- Academic Editor
†These authors contributed equally.
Background: Forceps-assisted
vaginal delivery is closely associated with postpartum pelvic floor muscle (PFM)
injury and postpartum pelvic floor
dysfunction. The present study utilized
Glazer PFM surface electromyography (sEMG)
and International Consultation on Incontinence Questionnaire-Urinary Incontinence
Short Form (ICIQ-UI-SF) for the objective assessment of postpartum PFM function
to determine the effects of different forceps delivery indications on early
postpartum pelvic floor function in primiparas. Methods: Four hundred
primiparas whose pregnancies had been terminated by forceps delivery were divided
into three groups based on the indication for forceps delivery: fetal distress
(FD) (n = 260), prolonged second stage of labor (PSSL) (n = 30), and intrapartum
fever combined with fetal distress (IFFD) (n = 110). Pelvic floor muscle surface
electromyography (EMG) performed according to the Glazer protocol at 42–60 days
postpartum was the primary outcome measure. Results: The overall Glazer
assessment scores of the PSSL (54.4
Pelvic floor dysfunctions (PFDs) refer to a group of conditions with diverse etiologies that damage the pelvic floor muscle (PFM) function. This leads to the weakening of PFM reaction and impairment of pelvic floor support functions, including stress urinary incontinence (SUI), anal incontinence (AI), pelvic organ prolapse, and postpartum sexual dysfunction [1]. Presently, PFDs are regarded as a hidden epidemic that affect approximately 21%–26% of women, with urinary incontinence (UI), having the highest incidence rates worldwide [2]. Pregnancy and delivery are independent risk factors for impaired pelvic floor function [3, 4], with operative vaginal delivery (OVD) being closely associated with PFDs [5]. OVD refers to a key means of delivery in which forceps or vacuum extraction is used to apply direct traction on the fetal head during the second stage of labor to accelerate or achieve vaginal delivery [6]. It currently serves as a crucial operating method for dealing with obstructed labor. However, there are relatively few reports regarding the effects of different indications for forceps delivery on postpartum pelvic floor function. The present study utilized Glazer PFM surface electromyography (sEMG) and International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI-SF) for the assessment of postpartum PFM function to determine the effects of different forceps delivery indications on early postpartum pelvic floor function in primiparas. Our results contribute to a better understanding of the effects of forceps delivery on the incidence of PFD and provide a theoretical basis for the early screening of PFDs.
This retrospective cohort study was conducted by the International Peace Maternity & Child Healthcare Hospital (IPMCH), Shanghai Jiaotong University. Four hundred primiparas who had undergone forceps delivery between September 2019 and December 2021 and pelvic function screening at 42–60 days postpartum at the International Peace Maternity and Child Health Hospital affiliated to Shanghai Jiao Tong University School of Medicine, China, were selected as study participants. All participants provided informed consent. This study was approved by the ethics committee of the hospital.
Indications for forceps delivery include intrauterine fetal distress, prolonged
second stage of labor, and intrapartum fever combined with fetal distress [7].
The participants were divided into three groups based on the indication for
forceps delivery. First, the fetal distress (FD) group: fetal distress refers to
the syndrome in which the health and life of the fetus are endangered by acute or
chronic hypoxia in utero, often manifested as acute fetal distress after labor.
Second, the prolonged second stage of labor (PSSL) group: currently, the American
College of Obstetricians and Gynecologists (ACOG) recommends that the second
stage of labor be no longer than 3 hours for primipara with epidural delivery and
4 hours for primipara without epidural delivery [8]. Third, the intrapartum fever
combined with fetal distress (IFFD) group: intrapartum fever defined as maternal
body temperature
The inclusion criteria were primipara, 18–40 years of age; singleton pregnancy,
delivery at
The exclusion criteria were history of long-term constipation or UI, history of PFDs or pelvic surgery, and severe hearing impairment and intellectual disability.
