- Academic Editor
†These authors contributed equally.
Background: To explore possible predictors of severe late ovarian
hyperstimulation syndrome (SL-OHSS) in fresh embryo transfer cycles.
Methods: We conducted a historical cohort study in a Chinese tertiary
hospital from January 2017 to December 2019, with a total of 6931 women who had
the first fresh embryo transfer included. SL-OHSS was defined as severe symptoms
of OHSS occurring 12–17 days after ovulation triggering. Possible determinants
of the occurrence of SL-OHSS were identified by a detection decision tree,
effects of which were estimated by conditional logistic regression and restricted
cubic spline. Results: Elevated estradiol (E2) on the day of ovulation
triggering and elevated Anti-Mullerian hormone (AMH) were associated with an
increased risk of SL-OHSS (p
In vitro fertilization (IVF) and embryo transfer (ET) is widely used in the treatment of infertility. Ovarian hyperstimulation syndrome (OHSS) is a severe iatrogenic complication of controlled ovarian stimulation (COS) which is an essential component in IVF treatment. An estimated 3% to 8% of IVF cycles are complicated by moderate or severe forms of OHSS [1]. Patients with OHSS may experience severe symptoms including abdominal distention, pain, dyspnoea, oliguria and intractable nausea/vomiting [2]. Two subtypes of OHSS (early OHSS and late OHSS) have been described, defined by the interval from the administration of trigger medicine to the onset of OHSS [3, 4, 5]. The symptoms of late OHSS are often far more severe than those of early OHSS and may extend through the first trimester [6].
With the development and utilization of vitrification technology, freezing all embryos followed by frozen ET is a treatment strategy with the ability to eliminate late OHSS. Indeed, this approach may also increase live birth rates among those women with hyper response to COS [7, 8]. However, frozen ET cycles are also associated with an increase in some pregnancy complications such as hypertensive disorders of pregnancy and large for gestational age [9, 10, 11, 12]. Furthermore, the freeze-all strategy was not cost-effective compared with fresh ET for women without polycystic ovary syndrome (PCOS) [13, 14, 15, 16, 17]. Therefore, many assisted reproductive technology centers around the world are still performing fresh ET, even if they could avoid the occurrence of late OHSS by a freeze-all approach.
Due to the severity of symptoms, protracted clinical course and morbidity associated with late OHSS, it would be preferable to identify factors within the patient or cycle that put them at risk of late OHSS to allow conversion to a freeze all cycle. Unfortunately, late OHSS is difficult to predict and prevent in fresh ET cycles [5]. Serum estradiol (E2) concentrations and oocytes number could represent risk factors for early OHSS, but do not accurately predict the risk of developing late OHSS [3, 5]. Ultrasound and hematological changes have also been explored as biomarkers for the onset of severe early OHSS, but its validation in late OHSS requires further study and current study populations have been restricted to patients at high risk of OHSS after a human chorionic gonadotropin (HCG) trigger [18]. The aim of the present study was to explore possible determinants of developing severe late OHSS (SL-OHSS) in fresh ET cycles.
This was a historical cohort study conducted in West China Second University Hospital of Sichuan University, which is one of the largest reproductive centers in Southwest China. A total of 14,348 patients who underwent COS and in vitro fertilization and embryo transfer treatment were enrolled from January 2017 to December 2019. Among them, 2887 patients were excluded due to their duplicated ID number, and 4530 did not proceed to fresh transfer due to a planned freeze all cycle or had no embryos available for transfer. Therefore, a total of 6931 women who had their first fresh ET were included in the statistical analysis. We reviewed each patient’s complete inpatient and outpatient medical record. Demographic information including age and body mass index, cycle details including peak estradiol levels and oocytes retrieved as well as treatment outcomes including SL-OHSS were collected (Supplementary Table 1).
The ovarian stimulation protocols included long gonadotrophin releasing hormone
(GnRH) agonist and GnRH antagonist protocols. The protocol selection was based on
female age, ovarian reserve and previous ovarian response to gonadotrophin
stimulation. All patients were treated according to standard clinic protocols and
at the discretion of the treating clinician. When 1–3 leading follicles reached
Luteal support was commenced on the day of oocyte retrieval for fresh cycles. Luteal progesterone support was administered via progesterone injection (H33020828, Shanghai General Pharmaceutical, Shanghai, China) 40 mg twice a day or progesterone gel (H20140552, Crinone 8%, Fleet laboratories limited, Hertfordshire, UK) 90 mg once daily plus dydrogesterone tablet (H20170221, Abbott Biologicals B.V., Overijssel, the Netherlands) 20 mg once daily.
