group were excluded.
A computerized search of the published literature was carried out using PubMed, MEDLINE, Science direct and Google Scholar databases. We considered RCTs published up to 2020. The search strategy included different terms such as ART, CFA, rFSH, IVF programs. In PubMed we utilized keywords as follows: (“IVF” OR in vitro fertilization) OR (“ART” OR assisted reproductive technology) and (“CFA” OR corifollitropin alpha) OR (“rFSH” OR recombinant follicle stimulating hormone).
Two investigators independently screened titles and abstracts of the studies. The same authors independently assessed the RCTs for inclusion according to the selection criteria and extracted data about study features. The investigators manually collected data from the studies. The items collected from each study were as follows: the first author’s name, the year of publication, study design, study setting, participant characteristics (intervention and control groups), CFA administration during COS and IVF outcomes. Any disagreements about inclusion were resolved through discussion or by consultation with a third researcher.
The critical assessment of the study quality was performed in accordance with the Cochrane Risk Assessment Tool [10] by two researchers who worked independently. The tool includes the following domains of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. Each domain was assessed and classified as low, unclear, or high risk of bias. Any discrepancies in the evaluation of studies quality were resolved by discussion with a third investigator.
The current meta-analysis was conducted using the R Package Metafor version
2.1–0 (Wolfgang Viechtbauer, Maastricht, The Netherlands) [11]. We considered a
group of women treated with CFA and a control group treated with daily rFSH. The
studies included in the analysis were pooled together. According to the nature of
data, we used as outcome measure the log odds ratio (OR) or the raw mean
difference (MD) and calculated the summary estimates along with the corresponding
95% confidence intervals (CI) by using a random effects model, i.e., assuming
that data were drawn from a hierarchy of different populations. Where studies
reported data in terms of mean and range instead of standard deviation (SD), the
SD was approximated by one-fourth of the range of data [12]. The Higgins index
(I
The studies selection is summarized in Fig. 1. After reading the article titles and abstracts, 116 studies were screened, and 102 studies were discarded because they did not respect the study selection criteria. Of the remaining 14 studies, 2 were excluded after a full text evaluation. A total of 12 studies were included in the present meta-analysis [13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24]. The publication years ranged from 2004 to date and the characteristics of the included studies are summarized in Table 1 (Ref. [13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24]).
 Fig. 1.
Fig. 1.Flow chart of the studies selection.
| Authors | Participants | Intervention | Comparator | Outcomes |
|---|---|---|---|---|
| Devroey et al. (2004) [13] | Patients randomized: | -From day 2 or 3 of the menstrual cycle: single s.c injection of CFA (120, 180, or 240 µg) + 150 IU rFSH from stimulation day 8 up to and including the day of hCG | -From day 2 or 3 of menstrual cycle: fixed daily s.c dose of 150 IU rFSH up to and including the day of hCG | -Higher mean number of oocytes recovered per started cycle in CFA group compared to rFSH group |
| n = 75 with CFA | -GnRH antagonist (ganirelix acetate, 0.25 mg) starting on the day that the leading follicle had reached 14 mm | -GnRH antagonist (ganirelix acetate, 0.25 mg) starting on the day that the leading follicle had reached 14 mm | -No differences in the number of good quality embryos between the two study groups | |
| n = 24 with rFSH | -Final oocyte maturation trigger: 10000 IU hCG | -Final oocyte maturation trigger: 10000 IU hCG | -Equal numbers of embryos available for ET between the two study groups | |
| Patients treated: | -Luteal phase support: vaginal micronized P (600 mg/d) or i.m P ( |
-Luteal phase support: vaginal micronized P (600 mg/d) or i.m progesterone ( |
||
| n = 74 with CFA | ||||
| n = 24 with rFSH | ||||
| Characteristics: | ||||
| -Women aged 18–39 years, BMI 17–31 kg/m |
||||
| -Regular menstrual cycle (24–35 days). | ||||
| Corifollitropin alfa dose-finding, (2008) [14] | Patients randomized: | -From day 2 or 3 of the menstrual cycle: single s.c dose of 60, 120, or 180 µg corifollitropin alfa + 150 IU (from stimulation day 8) rFSH (follitropin beta) up to the day of hCG | -From day 2 or 3 of the menstrual cycle: 150 IU rFSH up to the day of hCG | -Dose-related increase in multifollicular development and in the number of retrieved oocytes in CFA group |
| n = 242 with CFA | -GnRH antagonist (ganirelix acetate, 0.25 mg) from stimulation day 5 up to and including the day of hCG | -GnRH antagonist (ganirelix acetate, 0.25 mg) from stimulation day 5 up to and including the day of hCG | -The optimal dose for a 1-week interval is higher than 60 µg and lower than 180 µg | |
| n = 83 with rFSH (follitropin beta) | -Final oocyte maturation trigger: 10000 IU hCG | -Final oocyte maturation trigger: 10000 IU hCG | ||
| Patients treated: | -Luteal phase support: P administered daily | -Luteal phase support: P administered daily | ||
| n = 234 with CFA | ||||
| n = 81 with rFSH | ||||
| Characteristics: | ||||
| -Women aged 20–39 years | ||||
| -Normal menstrual cycle (24–35 days) | ||||
| -BMI 17–31 kg/m |
||||
| Devroey et al. (2009) [15] | Patients randomized: | -From menstrual cycle day 2 or 3: s.c injection of 150 µg CFA, or matching placebo + rFSH from day 8 up to and including the day of hCG administration | -From menstrual cycle day 2 or 3: placebo + 200 IU rFSH up to and including the day of hCG | -Ongoing pregnancy rates of 38.9% for the CFA group and 38.1% for rFSH |
| n = 757 with CFA | -GnRH antagonist (ganirelix, 0.25 mg) once daily s.c. starting on stimulation day 5 up to and including the day of hCG | -GnRH antagonist (ganirelix acetate, 0.25 mg) starting on stimulation day 5 up to and including the day of hCG | -Higher follicular response with CFA with higher number of COCs compared with rFSH | |
| n = 752 with rFSH (follitropin beta) | -Final oocyte maturation trigger: 5000–10000 IU urinary hCG | -Final oocyte maturation trigger 5000–10000 IU urinary hCG | -Equal median duration of stimulation and incidence of OHSS between the study groups | |
| Patients treated: | -Luteal phase support: P |
-Luteal phase support: P |
||
| n = 756 with CFA | ||||
| n = 750 with rFSH | ||||
| Characteristics: | ||||
| -Women aged 18–36 years with a body weight |
||||
| -BMI of 18–32 kg/m |
||||
| -Menstrual cycle length of 24–35 days | ||||
| Corifollitropin alfa Ensure study group (2010) [16] | Patients randomized and treated: | -From day 2 or 3 of menstrual cycle: single s.c injection of 100 µg CFA + |
-From day 2 or 3 of menstrual cycle: placebo + 150 IU rFSH (follitropin beta) + |
-The mean |
| n = 268 with CFA | -GnRH antagonist (ganirelix acetate, 0.25 mg) starting on stimulation day 5 | -GnRH antagonist (ganirelix acetate, 0.25 mg) starting on stimulation day 5 | -The incidence of moderate and severe OHSS of 3.4% for CFA group and 1.6% for rFSH | |
| n = 128 with rFSH | -Final oocyte maturation trigger: 5000–10000 IU urinary hCG | -Final oocyte maturation trigger: 5000–10000 IU urinary hCG | ||
| Characteristics: | -Luteal phase support: P |
-Luteal phase support: P |
||
| -Women aged 18–36 years with body weight |
||||
| -BMI 18–32 kg/m |
||||
| -Normal menstrual cycle length (24–35 days) | ||||
| Requena et al. (2013) [17] | Patients randomized: | -Oral contraceptive pill for a maximum of 21 days preceded ovarian stimulation | -Oral contraceptive pill for a maximum of 21 days preceded ovarian stimulation | -Significant difference in the median duration of stimulation, between stimulation with CFA and daily rFSH (10.83 |
| n = 63 with CFA | -After a wash-out period of 5 days after the last pill: single injection of 150 µg CFA + daily s.c. administration of rFSH 200 IU (if needed) from stimulation day 8 | -After a wash-out period of 5 days after the last pill: daily s.c doses of 200 IU rFSH | -No significant differences in clinical parameters between the two protocols | |
| n = 68 with rFSH (follitropin beta) | -GnRH antagonist (ganirelix acetate, 0.25 mg) started on day 5 of stimulation | -GnRH antagonist (ganirelix acetate, 0.25 mg) started on day 5 of stimulation | ||
| Patients treated: | -Final oocyte maturation trigger: single dose of 0.1 mg GnRH agonist | -Final oocyte maturation trigger: single dose of 0.1 mg GnRH agonist | ||
| n = 59 with CFA | ||||
| n = 61 with rFSH | ||||
| Characteristics: | ||||
| -Oocyte donors aged 18-35 years with a regular menstrual cycle | ||||
| -No hereditary or chromosomal diseases, normal karyotype, negative when screened for sexually transmitted diseases | ||||
| -At least 7 antral follicles at the beginning of the cycle | ||||
| -Body weight |
||||
| Kolibianakis et al. (2015) [18] | Patients randomized and treated: | -From day 2 of menstrual cycle: single s.c dose of 150 µg CFA + 450 IU of rFSH administered from Day 8 of stimulation until the day of hCG administration | -From day 2 of menstrual cycle: seven fixed daily doses of 450 IU rFSH | -Number of COCs retrieved not statistically different between the two study groups |
| n = 40 with CFA | -GnRH antagonist (ganirelix acetate, 0.25 mg) when the leading follicle reached 14 mm in average diameter up to the day of hCG administration | -GnRH antagonist (ganirelix acetate, 0.25 mg) when the leading follicle reached 14 mm in average diameter up to the day of hCG administration | -No significant difference regarding the probability of live birth between the two study groups | |
| n = 39 with rFSH (follitropin beta) | -Final oocyte maturation trigger: 250 µg of rhCG | -Final oocyte maturation trigger: 250 µg of rhCG | ||