The following obstetrics-related data were extracted from the electronic health records system of the hospital: indication for forceps delivery, educational attainment, body mass index (BMI), time of screening, maternal age, gestational age at delivery, neonatal birth weight, use of labor analgesia, gestational weight gain, and gestational complications. At 42–60 days postpartum, the women were subjected to PFM sEMG in accordance with the Glazer protocol at the pelvic floor clinic center of our hospital. The incidences of UI, overactive bladder (OAB), and AI were assessed using the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI-SF) [10] and Epidemiology of Prolapse and Incontinence Questionnaire (EPIQ) [11].
The primary outcome was the PFM sEMG value measured using a modified Glaze
protocol [12]. All procedures were performed by professionally trained healthcare
workers of the pelvic floor clinic center of our hospital. Electromyography (EMG)
signals were acquired using a customized metal vaginal probe (CACB04, MLD V1, Med
lander Medical Equipment Co., Ltd.,
Nanjing, Jiangsu, China), processed using a neuromuscular stimulator (SA9800, MLD
B4, Med lander Medical Equipment Co., Ltd., Nanjing, Jiangsu, China), and
analyzed using a MyoTrac Infiniti system V6.8.11.2 (Thought Technology Ltd.,
Montreal, Quebec, Canada). The final results were expressed in microvolts
(
In accordance with the PFM testing guide jointly formulated by the International Continence Society and International Urogynecology Association [13], the testing procedure consisted of four stages. (1) Pre-testing baseline resting assessment: prior to function testing, the relaxed state was maintained for the measurement of 10-second resting-state EMG values. The average EMG value was determined for the assessment of resting-state PFM tone. (2) Fast-twitch muscle assessment: short PFM contractions were performed, and the maximum peak EMG value was determined for the assessment of fast-twitch muscle function. (3) Slow-twitch muscle assessment: five slow and gentle PFM contractions were performed and held for 10 seconds at maximum tension. The average value of the five contractions was determined for the assessment of slow-twitch muscle strength and coordination between fast-twitch and slow-twitch muscle fibers. (4) Post-testing baseline resting assessment: the baseline state after 60 seconds was recorded for the assessment of PFM recovery function after the muscular activity described above. Prior to the assessment, participants were informed of the complete testing procedure and given instructions on PFM relaxation and contraction through vaginal palpation.
The secondary outcome was PFD prevalence determined using the ICIQ-UI-SF and EPIQ. Both questionnaires have demonstrated good validity and reliability in several studies [10, 11]. The participants completed the questionnaires under the guidance of a urogynecologist to ensure questionnaire validity.
The pre-testing resting stage allowed the assessment of muscle state during
relaxation (normal values,
Data were statistically analyzed using SPSS 25.0 (IBM Corp., Armonk, NY, USA).
Measurement data were tested for normality and expressed as mean
Between September 2019 and December 2021, postpartum pelvic floor function
screening was performed on 24,637 puerperas at the Pelvic Floor Screening and
Rehabilitation Center of our hospital. Four hundred puerperas were eventually
included. The participants were divided into three groups:
fetal distress (FD) (n = 260), prolonged
second stage of labor (PSSL) (n = 30), and
intrapartum fever combined with fetal
distress (IFFD) (n = 110) (Fig. 1). Age, educational attainment, pre-gestational
body weight, gestational BMI gain, method of conception, gestational
complications (gestational hypertension, gestational diabetes, gestation anemia),
gestational age at delivery, neonatal birth weight, and method of placental
delivery were not significantly different among the three groups (p
 Fig. 1.
Fig. 1.Flowchart of the subject screening process.