Freezing all embryos was recommended to patients if there was a moderate to high
risk of OHSS, where E2 concentrations were more than 5000 ng/mL on the day of HCG
administration or more than 15 oocytes were retrieved, or high progesterone
concentration (
We first describe the characteristics of cases and controls. The continuous data
were expressed as mean and standard deviation (SD), or median, p25 and p75 if
p of normality test is less than 0.05, and categorical data was
expressed as number or percent as appropriate. To explore the determinants of
SL-OHSS a two-step analysis strategy was conducted. (1) We used a Chi-squared
Automatic Interaction Detection (CHAID) decision tree, which can be performed on
most medical data for the purpose of differential diagnosis, prediction, and
determinants selection to select the potential determinants of SL-OHSS [21].
According to the literature and clinical experience, the possible significant
factors including women age, body mass index, Anti-Mullerian hormone (AMH),
history of polycystic ovary syndrome (PCOS), history of allergy, protocols of
COS, total gonadotrophin dose, E2 concentrations on the day of HCG
administration, type of medicine for oocyte maturation trigger, number of oocytes
retrieved, good quality embryos transferred were input into the CHAID decision
tree model [6]. Probability value for the chi-square statistic was estimated for
each independent variable, and independent variables with p
All statistical analyses were performed using R version 3.6.1 (R Foundation for
Statistical Computing, http://www.r-project.org). Two-tailed values of
p
A total of 6931 women who had fresh ET were eligible for the final analysis
(Fig. 1) and of these, 54 cycles (0.78%) were complicated by SL-OHSS. The mean
age in the SL-OHSS group (30.51
 Fig. 1.
Fig. 1.Flow chart of women with SL-OHSS and without SL-OHSS. SL-OHSS, severe late ovarian hyperstimulation syndrome.
The agonist trigger use was similar in the SL-OHSS group (3.70%) and the non-SL-OHSS group (3.62%). E2 values on the day of HCG administration and the number of oocytes retrieved were significantly higher in the SL-OHSS group (2965 pg/mL and 11.17, respectively) than that in the non-SL-OHSS group (1953 pg/mL and 8.04, respectively). There was no difference in the rate of blastocyst transfer between the two groups (3.7% in SL-OHSS group and 2.36% in non-SL-OHSS group) (Supplementary Table 1).
Fig. 2 shows a simplified classification tree model, which had a depth of two
levels and a total of two nodes. From CHAID analysis we can see that serum
estradiol concentration greater than 3320.2 pg/mL on HCG day, or the combination
of serum estradiol less than 3320.2 pg/mL on HCG day as well as AMH concentration
greater than 4.62 ng/mL were risk factors of SL-OHSS occurrence (p
 Fig. 2.
Fig. 2.Factors associated with SL-OHSS occurrence based on CHAID decision tree model. SL-OHSS, severe late ovarian hyperstimulation syndrome; CHAID, Chi-squared Automatic Interaction Detection; E2, estradiol; HCG, human chorionic gonadotrophin; AMH, anti-Müllerian hormone.
Table 1 shows that apart from PCOS history (SMD = 28.9%), allergic history (SMD
= 17.7%) and trigger medicine (SMD = 1.4%), all the other covariates had a SMD
over 30%. Furthermore, distribution of covariates was statistically significant
between women with SL-OHSS and without SL-OHSS (p