| Characteristics: | -Luteal phase support: vaginal micronized P (600 mg/day) | -Luteal phase support: vaginal micronized P (600 mg/day) | ||
| -Women with previous poor response to ovarian stimulation ( |
||||
| -Age |
||||
| -Regular spontaneous menstrual cycle | ||||
| -BMI of 18–32 kg/m |
||||
| Boostanfar et al. (2015) [19] | Patients randomized: | -From day 2 or 3 of menstrual cycle: single injection of 150 µg of CFA + seven injections of placebo rFSH from stimulation days 1–7 + treatment with open-label daily |
-From day 2 or 3 of menstrual cycle: injection of placebo CFA + seven injections of 300 IU rFSH from stimulation days 1–7 + treatment with open-label daily |
-Vital PRs per started cycle of 23.9% in the CFA group and 26.9% in the rFSH group |
| n = 695 with CFA | -GnRH antagonist (ganirelix acetate, 0.25 mg/d) starting on stimulation day 5 | -GnRH antagonist (ganirelix acetate, 0.25 mg/d) starting on stimulation day 5 | -Mean (SD) number of recovered oocytes per started cycle of 10.7 (7.2) and 10.3 (6.8) in the CFA and the r FSH groups, respectively | |
| n = 696 with rFSH | -Final oocyte maturation trigger: rhCG | -Final oocyte maturation trigger: rhCG | -LBRs per started cycle of 21.3% in the CFA group and 23.4% in the rFSH group | |
| Patients treated: | -Luteal phase support: intravaginal P gel | -Luteal phase support: intravaginal P gel | -Incidence of SAEs of 0.4% versus 2.7% in the CFA and rFSH groups | |
| n = 694 with CFA | -OHSS (all grades) of 1.7% in both groups | |||
| n = 696 with rFSH | ||||
| Characteristics: | ||||
| -Women aged |
||||
| -History of regular spontaneous menstrual cycles (cycle length, 24–35 days) | ||||
| -Patients with normal thyroid function | ||||
| -Access to ejaculatory sperm for IVF or intracytoplasmic sperm injection (ICSI) | ||||
| Drakopoulos et al. (2017) [20] | Patients randomized: | - From day 2 of menstrual cycle: single s.c injection of 150 |
-From day 2 of the menstrual cycle: daily dose of rFSH (300 IU/day) administered up to the day of hCG administration | -No differences in the ongoing pregnancy rates between the two study groups |
| n = 77 with CFA | -GnRH antagonist ganirelix acetate (0.25 mg/d) starting on stimulation day 6 | -GnRH antagonist ganirelix acetate (0.25 mg/d) starting on stimulation day 6 | -Biochemical pregnancy rate, CPRs, LBR and number of oocytes retrieved comparable between the two groups | |
| n = 75 with rFSH | -Final oocyte maturation trigger: 10000 IU hCG | -Final oocyte maturation trigger: 10000 IU hCG | -More patients in the CFA group with cryopreserved embryos compared to the rFSH group (28.6% versus 14.3%, respectively) | |
| Patients treated: | -Luteal phase support: progesterone tablets intravaginally | -Luteal phase support: progesterone tablets intravaginally | -Asian patients with significantly lower cancellation rates compared to European poor responders (3.1% versus 20.4%, respectively) | |
| n = 77 with CFA | ||||
| n = 72 with rFSH | ||||
| Characteristics: | ||||
| -Patients younger than 40 years old, fulfilling the Bologna criteria for poor ovarian response | ||||
| -Patients with the cut-off of AMH |
||||
| -Patients with AFC (measured on Day 2–4 of a previous cycle) with the cut-off |
||||
| Cruz et al. (2017) [21] | Patients randomized: | -Oral contraceptive pill taken for a maximum of 21 days, starting on day 1 or 2 of menses of the previous cycle | -Oral contraceptive pill taken for a maximum of 21 days, starting on day 1 or 2 of menses of the previous cycle | -No statistical differences in the mean of transferred embryos or frozen embryos in each treatment group |
| n = 68 with CFA | -After a wash-out period of 5 days after the last pill: 100 µg of CFA + daily administration of rFSH from stimulation day 8 | -After a wash-out period of 5 days after the last pill: daily doses of 150 IU rFSH or 225 IU hp-HMG | -Implantation rate and CPRs similar among the groups of study | |
| n = 69 with rFSH | -GnRH antagonist ganirelix acetate (0.25 mg/d) starting on stimulation day 6 | -GnRH antagonist ganirelix acetate (0.25 mg/d) starting on stimulation day 6 | ||
| Patients treated: | -Final oocyte maturation trigger: 0.1 mg GnRH agonist | -Final oocyte maturation trigger: 0.1 mg GnRH agonist | ||
| n = 59 with CFA | ||||
| n = 63 with rFSH | ||||
| Characteristics: | ||||
| -Healthy women aged between 18 and 35 years | ||||
| -Regular menstrual cycles | ||||
| -No hereditary or chromosomal diseases, with normal karyotype and negative for sexually transmitted | ||||
| -At least six antral follicles per ovary at the beginning of the cycle | ||||
| -Weigh less than 60 kg | ||||
| Vuong et al. (2017) [22] | Patients randomized and treated: | -From day 2 or 3 of the menstrual cycle: single s.c injection of CFA 150 µg + daily doses of rFSH from stimulation day 8, up to the day before the final trigger of ovulation | -From day 2 or 3 of the menstrual cycle: daily injection of rFSH 300 IU/day continuing up to and including stimulation day 7 + daily dose of rFSH from stimulation day 8 up to the day before the final trigger of ovulation | -No significant difference between the CFA and rFSH groups for the number of oocytes retrieved |
| n = 200 with CFA | -GnRH antagonist (ganirelix acetate 0.25 mg in 0.5 ml s.c) from day 5 of stimulation | -GnRH antagonist (ganirelix acetate 0.25 mg in 0.5 mL SC) from day 5 of stimulation | -Similar ongoing pregnancy rate and LBRs in both the treatment groups | |
| n = 200 with rFSH (follitropin beta) | -Final oocyte maturation trigger: rhCG | -Final oocyte maturation trigger: rhCG | -Low and similar complication rates in the CFA and rFSH groups | |
| Characteristics: | -Luteal phase support: 50 mg P i.m and estradiol (2 mg/day orally) | -Luteal phase support: 50 mg P i.m and estradiol (2 mg/day orally) | -No significant differences in obstetric outcomes between the study groups | |
| -Patients from Vietnam aged 35–42 years with a body weight of |
||||
| -Regular spontaneous menstrual cycle | ||||
| -AMH |
||||
| Sorouri et al. (2019) [23] | Patients randomized and treated: | -From day 2 or 3 of the menstrual cycle: single s.c injection of CFA 150 µg + daily doses of rFSH from stimulation day 8, up to the day before the final trigger of ovulation | - From day 2 or 3 of the menstrual cycle: 150 IU of daily r-FSH | -No significant difference between the two groups in terms of stimulation duration, number of follicles, number of oocytes, total number of embryos, and number of transferred embryos |
| n = 54 with CFA | -GnRH antagonist (ganirelix acetate 0.25 mg in 0.5 mL SC) from day 5 of stimulation | -GnRH antagonist (ganirelix acetate 0.25 mg in 0.5 mL SC) from day 5 of stimulation | -No significant differences regarding the pregnancy outcomes including chemical pregnancy rate (positive pregnancy test), clinical pregnancy rate (detection of fetal heart), the rate of ovarian hyper-stimulation syndrome, multiple pregnancy, ectopic pregnancy, and miscarriage between the two study groups | |
| n = 55 with rFSH | -Final oocyte maturation trigger: rhCG | -Final oocyte maturation trigger: rhCG | ||
| Characteristics: | -Luteal phase support: 100 mg per day progesterone from the day of OPU and 150 mg per day after embryo transfer | -Luteal phase support: 100 mg per day progesterone from the day of OPU and 150 mg per day after embryo transfer | ||
| -Age between 18–36 years | ||||
| -Regular menstruations | ||||
| -Body mass index (BMI) between 19–30 kg/m |
||||
| -Presence of two ovaries, having an ultrasound within the last 6 weeks and no problems in the uterus | ||||
| -FSH on second-fourth day of menstruation below 10 | ||||
| -Normal thyroidstimulating hormone | ||||
| -Sperm analysis at acceptable level for ICSI (sperm count being not less than 5 million) | ||||
| Fusi et al. (2020) [24] | Patients randomized and treated: | -From day 1 or 2 of the menstrual cycle: injection of CFA 100 µg or 150 µg + 300 IU rFSH and 150 IU rLH from the 5th day after CFA injection | -From day 3 of the menstrual cycle: administration of 300 IU of rFSH and 150 IU rLH or 300 IU HMG | -Number of retrieved oocytes different between CFA protocols and the control group |
| n = 136 with CFA | -GnRH antagonist (ganirelix acetate 0.25 mg) when the leading follicle reached 13 mm | -GnRH antagonist (ganirelix acetate 0.25 mg) when the leading follicle reached 13 mm | -Higher pregnancy rates, especially in the long protocol with CFA | |
| n = 136 with rFSH | -Final trigger of oocyte maturation: 10000 IU of hCG | -Final trigger of oocyte maturation: 10000 IU of hCG | -Shorter length of stimulation with CFA treatments compared to rFSH | |
| Characteristics: | ||||
| -AFC |
||||
| -AMH |
||||
| -Less than three oocytes obtained in the previous cycle | ||||
| -Age |
P, Progesterone; AFC, antral follicle count; AMH, anti-mullerian hormone; AEs, adverse effects; BMI, body mass index; CFA, corifollitropin alfa; COCs, cumulus-oocyte complexes; COS, controlled ovarian stimulation; CPRs, clinical pregnancy rates; ET, embryo transfer; FSH, follicle stimulating-hormone; hCG, human chorionic gonadotropin; hMG, human menopausal gonadotropin; HP-HMG, highly purified human menopausal gonadotropin; i.m, intramuscular; LBR, live birth rate; LH, luteinizing-hormone; OHSS, ovarian hyperstimulation syndrome; P, progesterone; PRs, pregnancy rates; rFSH, recombinant follicle stimulating-hormone; rhCG, recombinant human chorionic gonadotropin; s.c, subcutaneous; SAEs, severe adverse effects; SD, standard deviation.
We notice that all analyses were carried out with an intention-to-treat approach. Twelve RCTs have been included in the analysis, considering a total of 4980 patients (2664 receiving CFA and 2316 receiving daily rFSH). Data were typically presented per woman randomized or started cycle. However, the implantation and fertilization rates were restricted only to patients with embryo transfer and subjects undergoing IVF and/or ICSI, respectively, whereas the miscarriage rate was presented per clinical pregnancy.