| Characteristic | Indications for forceps delivery | p | |||
|---|---|---|---|---|---|
| Fetal distress (n = 260) | Prolonged second stage of labor (n = 30) | Intrapartum fever combined with fetal distress (n = 110) | |||
| Maternal age (years) | 30.5 |
30.5 |
30.2 |
0.703 | |
| Prenatal BMI (kg/m |
0.500 | ||||
| 41 (15.8) | 4 (13.3) | 17 (15.5) | |||
| 18.5–24 | 188 (72.3) | 22 (73.4) | 86 (78.2) | ||
| 31 (11.9) | 4 (13.3) | 7 (6.3) | |||
| Gestational BMI gain (kg) | 0.070 | ||||
| 236 (90.8) | 23 (76.7) | 98 (89.1) | |||
| 24 (9.2) | 7 (23.3) | 12 (10.9) | |||
| Educational attainment | 0.700 | ||||
| Less than a diploma | 11 (4.2) | 2 (6.6) | 3 (2.7) | ||
| Diploma | 48 (18.5) | 8 (26.7) | 17 (15.5) | ||
| Bachelor’s degree | 136 (52.3) | 12 (40.0) | 57 (51.8) | ||
| Postgraduate degree or above | 65 (25.0) | 8 (26.7) | 33 (30.0) | ||
| Gestational hypertension | 14 (5.4) | 0 (0.0) | 3 (2.7) | 0.400 | |
| Gestational diabetes | 31 (11.9) | 2 (6.7) | 15 (13.6) | 0.700 | |
| Gestational anemia | 53 (20.4) | 10 (33.3) | 27 (24.5) | 0.200 | |
| ART | 22 (8.5) | 5 (16.7) | 8 (7.3) | 0.300 | |
| Gestational age at delivery (weeks) | 0.900 | ||||
| 34–37 | 8 (3.1) | 1 (3.3) | 4 (3.6) | ||
| 252 (96.9) | 29 (96.7) | 106 (96.4) | |||
| Method of placental delivery | 0.600 | ||||
| Manual removal | 12 (4.6) | 0 (0.0) | 3 (2.3) | ||
| Spontaneous delivery | 248 (95.4) | 30 (100.0) | 107 (97.3) | ||
| Neonatal birth weight (g) | 3290.0 |
3430.0 |
3330.0 |
0.090 | |
Data expressed as mean
BMI, body mass index; ART, assisted reproductive technology.
The overall scores of the PSSL and IFFD groups were significantly lower than
that of the FD group, with the IFFD and FD groups being significantly different
(p
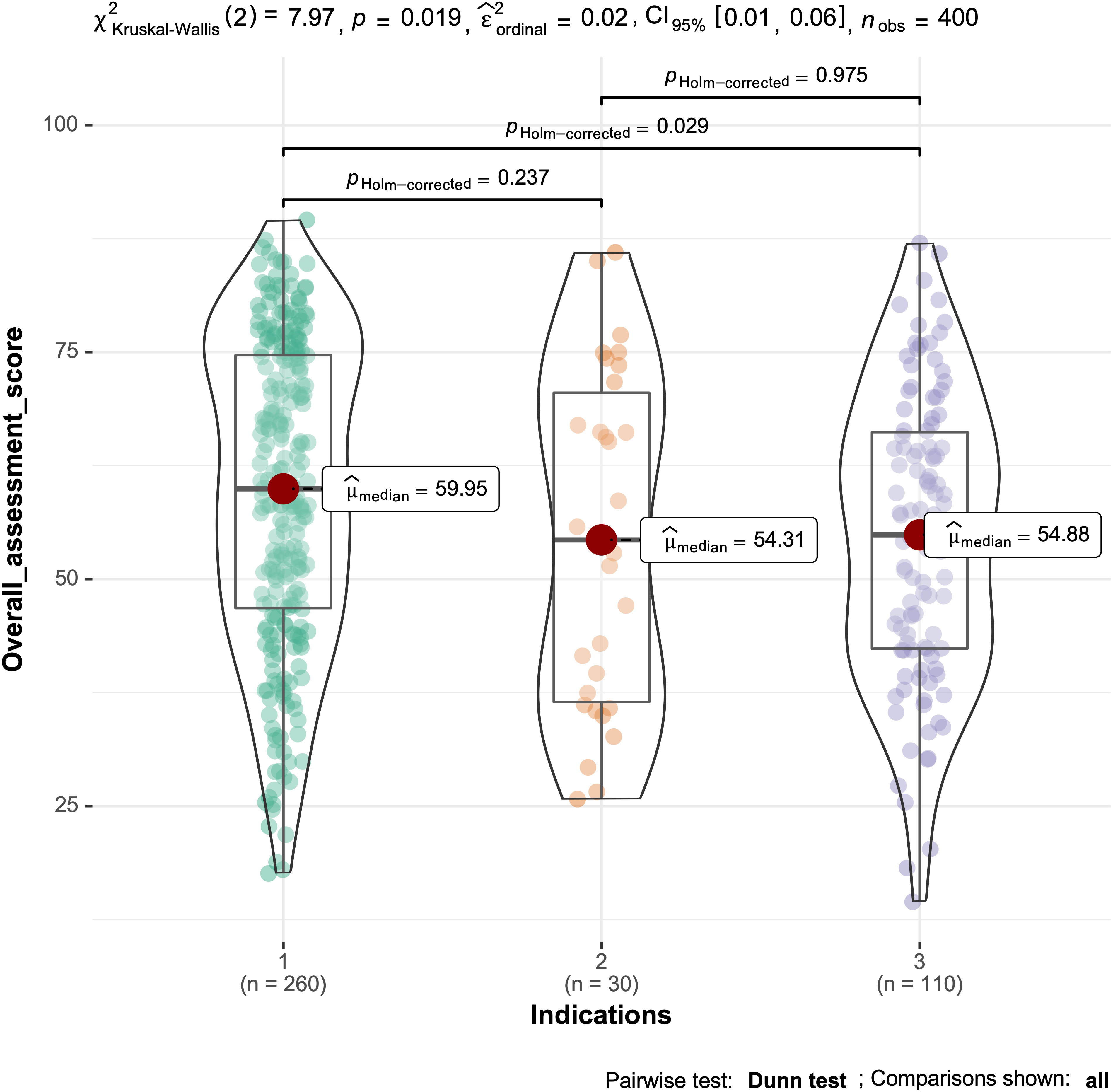 Fig. 2.
Fig. 2.Total Glazer score for the three groups.
| Stage | Indications | p | |||
|---|---|---|---|---|---|