| Covariates | Before PSM | After PSM* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Women with SL-OHSS | Women without SL-OHSS | p | SMD (%) | Women with SL-OHSS | Women without SL-OHSS | p | SMD (%) | ||
| Total | 49 | 6524 | 49 | 196 | |||||
| Female age-yr Median (p25, p75) | 30.00 (29.00, 32.00) | 31.00 (28.00, 35.00) | 0.092 | 30.7 | 30.00 (29.00, 32.00) | 30.00 (29.00, 32.00) | 0.860 | 2.0 | |
| BMI-kg/m |
21.10 (19.00, 23.10) | 21.50 (19.89, 23.63) | 0.084 | 35.0 | 21.10 (19.00, 23.10) | 21.16 (19.10, 22.89) | 0.972 | 2.2 | |
| History of PCOS n (%) | |||||||||
| No | 43 (87.8) | 6240 (95.6) | 0.020 | 28.9 | 43 (87.8) | 173 (88.3) | 1 | 1.6 | |
| Yes | 6 (12.2) | 284 (4.4) | 6 (12.2) | 23 (11.7) | |||||
| History of allergy n (%) | |||||||||
| No | 46 (93.9) | 6289 (96.4) | 0.577 | 11.7 | 46 (93.9) | 186 (94.9) | 1 | 4.4 | |
| Yes | 3 (6.1) | 235 (3.6) | 3 (6.1) | 10 (5.1) | |||||
| Protocols of COS n (%) | |||||||||
| Agonist | 37 (75.5) | 3865 (59.2) | 0.064 | 36.7 | 37 (75.5) | 148 (75.5) | 1.000 | ||
| Antagonist | 12 (24.5) | 2602 (39.9) | 12 (24.5) | 48 (24.5) | |||||
| Others | 0 | 57 (0.9) | 0 | 0 | |||||
| Total Gn dose (IU) | 2100.00 (1800.00, 2550.00) | 2400.00 (1909.38, 2925.00) | 0.007 | 39.2 | 2100.00 (1800.00, 2550.00) | 2100.00 (1800.00, 2550.00) | 0.900 | 1.0 | |
| Median (p25, p75) | |||||||||
| Type of medicine triggered for oocyte maturation n (%) | |||||||||
| HCG | 47 (95.9) | 6276 (96.2) | 1.000 | 1.4 | 47 (95.9) | 189 (96.4) | 1.000 | 2.7 | |
| GnRH agonist | 2 (4.1) | 248 (3.8) | 2 (4.1) | 7 (3.6) | |||||
| No. of oocytes retrieved mean |
11.18 |
8.07 |
85.8 | 11.18 |
11.14 |
0.929 | 1.4 | ||
| Good quality embryo transfer n (%) | |||||||||
| No | 4 (8.2) | 1341 (20.6) | 0.049 | 35.9 | 4 (8.2) | 16 (8.2) | 1.000 | ||
| Yes | 45 (91.8) | 5183 (79.4) | 45 (91.8) | 180 (91.8) | |||||
*358 women with any missing data of the above variable were excluded from the analysis. SL-OHSS, severe late ovarian hyperstimulation syndrome; IU, International Unit; BMI, Body Mass Index.
Table 2 displays the effects of E2 concentrations on HCG day and AMH levels on
the occurrence of SL-OHSS. We found that the incidence of SL-OHSS in the group
with peak E2 concentration
| Variable | Original data (n = 6573) | Matched data after PSM (n = 245) | |||||
|---|---|---|---|---|---|---|---|
| Cases (n = 49) | Controls (n = 6524) | Crude OR (95% CI) | Cases (n = 49) | Controls (n = 196) | OR (95% CI) | ||
| E2 on HCG day-pg/mL n (%) | |||||||
| 32 (65.31) | 5760 (88.29) | 1 | 32 (65.31) | 162 (82.65) | 1 | ||
| 17 (34.69) | 764 (11.71) | 4.01 (2.17–7.15) | 17 (34.69) | 34 (17.35) | 2.20 (1.03–4.68) | ||
| AMH levels-ng/mL n (%) | |||||||
| 9 (18.37) | 4635 (71.05) | 1 | 9 (18.37) | 102 (52.04) | 1 | ||
| 40 (81.63) | 1889 (28.95) | 10.91 (5.53–24.02) | 40 (81.63) | 94 (47.96) | 5.44 (2.29–12.90) | ||
| E2 on HCG day + AMH | |||||||
| HCG |
6 (12.24) | 4250 (65.14) | 1 | 6 (12.24) | 91 (46.43) | 1 | |
| HCG |
26 (53.06) | 1510 (23.15) | 12.20 (5.36–32.82) | 26 (53.06) | 71 (36.22) | 7.53 (2.52–22.54) | |
| HCG |
3 (6.12) | 385 (5.90) | 5.52 (1.16–21.02) | 3 (6.12) | 11 (5.61) | 5.26 (1.03–27.00) | |
| HCG |
14 (28.57) | 379 (5.81) | 26.17 (10.43–74.27) | 14 (28.57) | 23 (11.73) | 13.20 (3.87–45.02) | |
SL-OHSS, severe late ovarian hyperstimulation syndrome; E2, estradiol; HCG, human chorionic gonadotrophin.