A summary of risk of bias is presented in Fig. 2. Selection bias in the included studies was “unclear” considering that not all the trials reported adequate random sequence generation and detailed methods of allocation concealment. All the studies are blinded with “low” risk of performance with the exception of Devroey et al. [13] Corifollitropin alfa dose-finding [14], Requena et al. [17], Drakopoulos et al. [20], Cruz et al. [21], Sorouri et al. [23] and Fusi et al. [24]. No studies were assessor blinded and for this reason they were judged to be at “unclear” risk of detection bias, while regarding the attritition bias not all the studies reported complete outcome data with the exception of Devroey et al. [15], Boonstanfar et al. [19] and Vuong et al. [22]. Low risk of bias was reported concerning the reporting bias and no other sources of bias were detected but we judged these as “unclear” risk of bias.
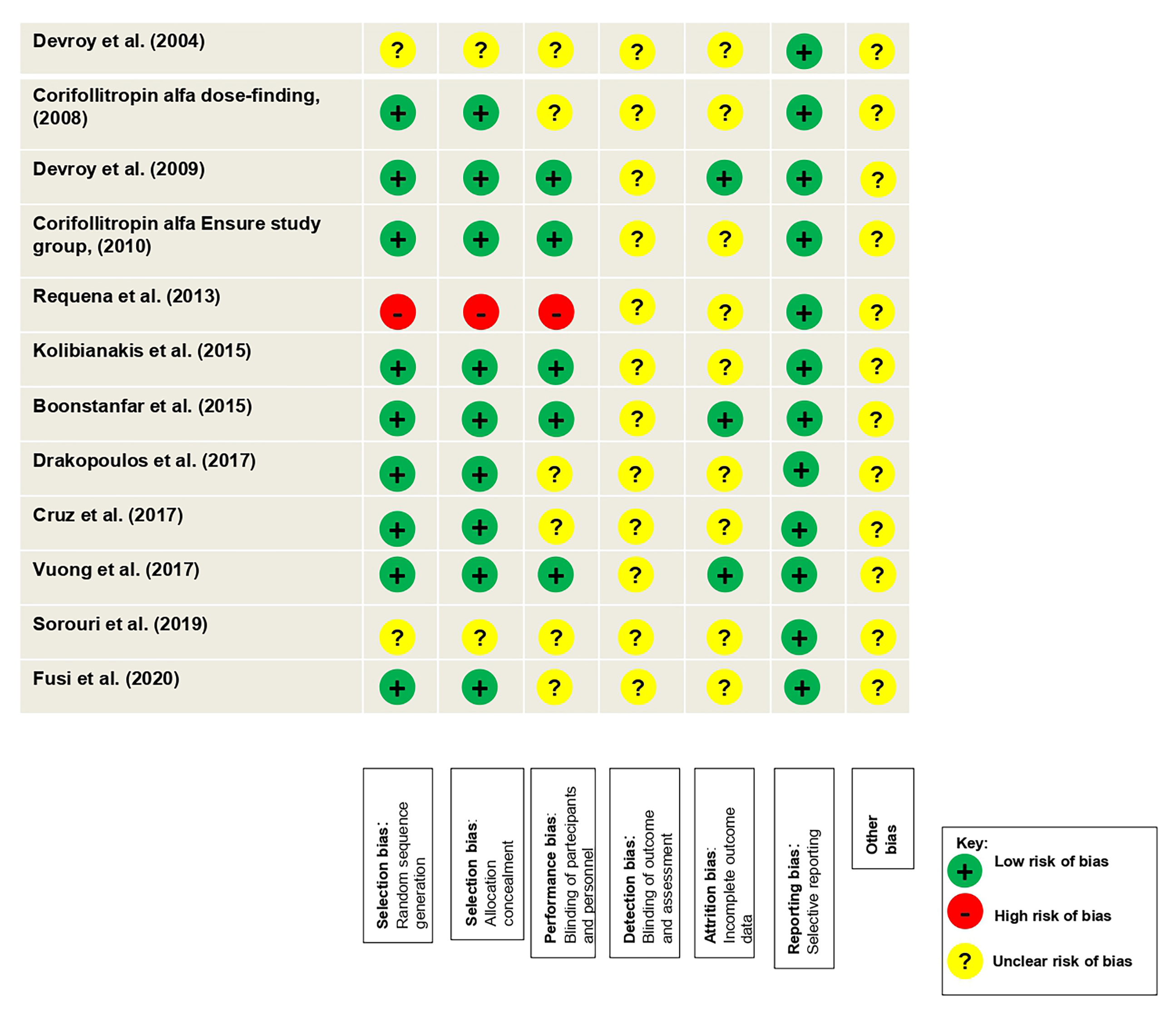 Fig. 2.
Fig. 2.Risk assessment of bias for the randomized controlled studies (RCTs) included in the meta-analysis.
3.3.1.1 Total duration of stimulation
There was no evidence of a statistically significant difference (MD –0.17, 95%
CI [–1.13, 0.79], p = 0.73; 9 RCTs, n = 4420; substantial
heterogeneity: I
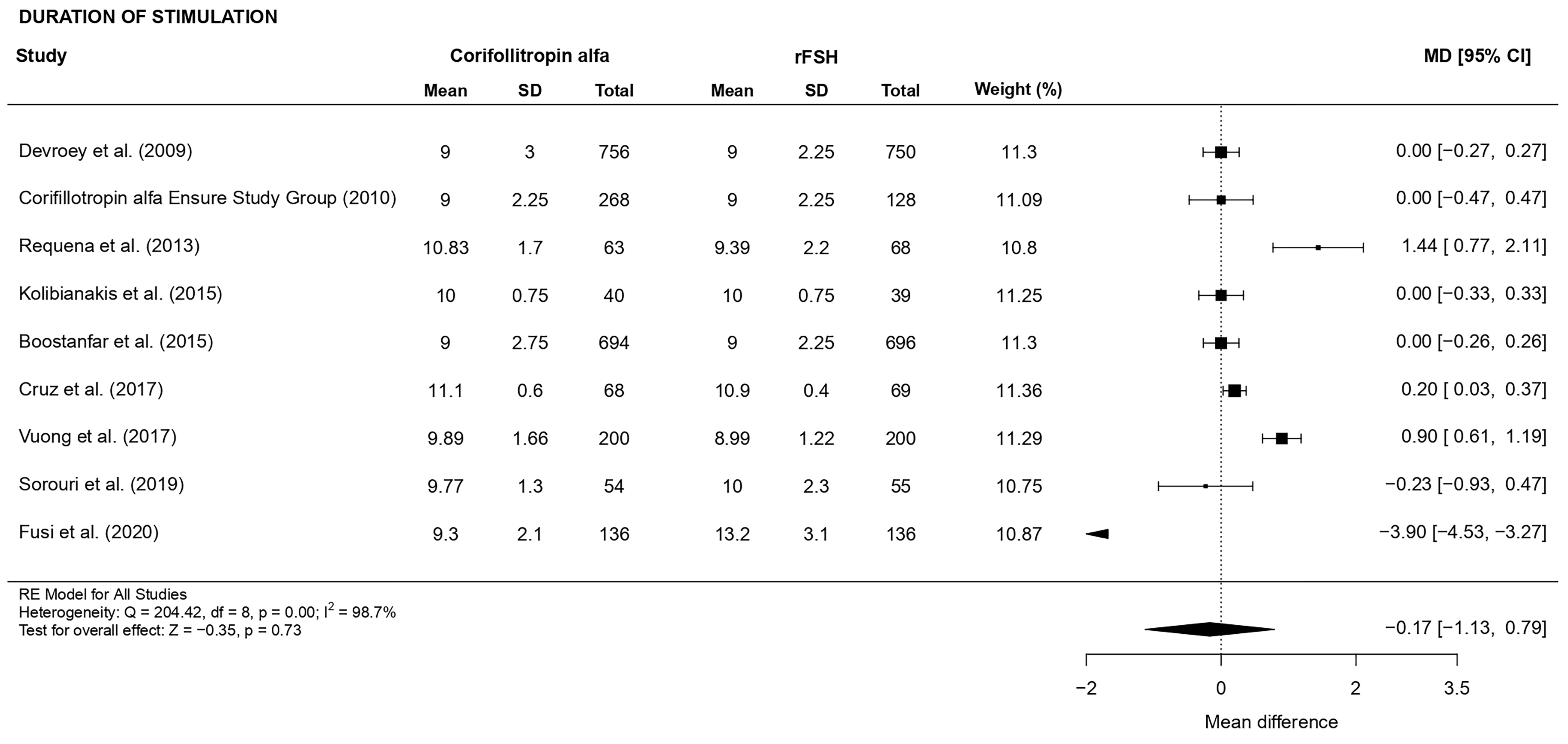 Fig. 3.
Fig. 3.Forest plot of the total duration of stimulation.