| Fetal distress (n = 260) | Prolonged second stage of labor (n = 30) | Intrapartum fever combined with fetal distress (n = 110) | |||
| Pre-testing resting stage | |||||
| Average | 4.3 |
7.8 |
4.0 |
0.305 | |
| Variability | 0.2 |
0.2 |
0.2 |
0.070 | |
| Muscle overactivity (%) | 96 (36.9) | 16 (53.3) | 34 (30.9) | 0.080 | |
| Fast-twitch (type II muscle fibers) stage | |||||
| Maximum | 32.4 |
31.7 |
26.5 |
0.023* | |
| Rise time | 0.4 |
0.4 |
0.5 |
0.171 | |
| Recovery time | 0.5 |
0.5 |
0.6 |
0.750 | |
| Decrease in fast-twitch muscle strength (%) | 190 (73.1) | 23 (76.7) | 97 (88.2) | 0.006* | |
| Slow-twitch (type I muscle fibers) stage | |||||
| Average | 21.3 |
19.7 |
17.9 |
0.075 | |
| Variability | 0.3 |
0.3 |
0.3 |
0.355 | |
| Rise time | 0.6 |
0.7 |
0.6 |
0.588 | |
| Recovery time | 1.3 |
1.5 |
1.4 |
0.278 | |
| Decrease in slow-twitch muscle strength (%) | 225 (86.5) | 28 (93.3) | 104 (94.5) | 0.050 | |
| Post-testing resting stage | |||||
| Average | 4.2 |
6.5 |
3.6 |
0.384 | |
| Variability | 0.2 |
0.2 |
0.2 |
0.998 | |
| Muscle overactivity (%) | 106 (40.1) | 15 (50.0) | 39 (35.5) | 0.300 | |
| Overall score | 59.3 |
54.4 |
54.6 |
0.019* | |
Data expressed as mean
*, p
The incidence of postpartum SUI was significantly higher in the IFFD and PSSL
groups compared with the FD group, with the IFFD and FD groups being
significantly different (p
| PFD | Indications | p | ||
|---|---|---|---|---|
| Fetal distress (n = 260) | Prolonged second stage of labor (n = 30) | Intrapartum fever combined with fetal distress (n = 110) | ||
| UI | 44 (16.9) | 6 (20.0) | 31 (28.2) | a vs. b p: 0.357; a vs. c p: 0.013; b vs. c p: 1.000 |
| SUI | 26 (10.0) | 6 (20.0) | 24 (21.8) | a vs. b p: 1.000; a vs. c p: 0.049; b vs. c p: 1.000 |
| OAB | 2 (0.8) | 1 (3.3) | 1 (0.9) | 0.300 |
| FOS | 66 (25.4) | 8 (26.7) | 27 (24.5) | 1.000 |
a, FD group; b, PSSL group; c, IFFD group.