Fig. 3 presented the nonlinear association between AMH and E2 concentrations on
HCG day and SL-OHSS occurrence. Taking 4.62 ng/mL and 3320.2 pg/mL as the
reference values of AMH and E2 concentrations on HCG day respectively, the risk
of SL-OHSS was significantly increased with the increase of AMH between 3 ng/mL
and 7.5 ng/mL, and E2 concentrations on HCG day between 2400 pg/mL and 5000 pg/mL
(p
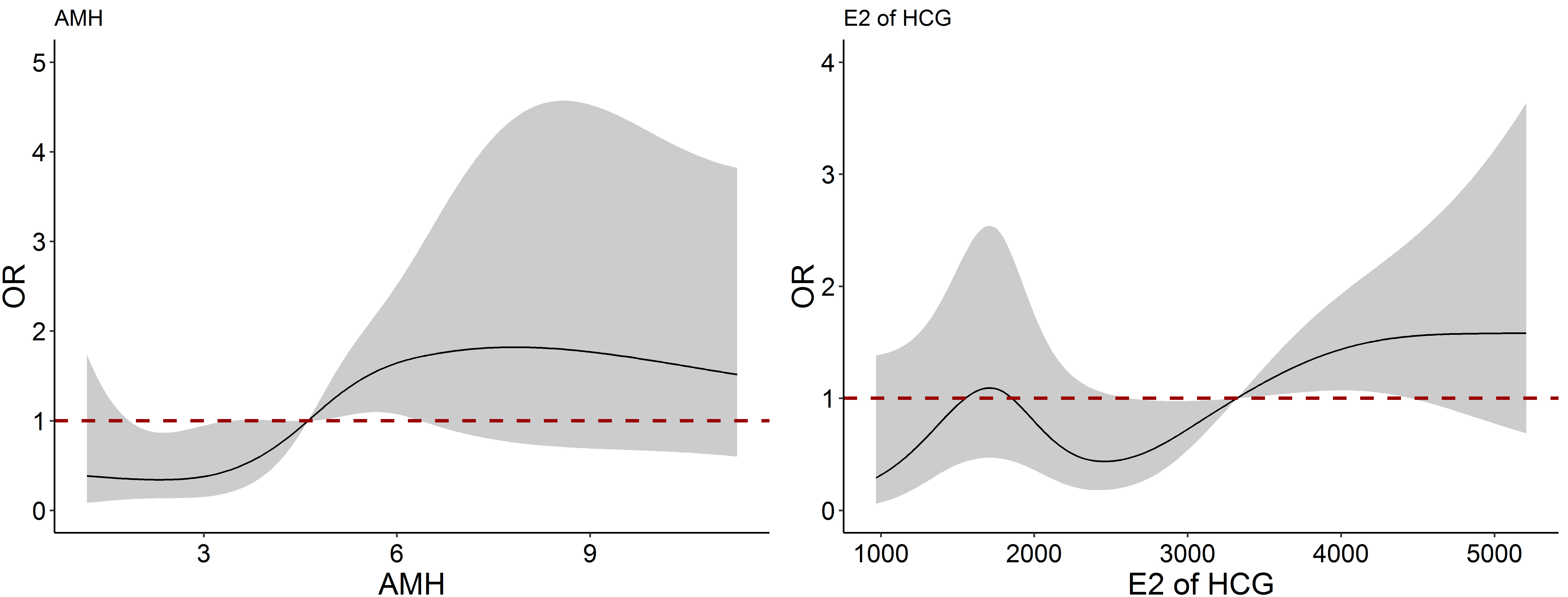 Fig. 3.
Fig. 3.The nonlinear association between AMH and E2 on HCG day and SL-OHSS occurrence. SL-OHSS, severe late ovarian hyperstimulation syndrome; E2, estradiol; HCG, human chorionic gonadotrophin; AMH, anti-Müllerian hormone.
Our CHAID decision tree analysis and conditional logistic regression demonstrate
that serum E2 concentrations on HCG day
Supraphysiological estradial (E2) concentrations due to COS and the administration of human chorionic gonadotrophin (hCG) as an ovulation trigger in fresh cycles can be the crucial stimulus of OHSS [23]. And the Endogenous HCG from the initiated pregnancy after fresh embryo transfer has the chance to induce the onset of late OHSS. With the increasing permeability of capillary, the fluid in the intravascular shift into the third space such as pleural and pericardial spaces. As a result, there is possible to occur haemoconcentration, hypercoagulation, electrolyte imbalance, even life threaten. So, it is necessary to find biomarkers to help clinicians to make decisions regarding fresh or frozen transfer to decrease SL-OHSS and increase safety of assisted reproductive treatment.