3.3.1.2 Number of oocytes retrieved (Primary outcome)
There was a statistically significant higher number of oocytes retrieved (MD
0.91, CI [0.34, 1.49], p = 0.001; 8 RCTs, n = 3700; substantial
heterogeneity: I
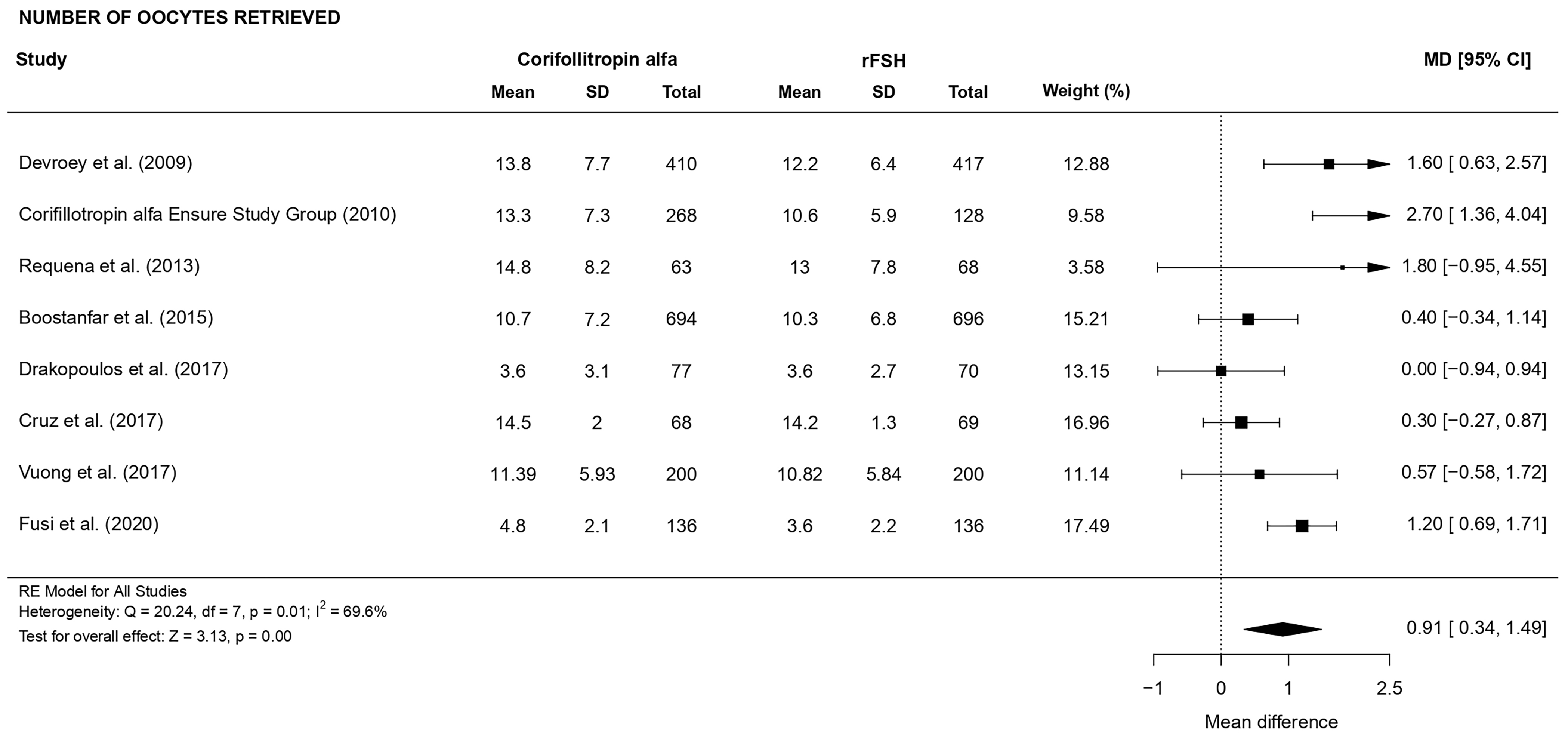 Fig. 4.
Fig. 4.Forest plot of the number of oocytes retrieved.
3.3.1.3 Number of MII oocytes retrieved
There was a statistically significant higher number of MII oocytes retrieved (MD
1.00, CI [0.37, 1.62], p = 0.002; 9 RCTs, n = 3314; substantial
heterogeneity: I
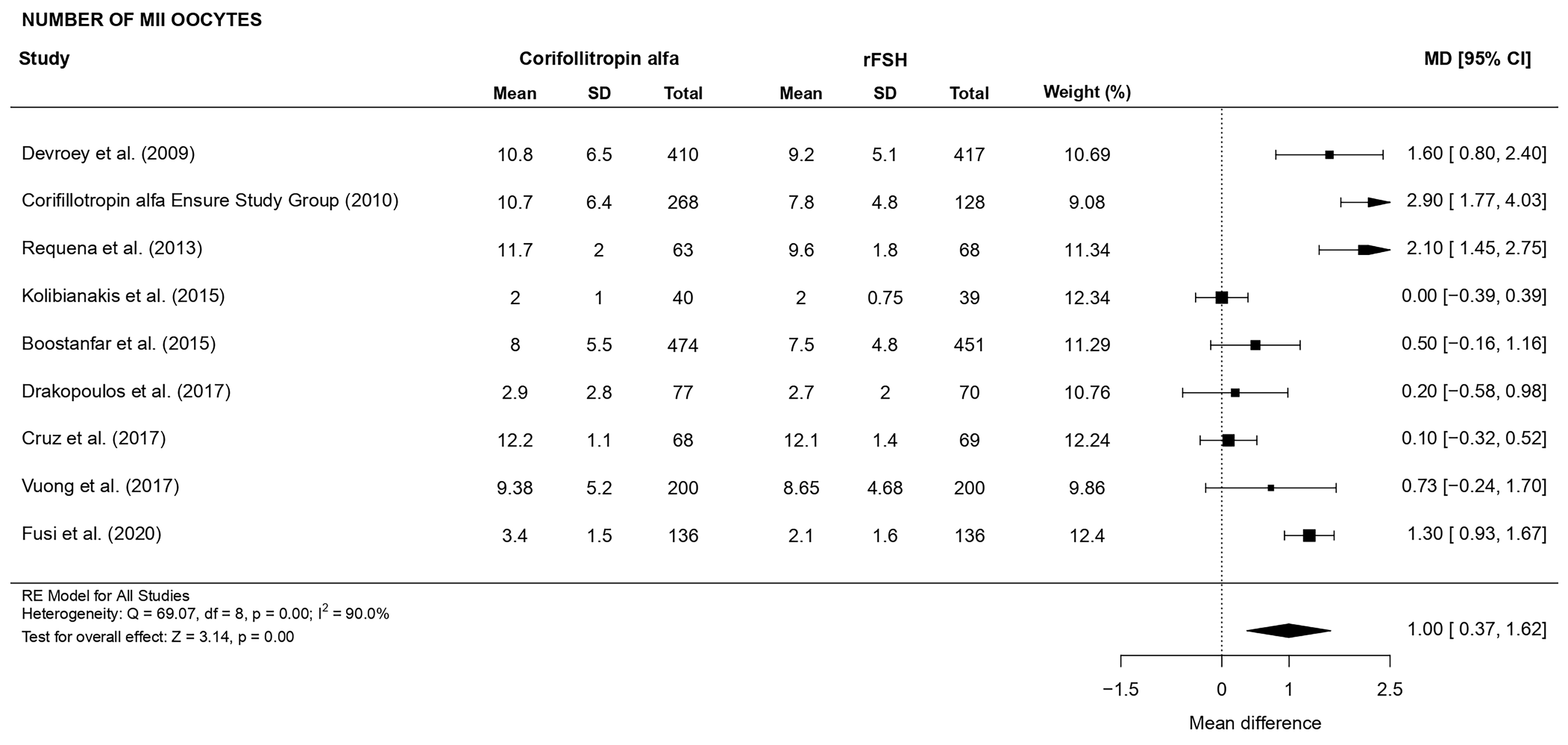 Fig. 5.
Fig. 5.Forest plot of the number of MII oocytes.
3.3.1.4 Fertilization rate
There was no evidence of a statistically significant difference (OR 0.94, CI
[0.81, 1.08], p = 0.38; 6 RCTs, n = 3584; no heterogeneity: I
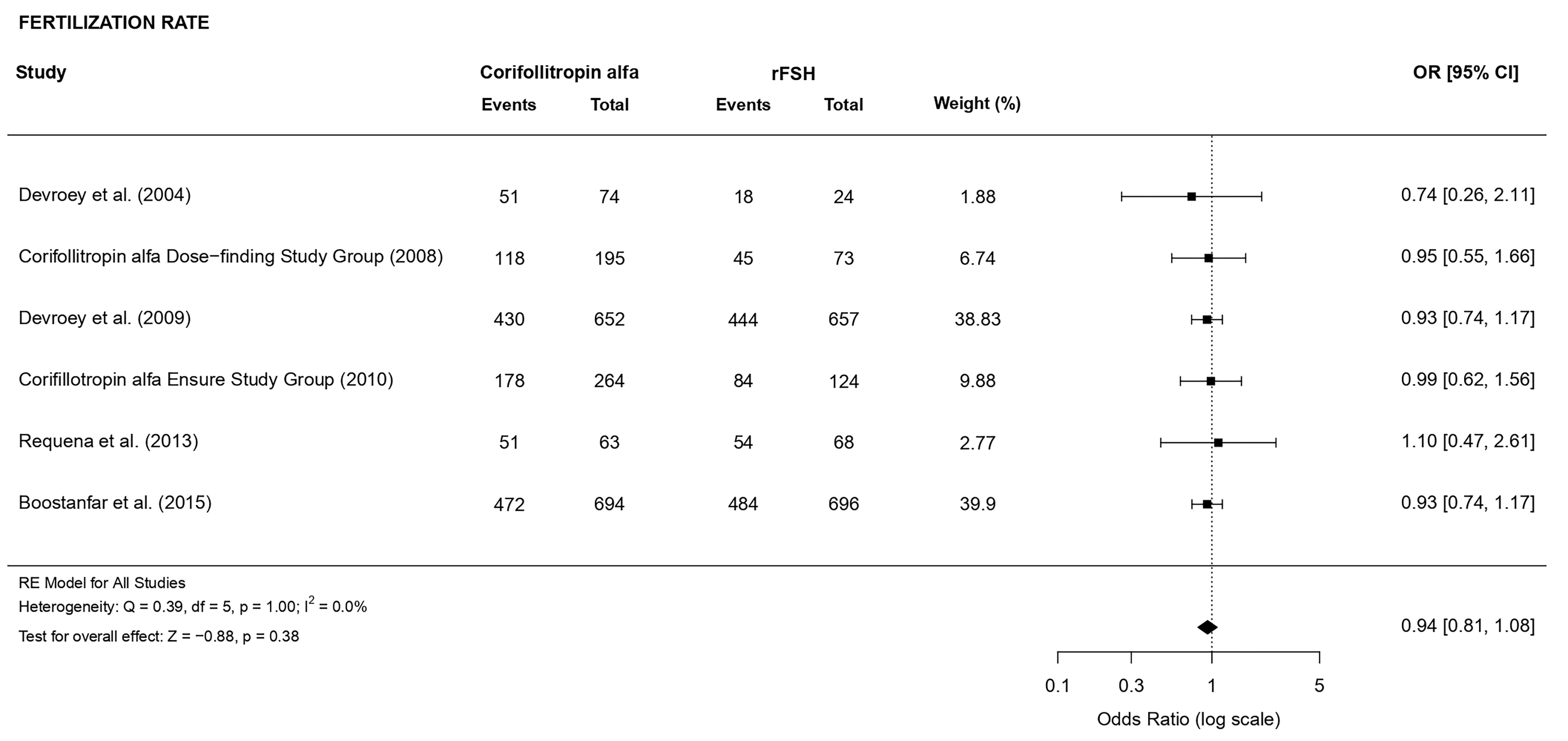 Fig. 6.
Fig. 6.Forest plot of the fertilization rate.