PFD, pelvic floor dysfunction; UI, urinary incontinence; SUI, stress urinary incontinence; OAB, overactive bladder; FOS, constipation (full of stool); FD, fetal distress; PSSL, prolonged second stage of labor; IFFD, intrapartum fever combined with fetal distress.
Studies have proposed the following reasons for the increased incidence of PFDs with OVD: (1) long-term elevation of hormone levels during gestation causes changes in the metabolism of PFM collagen fibers, thereby leading to abnormalities in pelvic floor support structures [14], and (2) OVD increases the risk of PFM injury [5]. This study assessed the effects of different forceps delivery indications on early postpartum pelvic floor function in primiparas using the Glazer protocol.
Despite the inability of sEMG to directly measure muscle strength, it has been demonstrated that the levels of rapid and sustained contractions measured by sEMG are closely associated with the motor and resting functions of muscles [13].
Our results revealed that the participants who underwent forceps delivery owing to IFFD had a significantly lower overall Glazer assessment score at 42–60 days postpartum compared with those who underwent forceps delivery solely because of FD. The overall score of participants who underwent forceps delivery owing to PSSL was not significantly different from that of the IFFD group but was significantly lower than that of the FD group. Moreover, studies have reported that prolongation of the second stage is an independent risk factor for the occurrence of PFDs. This may be related to the persistent exertion of pressure by the fetal head on the nervous tissues and PFMs during the second stage of labor, which results in tissue edema, hyperemia, and muscle relaxation [15]. The fetus exerts the strongest effects on the birth canal, and the pelvic floor soft tissues are subjected to great forces during the second stage of labor [16]. Caudwell-Hall et al. [17] has reported that a prolonged second stage causes an increase in the time that the PFMs are subjected to pressure. Under such persistent and strong action, the probability of the occurrence of tears and ruptures in muscle fibers (especially type I muscle fibers) is significantly increased, and patients who sustain perineal tears are more prone to developing severe PFDs [17]. Research has further shown that PSSL is associated with an increased possibility of postpartum incontinence in women and increases the long-term risk of UI [18]. In the present study, the incidence of early postpartum UI in the PSSL group was higher than that in the FD group, with the incidence of SUI being twice as high as that of the FD group. This is consistent with the results of a study on an Australian population by Brown et al. [19], who reported that the probability of SUI occurrence in puerperas who experienced PSSL was 1.9 times that of puerperas with a normal second stage of labor.
Additionally, we found that the IFFD group
had the lowest overall Glazer assessment score and was prone to decreased
strength in the pelvic floor fast-twitch muscles (type II muscle fibers). The
maximum EMG values of the three groups during the fast-twitch (type II muscle
fibers) stage were 32.4
The main pathophysiological mechanism of PFD onset is the occurrence of pathological changes in muscle fibers, which induces abnormalities in overall muscle tone, or contraction function of muscles, resulting in the transposition, or dysfunction, of pelvic organs such as the uterus, bladder, and rectum [21]. The incidence of intrapartum fever is 1.6–14.6% [9]. A case-control study has shown fever was associated with epidural analgesia, nulliparity, and a long duration of labor. Multiple regression analysis showed that all three variables were independently associated with maternal temperature [22]. First-time delivery, a higher rate of epidural analgesia and a longer second stage of labor are associated with an increased incidence of intrapartum fever. Current research indicates that intrapartum fever is not entirely attributable to maternal infection, as most cases of intrapartum fever are caused by non-infective inflammation [23]. Possible mechanisms of intrapartum fever occurrence include endogenous heat generated by skeletal muscle contractions, infective inflammation following amnion rupture, and epidural analgesia [24]. In the present study, the white blood cell count and C-reactive protein (CRP) inflammation indicator values of all the participants in the IFFD group were higher than the normal values.