Peak E2 concentrations play an important role in predicting the occurrence of
OHSS [24], but its threshold values vary from literature. The American Society
for Reproductive Medicine suggests that peak E2 concentration
In contrast to studies examining early onset OHSS risk, Mathur et al. [5] and Lyons et al. [3] have previously reported that serum peak E2 concentrations and the number of oocytes retrieved does not accurately predict the risk of developing late OHSS. In the study conducted by Lyons et al. [3], they proposed that the serum estradiol and progesterone concentrations 11–13 days after HCG administration and gestational sac number were superior predictors of late OHSS. This finding is of limited clinical utility in guiding decision making with regard to embryo transfer. In our study, we included all fresh embryo transfer cycles, and our positive cases (severe late OHSS) were larger than previous studies. We found that threshold E2 concentration on HCG day greater than 3320.2 pg/mL was an independent determinant of SL-OHSS.
Serum AMH concentration is strongly related to the quantitative aspect of ovarian reserve and ovarian response to COS [26]. It has been also found to be associated with the onset of OHSS [27, 28]. Lee et al. [29] concluded that basal serum AMH concentration (greater than 3.36 ng/mL) was the strongest predictor for OHSS, and their multivariate conditional logistic regression model showed that the basal AMH and E2 concentrations on the day of HCG administration were the significant predictors of moderate and severe OHSS. In our study, we focused on SL-OHSS and found the two risk factors of SL-OHSS were also E2 on HCG day and serum AMH concentration. Our findings suggest that the combination of these two factors might be more valuable for predicting SL-OHSS due to the higher adjusted OR.
In our study, although the number of oocytes retrieved in the SL-OHSS group was significantly higher than that in the non-SL-OHSS, we did not find the number of oocytes retrieved was associated with SL-OHSS risk. This result differed from existing literature suggesting that the number of oocytes retrieved was associated with OHSS [6, 30, 31]. The possible reason was that our study focused on SL-OHSS.
Apart from a freeze-all strategy, GnRH antagonist protocols with the use of GnRH agonist to trigger final oocyte maturation is considered an effective strategy to reduce the risk of OHSS [6, 32]. But we should mind that severe early OHSS cases have been reported after GnRH agonist triggering and freeze-all [33]. In our study, we also found 2 cases of SL-OHSS which occurred after GnRH agonist triggering in antagonist protocol.
The combination of Serum E2 concentrations on HCG day and AMH levels may be a stronger predictor of SL-OHSS and inform clinical decision making regarding fresh or frozen transfer to decrease SL-OHSS and increase safety of assisted reproductive treatment. In the future, the multi-center, large-sample, and prospective methodologies should be considered to find more determinants of SL-OHSS occurrence.
Though total size in the study was large, the SL-OHSS cases were relatively small due to the rarity of the disease. Therefore, we were limited to find more determinants of SL-OHSS occurrence, and the 95% confidence intervals of OR in the study were generally wide, though they narrowed in matched group after PSM. Another limitation is the single center nature of this study. Indeed, West China Second University Hospital is the central hospital for the treatment of couples with infertility in the Southwest of China, and serves more than half of the infertile couples in this region.
Serum E2 concentrations on HCG day and AMH levels may represent important determinants of SL-OHSS occurrence in fresh ET cycles. The combination of these two biomarkers may be a stronger predictor of SL-OHSS and inform clinical decision making regarding fresh or frozen transfer to decrease SL-OHSS and increase safety of assisted reproductive treatment.
SL-OHSS, severe late ovarian hyperstimulation syndrome; E2, Elevated estradiol; AMH, Anti-Mullerian hormone; ET, embryo transfer; OHSS, Ovarian hyperstimulation syndrome; COS, controlled ovarian stimulation; IVF, in vitro fertilization; PCOS, polycystic ovary syndrome; OHSS, Ovarian hyperstimulation syndrome; HCG, human chorionic gonadotropin; ICSI, intracytoplasmic sperm injection; IU, International Unit; BMI, Body Mass Index; CHAID, Chi-squared Automatic Interaction Detection; SMD, standardized mean differences; CI, confidence interval.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
LJL and XLY collected and analyzed the data, and drafted the manuscript. XHL (non- corresponding author) contributed to the analysis and interpretation of the data. SH contributed to the interpretation of the data. GC contributed to the data collection. XHL (corresponding author) and SL designed the study and oversaw the data interpretation. All authors revised the manuscript and approved the version to be published.
The Ethics Committee of West China Second Hospital, Sichuan University approved this study (Permit Number: 201662). Informed consent was obtained from the participants before entering the study.
Not applicable.
This work was supported by Key R & D projects of Sichuan Provincial Department of Science and Technology under Grant 2021YFS0243.
The authors declare no conflict of interest. Sarah Hunt is serving as one of the Guest editors of this journal. We declare that Sarah Hunt had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Andrea Tinelli.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