3.3.1.5 Number of embryos obtained
There was no evidence of a statistically significant difference (MD 0.30, CI
[–0.35, 0.96], p = 0.36; 7 RCTs, n = 3999; substantial heterogeneity:
I
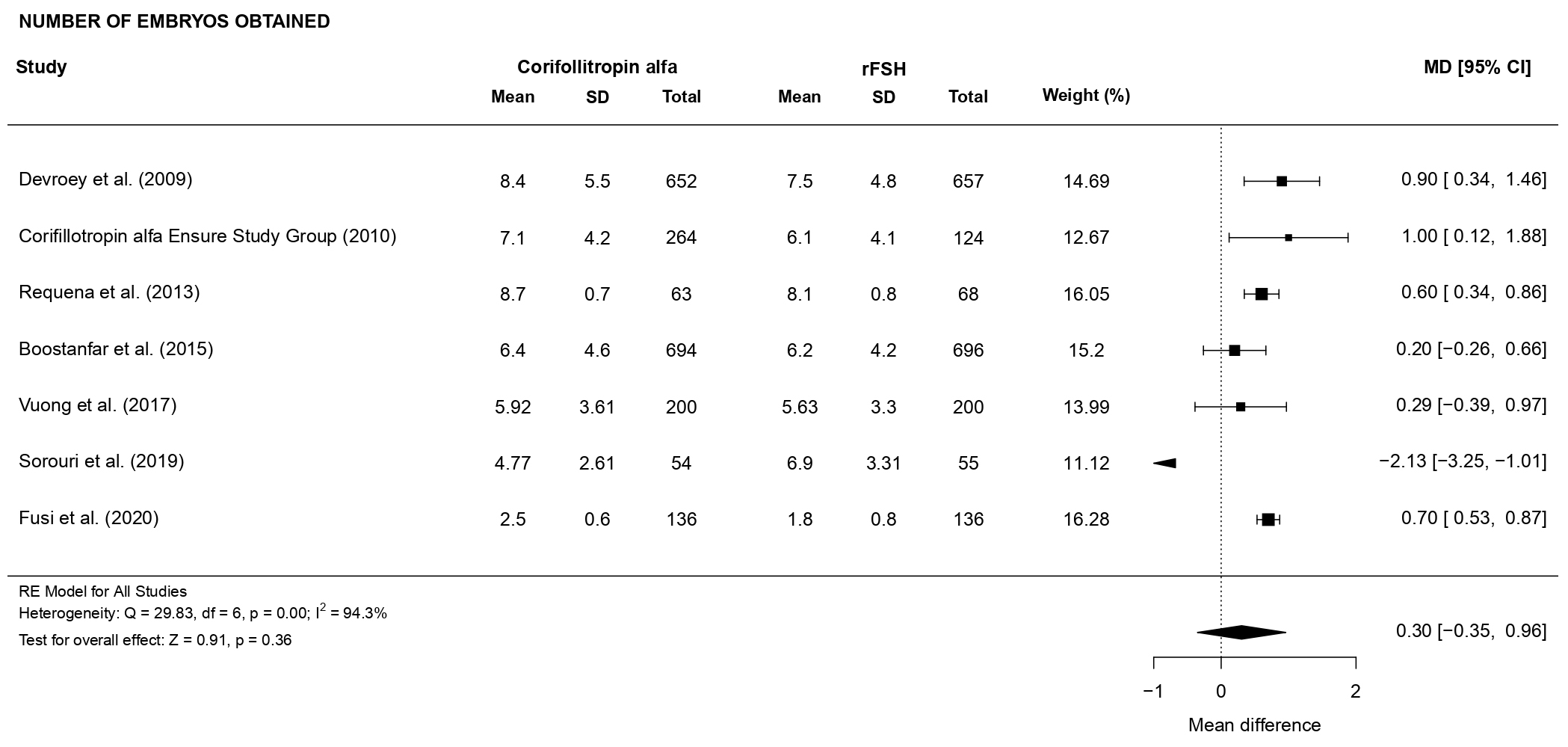 Fig. 7.
Fig. 7.Forest plot of the number of embryos obtained.
3.3.2.1 Implantation rate
There was no evidence of a statistically significant difference (OR 0.92, 95%
CI [0.75, 1.13], p = 0.44; 5 RCTs, n = 2300; no heterogeneity: I
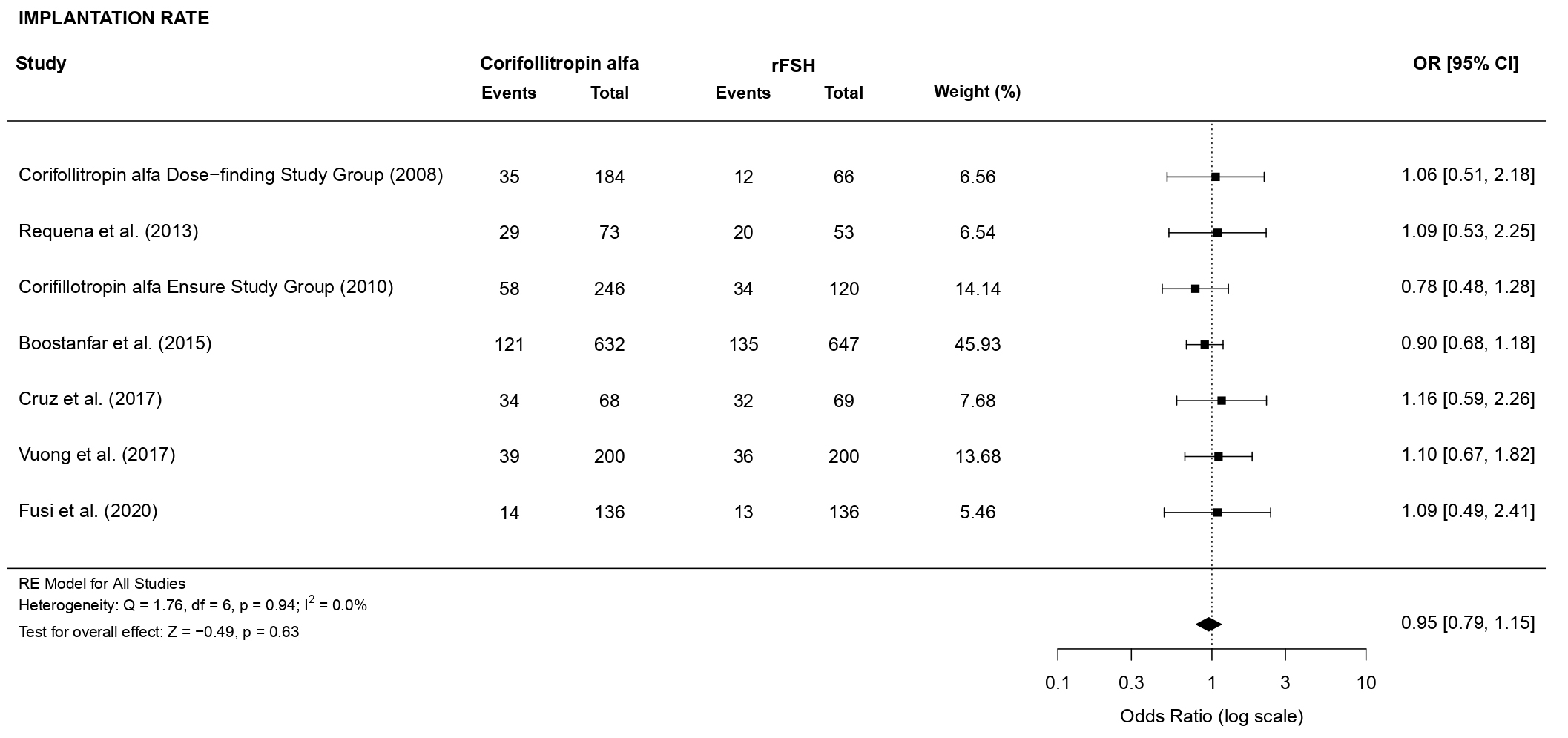 Fig. 8.
Fig. 8.Forest plot of the implantation rate.
3.3.2.2 Clinical pregnancy rate
As for the clinical pregnancy rate there was no evidence of a statistically
significant difference (OR 0.94, 95% CI [0.82, 1.08], p = 0.41; 10
RCTs, n = 4515; low heterogeneity: I
 Fig. 9.
Fig. 9.Forest plot of the clinical pregnancy rate.
3.3.2.3 Ongoing pregnancy rate
Concerning the ongoing pregnancy rate, there was no evidence of a statistically
significant difference (OR 0.93, 95% CI [0.81, 1.08], p = 0.35; 8 RCTs,
n = 4524; low heterogeneity: I
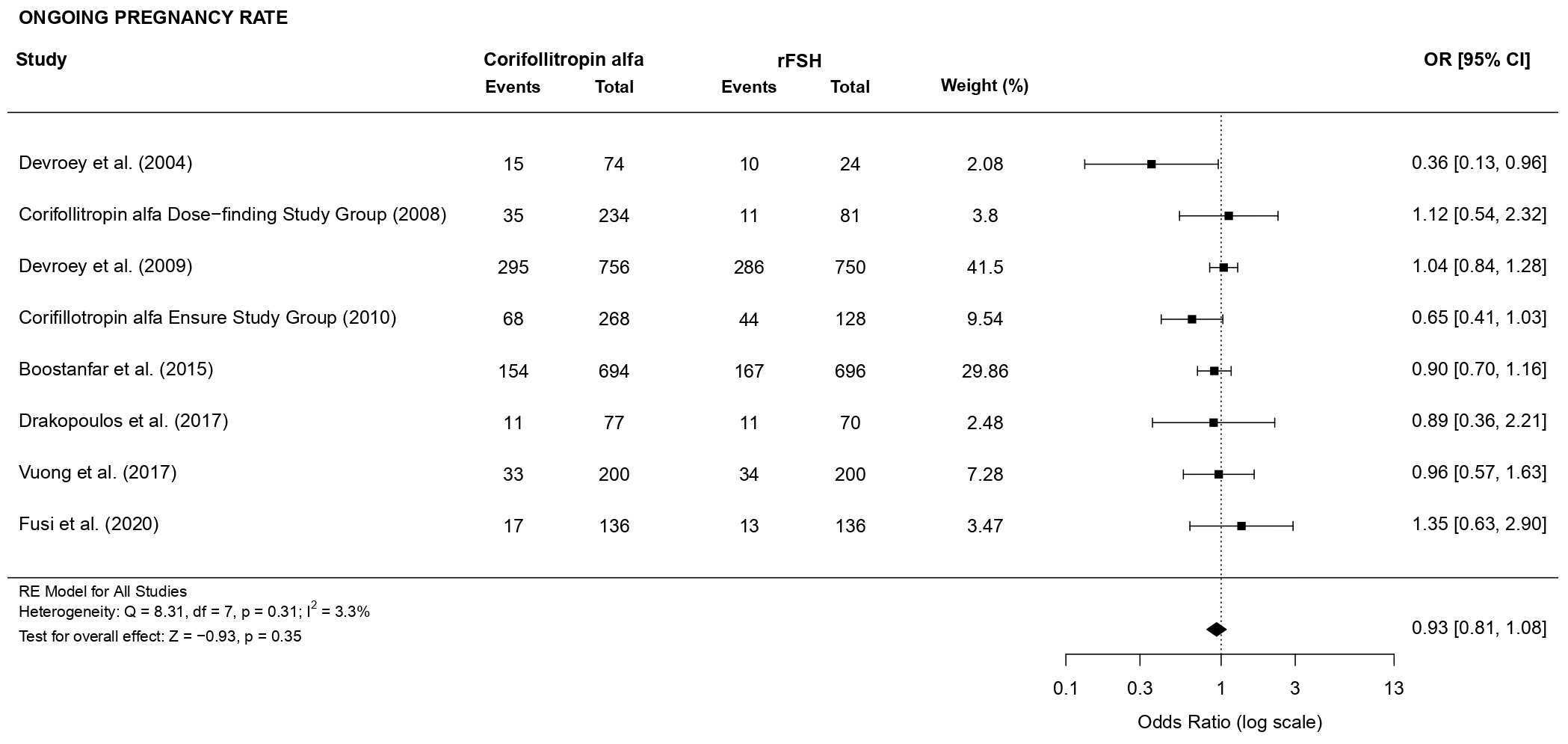 Fig. 10.