Furthermore, the inflammatory response may aggravate muscle injury [25, 26]. Since anaerobic metabolism does not favor the absorption and excretion of inflammatory cytokines [27, 28], the intrapartum fever-induced decrease in pelvic floor fast-twitch (type II muscle fibers) muscle strength may be related to the fact that metabolism is predominantly anaerobic in the fast-twitch muscles (type II muscle fibers). Impairment of the rapid contraction function of PFMs is mainly manifested as decreased muscle strength, rhythm disturbances, and weakening of intentionality in fast-twitch muscles; impairment of the persistent contraction function is mainly manifested as a reduction in the supportive and immobilization abilities of slow-twitch muscles, which causes the weakening of support strength.
The fast-twitch (type II muscle fibers) stage is mainly aimed at testing dynamic fast-twitch muscle (type II muscle fibers) strength and reaction speed. Individuals with inadequate fast-twitch muscle strength are prone to UI, AI, sexual indifference, and decline in sexual experience [29]. Our results further indicated that the IFFD group had a higher incidence of UI compared with the PSSL and FD groups, with the incidence of SUI being 2.18 times that of the FD group. This may be related to the decline in fast-twitch muscle strength caused by intrapartum fever. Further research is required on the mechanism of the decreased pelvic floor fast-twitch muscle strength caused by intrapartum fever. Pelvic floor muscle exercises decrease urinary incontinence in pregnancy, postpartum period, and later in life. A study has shown that there is a lack of knowledge about the relationship between pelvic floor muscle exercises and pelvic floor disorders [30]. The present study faces the same problem, and that is what we are going to work on in the future.
The main limitation of this study was that we only focused on vaginal sEMG tests in different indications for forceps delivery; sEMG values were not obtained for prenatal and non-pregnant women. The sEMG evaluation at 6–8 weeks after delivery may be insufficient. Moreover, the long-term pelvic floor function outcome of puerpera warrants further clinical follow-up study.
Intrapartum fever affects the early postpartum pelvic floor function of primiparas who underwent forceps delivery, which mainly manifests in the short term as reduced fast-twitch muscle (type II muscle fibers) strength and the occurrence of SUI. Therefore, vaginal examinations should be minimized as much as possible prior to the administration of labor analgesics to avoid the occurrence of intrapartum fever and consequent adverse effects of forceps delivery on the pelvic floor function of primiparas. Furthermore, the screening of pelvic floor function should be performed during the early postpartum period in primiparas indicated for forceps delivery due to intrapartum fever combined with fetal distress. Future studies should use larger sample sizes and longer follow-up times to assess whether intrapartum fever and a prolonged second stage of labor increase the risk of pelvic floor diseases.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
XLC—conception and design, data analysis and writing original draft; SSY—conception and design, data analysis and writing original draft; KZ—Data analysis and questionnaires; CCX—data acquisition and questionnaires; XHS—data acquisition and questionnaires; XLC—conception and design, funding acquisition, and writing review & editing. All authors read and approved the final manuscript.
This study was approved by the ethics committee of the International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University (No. GKLW 2021–55). All participants gave their informed consent for inclusion before they participated in the study.
We sincerely thank all women who participated in the study, and the medical staff at the Pelvic Floor Screening and Rehabilitation Center. And we would like to express our appreciation to the peer reviewers for the contributions they made to the study.
This research was funded by the Project of National Natural Science Foundation of China (CR2018WX01) and the Shanghai Municipal Key Clinical Specialty, Shanghai, China (GFY1808004).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