Fig. 10.Forest plot of the ongoing pregnancy rate.
3.3.2.4 Live birth rate
A total of 2013 women have been analyzed. There was no evidence of a
statistically significant difference in live birth rate (OR 0.90, 95% CI [0.73,
1.13], p = 0.37; 4 RCTs, n = 2013; no heterogeneity: I
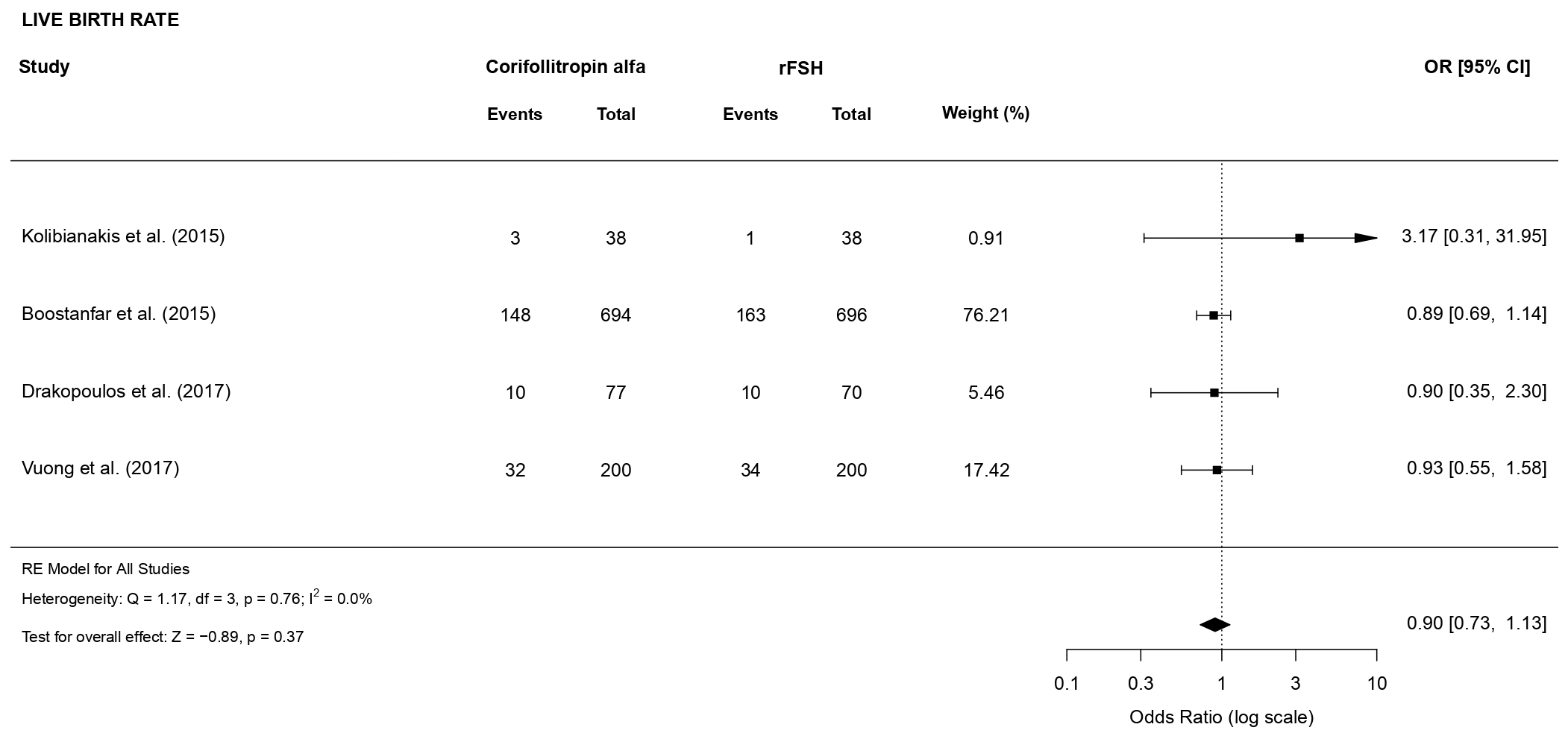 Fig. 11.
Fig. 11.Forest plot of the live birth rate.
3.3.2.5 Miscarriage rate
There was no evidence of a statistically significant difference (OR 1.03, 95%
CI [0.68, 1.55], p = 0.90; 4 RCTs, n = 1191; low heterogeneity: I
 Fig. 12.
Fig. 12.Forest plot of the miscarriage rate.
3.3.3.1 Incidence of OHSS
There was no evidence of a statistically significant difference (OR 1.08, 95%
CI [0.79, 1.49], p = 0.63; 7 RCTs, n = 4214; no heterogeneity: I
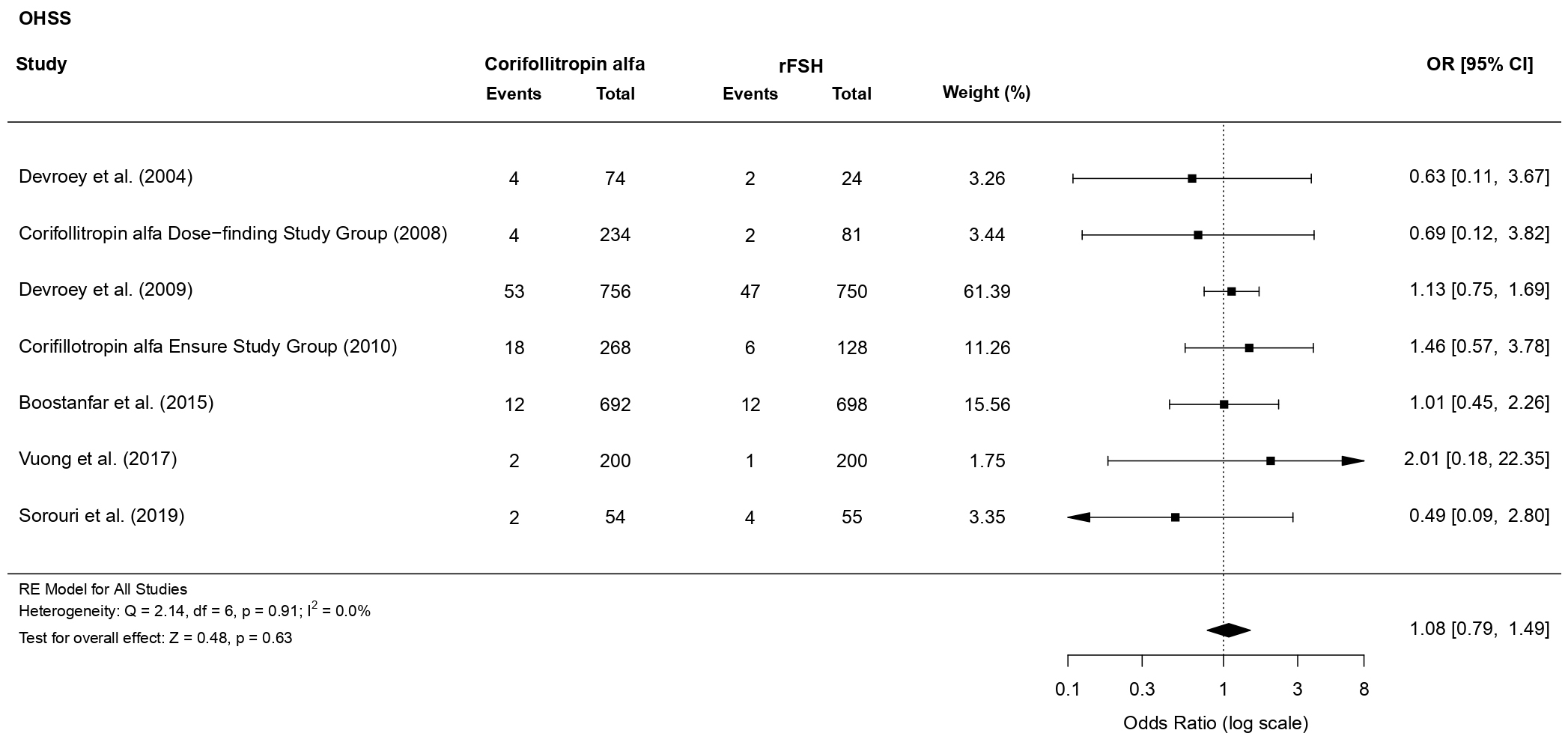 Fig. 13.
Fig. 13.Forest plot of the risk of OHSS.
3.3.3.2 Cycle cancellation
A total of 4557 women have been analyzed. There was no evidence of a
statistically significant difference (OR 1.25, 95% CI [0.89, 1.75], p =
0.20; 8 RCTs, n = 4557; moderate heterogeneity: I
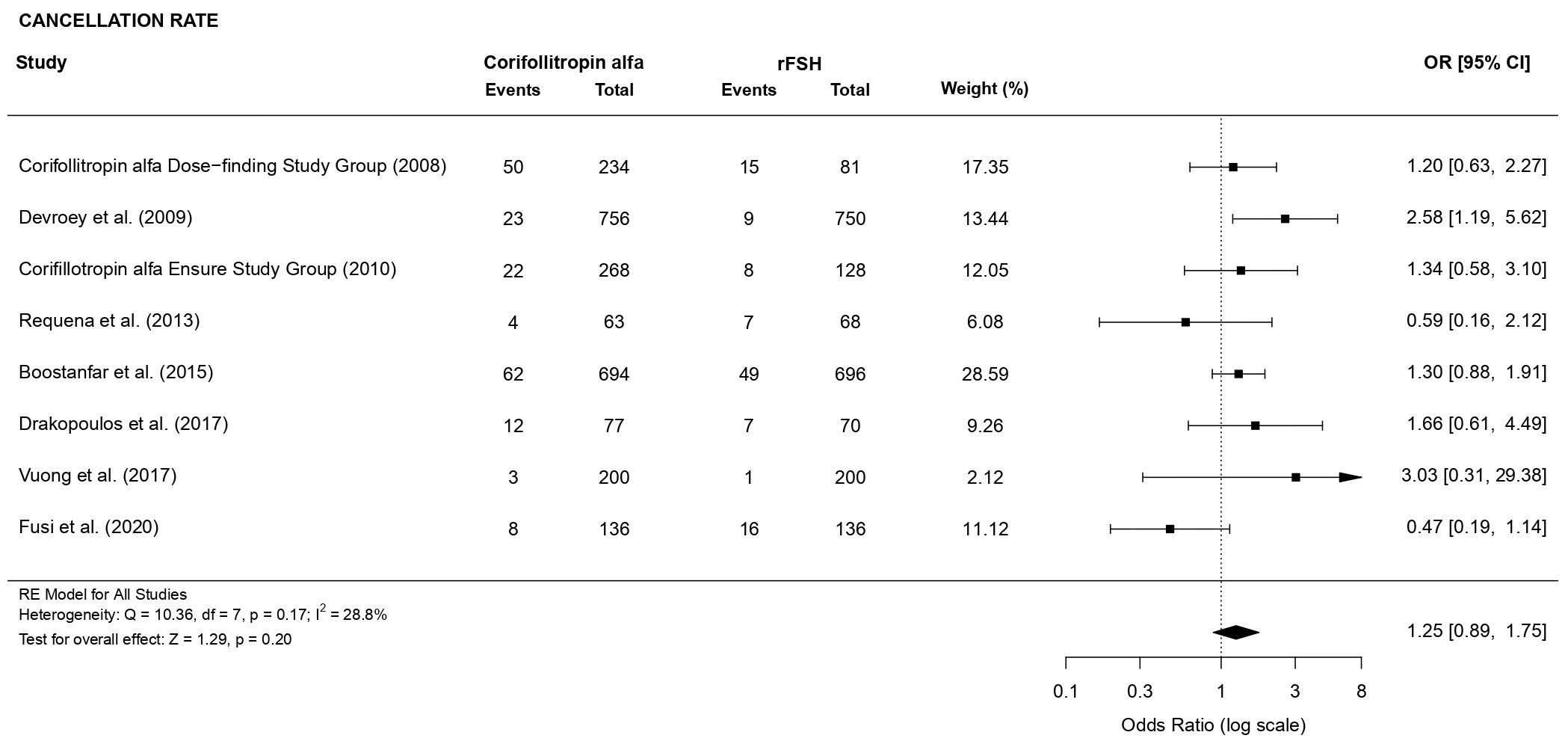 Fig. 14.
Fig. 14.Forest plot of the cycle cancellation rate.
To address the problem of heterogeneity and evaluate the robustness of our findings, we have carried out two subgroup analyses and performed, for each outcome, the sensitivity analysis.
The first subgroup analysis, identified according to the patient characteristic
“normal” versus “poor” responders, did not reveal significant differences
between subgroups for all the considered outcomes, except for the number of
oocytes retrieved (primary outcome), number of MII oocytes and cancellation rate,
which exhibited, for the normal responders’ group, a significant difference
between patients receiving CFA and daily rFSH. Specifically, normal responders
receiving CFA showed an increased number of oocytes retrieved (MD 1.05, 95% CI
[0.28, 1.82], p = 0.01; 6 RCTs, n = 3281; high heterogeneity: I
 Fig. 15.
Fig. 15.Forest plot of number of oocytes retrieved (Subgroup analysis).
 Fig. 16.
Fig. 16.Forest plot of number of MII oocytes retrieved (Subgroup analysis).
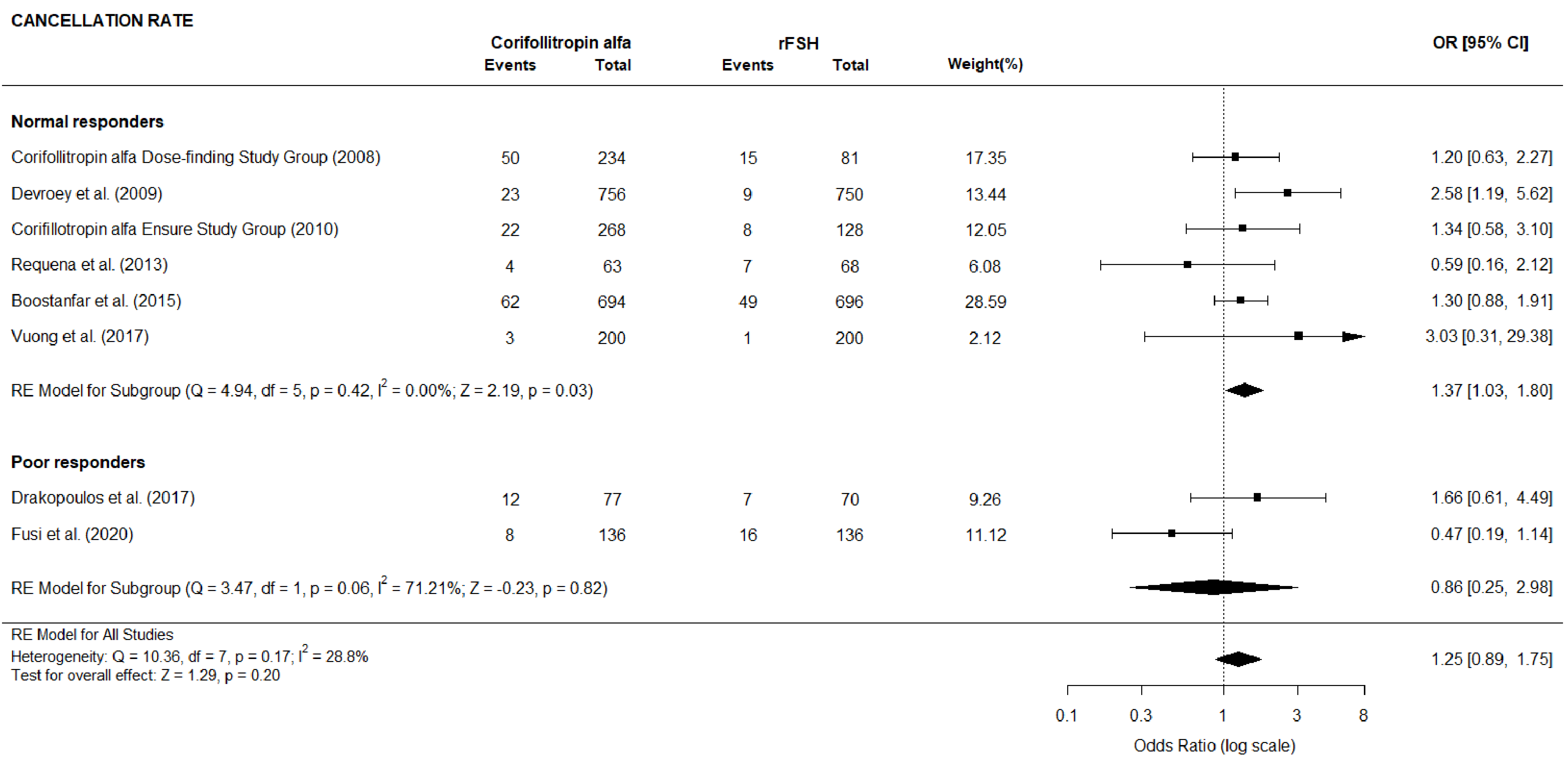 Fig. 17.
Fig. 17.Forest plot of the cycle cancellation rate (Subgroup analysis).
The second subgroup analysis has been performed according to the starting dose
of daily rFSH. For the group where daily doses greater than 150 International unit (IU) of daily rFSH
were given, we obtained a higher number of oocytes retrieved (MD 0.82, 95% CI
[0.29, 1.35], p = 0.002; 6 RCTs, n = 3167; moderate heterogeneity:
I
 Fig. 18.
Fig. 18.Forest plot of the number of oocytes retrieved (Subgroup analysis).
 Fig. 19.
Fig. 19.Forest plot of the number of MII oocytes (Subgroup analysis).
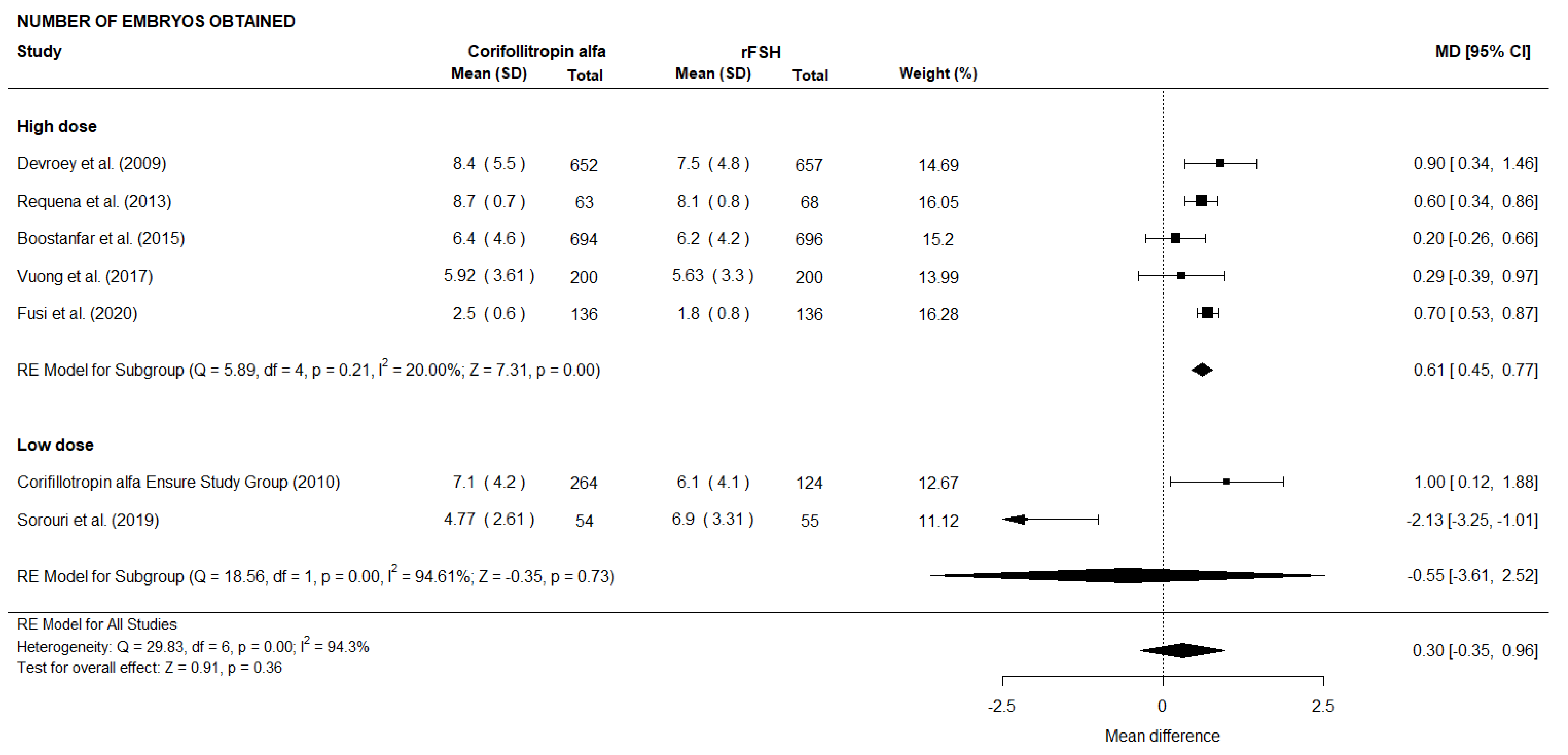 Fig. 20.
Fig. 20.Forest plot of the number of embryos obtained (Subgroup analysis).
Both the subgroup analyses did not reveal any significant difference between
subgroups in terms of duration of stimulation, which is an outcome with
substantial heterogeneity. However, we noticed that, in the group of patients
treated with a low dose of daily rFSH, when excluding the study of Sorouri
et al. [23], the duration of stimulation appears to be significantly
higher in the group of patients treated with CFA (MD 0.18, 95% CI [0.02, 0.34],
p = 0.03; 2 RCTs, n = 533; no heterogeneity: I
The leave-one-out sensitivity analysis, conducted by serially excluding each
study, confirmed the pooled results for almost all the outcomes, with the two
following exceptions. When excluding the study by Fusi et al. [24] we
obtained a significantly higher cycle cancellation rate in the CFA group (OR
1.39, 95% CI [1.06, 1.81], p = 0.02; 7 RCTs, n = 4285; no
heterogeneity: I
The present meta-analysis pooled the data from twelve RCTs focusing on the clinical effectiveness and safety of CFA compared to conventional daily rFSH.
The analysis shows that treatment with CFA results in an increased number of total oocytes retrieved at ovum pick-up and increased number of MII oocytes compared to patients receiving daily conventional rFSH during COS. No statistically significant differences were noted for the other outcomes analyzed in this study.
Previous meta-analyses have been published with the aim to compare the ovarian stimulation with CFA and daily rFSH [25, 26, 27, 28]. With respect to the latest recently published meta-analysis [28], the strengths of the present study comprise that our data updated the results by including four additional studies [17, 21, 23, 24] and 640 more patients. More to the point, the present meta-analysis also combined the data regarding five outcomes previously not examined by Cozzolino and colleagues (2019) (duration of stimulation, cancellation rate, fertilization rate, implantation rate and miscarriage rate). We also included RCTs on egg donors [17, 21] and poor responders [18, 20, 24] that represent two subgroups of IVF patients.
The number of retrieved oocytes at ovum pick-up represents one of the main parameters in the comparison between gonadotropins according to the European Medicines Agency (EMA) [9]. This parameter was considered as primary endpoint in the majority of the studies included in the present meta-analysis [14, 15, 16, 24]. The CFA protocol resulted in an higher number of oocytes retrieved at ovum pick up compared to daily rFSH [14, 16, 24]. These data reveal the efficacy of this novel FSH formulation but advises against the possible increased risk of developing OHSS during COS with CFA [6]. The present meta-analysis reassured about this concern considering that no differences in OHSS incidence have been found between CFA and daily rFSH treatments (OR 1.08, 95% CI [0.79, 1.49], p = 0.63). However, the heterogeneity between the included studies in relation to the patients’ characteristics and the ovarian stimulation protocols recall the need for specific studies on this matter. In this context, the study of Tarlatzis et al. [29] was conducted with the aim to assess the incidence of OHSS after CFA treatment by pooling the cases of OHSS from three large phase III trials primarily designed to analyse the efficacy of CFA treatment in a GnRH antagonist protocol [15, 16, 30]. The pooled data demonstrated that the risk of OHSS tends to be slightly higher with CFA than with daily rFSH treatment, but the overall incidence of OHSS (5.6%) together with the timing of occurrence and the severity in all the three phase III trials are in line with those obtained with daily rFSH treatment [29].
In addition, some RCTs included in the present meta-analysis were carried on
considering the ongoing pregnancy rate (defined as presence of at least one fetus
with heart activity at least 10 weeks after embryo transfer) as primary outcome
instead of the number of oocytes retrieved at ovum pick-up [15, 19, 20]. No
significant differences were noted between the percentage of women getting
pregnant following treatment with CFA or rFSH in the studies of Devroey
et al. [15] and Drakopoulos et al. [20]. In addition,
Boostanfar et al. [19] confirmed the non-inferiority of CFA to daily
rFSH with respect to the vital pregnancy rate (defined as the presence of at
least one fetus with heart activity assessed at least
It is noteworthy that several studies different from RCTs have been published with the aim to compare the clinical efficacy of CFA and the treatment with daily gonadotropins. The majority of these studies suggested that CFA represents an efficient alternative to daily rFSH formulations [31, 32, 33]. Contrarily to these data, Siristatidis et al. [34] found that live birth and clinical pregnancy rates were significantly reduced in women treated with CFA compared to those treated with follitropin beta, suggesting that CFA does not represent an equally method of ovarian stimulation compared with follitropin beta [34].
Three of the studies included in the present meta-analysis were carried out on poor responder patients [18, 20, 24] demonstrating that those treated with CFA showed a higher number of oocytes [24], and higher cryopreserved embryos [20], together with a shorter length of stimulation and reduced suspended treatments [24] compared to those treated with daily rFSH. In the current study, the subgroup analysis performed in order to compare “normal” versus “poor” responders reveals a significantly higher number of oocytes retrieved (MD 1.05, 95% CI [0.28, 1.82], p = 0.01), number of MII oocytes (MD 1.27, 95% CI [0.43, 2.11], p = 0.003), and cancellation rate (OR 1.37, 95% CI [1.03, 1.80], p = 0.03) in the group of normal responders receiving CFA.
In this context, the retrospective study performed by Adrisani et al.
[35] added significant information in this field [35]. The treatments with CFA
and daily gonadotropins resulted comparable in terms of clinical outcomes in poor
responders with antral follicle count (AFC)
Regarding the methodological quality of the trials included in the present
meta-analysis, six studies are open label-designed [13, 14, 17, 18, 20, 24] and three
studies are double blind-designed [15, 16, 19]. A potential selection bias must be
recognized since two studies not reported the methods of randomization and
allocation [13, 23] and in the study of Requena et al. [17] patients were
assigned to each protocol directly by investigators. In addition, not all the
studies detailed the blinding of participant and personnels and no study was
assessor-blinded. We have graphically detected the presence of publication bias
using both funnel and normal quantile plots. However, since the number of
studies, for almost all the outcomes, is less than ten, this latter has been
judged more reliable in revealing the presence of publication bias. In addition
it was recognized a clinical heterogeneity among the trials about the inclusion
criteria of the patients and the ovarian stimulation protocols, with particular
regard for the starting dose of daily rFSH. At this purpose, the subgroup
analysis highlighted a higher number of both oocytes retrieved (MD 0.82, 95% CI
[0.29, 1.35], p = 0.002), MII oocytes (MD 0.91, 95% CI [0.33, 1.50],
p = 0.002), and embryos obtained (MD 0.61, 95% CI [0.45, 0.77],
p
The main limitations of the present meta-analysis are related to the existing heterogeneity among the included studies, as represented by discrepancies in COS. In fact, in addition to differences in the starting dose of daily rFSH, two authors (Requena et al. [17] and Cruz et al. [21]) investigated oocyte donors and assigned an oral contraceptive pill to patients on day 1 or 2 of menses of the previous cycle before starting the assigned stimulation protocol.
In addition, differences in primary endpoints considered, methodological quality and patients characteristics among the studies represent possible sources of bias.
In view of the EMA statement that recommended to consider the number of oocytes retrieved as the primary endpoint to compare gonadotropins, our study demonstrated that CFA treatment represents an effective method in comparison to daily rFSH. The association between CFA and increased number of retrieved oocytes at ovum pick-up together with a higher number of MII oocyte is possibly due to the capacity of CFA to recruit an increased cohort of developing follicles. However, given the existing heterogeneity between the studies, further comparable RCTs are needed.
MCB—conceptualization, methodology, literature search, writing original draft, data curation, formal analysis; SF—conceptualization, methodology, literature search, writing original draft, data curation, formal analysis; MDM—conceptualization, methodology, literature search, writing original draft, writing-review and editing, investigation; GMT—conceptualization, methodology, literature search, writing original draft, writing-review and editing, investigation, supervision. All authors read and approved the final version of the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
