1 Department of Gynecology and Obstetrics, the Second Affiliated Hospital of Xinjiang Meidical University, 830063 Urumqi, Xinjiang, China
Abstract
Background: Sleep disorder, frequently observed among perimenopausal women, decreases quality of life. Melatonin is reported to ameliorate circadian imbalances and thus can be employed as a treatment for perimenopausal sleep disorder patients. We attempted to clarify whether and how melatonin affects perimenopausal sleep disorders. Methods: Study patients consisted of 120 perimenopausal women divided into 3 groups: (i) perimenopausal women without sleep disorder (n = 60); (ii) those with sleep disorder but without melatonin treatment (n = 30); and (iii) those with sleep disorder with melatonin treatment (n = 30). During the period March 2019 to December 2019, the following data was collected and analyzed: Pittsburgh Sleep Quality index (PSQI) score, sex hormones, melatonin, melatonin 1A receptor (MTNR1A), protein kinase A (PKA), extracellular signal-regulated kinase 1/2 (ERK1/2), phosphorylation–PKA (p-PKA), and p-ERK1/2 levels. We compared these data between the groups. Results: Melatonin administration showed the following in female patients with postmenopausal sleep disorder: (1) significantly decreased the PSQI scores, (2) up-regulated melatonin levels and MTNR1A protein expression, (3) promoted the phosphorylation pathway of the PKA-ERK1/2 pathway in peripheral blood, and (4) significantly improved follicle-stimulating hormone (FSH), luteinizing hormone (LH) and estradiol (E2) levels. Melatonin administration had no significant effect on progesterone (P), testosterone (T) or prolactin (PRL). Conclusions: Melatonin therapy alleviated perimenopausal sleep disorders. Up-regulation of MTNR1A expression and improvement of the hormone balance were also observed, which may the reason for the observed sleep-disorder-amelioration. Melatonin has the potential to be a useful option for perimenopausal sleep disorders.
Keywords
- melatonin
- perimenopausal sleep disorders
- MTNR1A
- hormone balance
Clinical studies have reported that perimenopausal women are more likely to have sleep disorders with late perimenopausal women being 1.3 times more likely to have sleep disorders than early perimenopausal women [1]. Sleep problems increase with age and are more common in women than men and increased in women over 45 years of age [2]. Studies of perimenopausal sleep disorders have revealed that difficulty falling sleep and frequent nighttime awakenings are due to insufficient melatonin production [3, 4]. In vertebrates, melatonin is centrally synthesized by the pineal gland as well as being produced in peripheral tissues and acts as an autocrine and paracrine signal [5]. The main hormonal system synthesis of melatonin is the adaptation of the central nervous/endocrine system to environmental geophysical days and seasons through coordinated behavior and physiology [6]. During the daily secretion phase, melatonin coordinates the nocturnal adaptive physiology through immediate effects while initiating the diurnal adaptive response through anticipatory effects that occur only when melatonin is deficient during the day [7]. This suggests the importance of exploring the potential role of melatonin in the process of perimenopausal sleep disorders.
Melatonin and melatonin 1A receptor (MTNR1A) are part of the circadian entrainment pathway, which is the cycle of the internal biological clock driven by repeated exogenous signals in order to synchronize the endocrine and behavioral rhythms with environmental cues [8]. Melatonin is an endogenous hormone produced by the pineal gland that synchronizes circadian rhythms, regulates sleep cycles, and improves the onset, duration and quality of sleep [9]. Circadian rhythm is one of the main processes controlling sleep, and sleep disorders are related to alterations in circadian rhythm [10]. Animal studies have shown that acupuncture can treat sleep disorders by up-regulating the expression of MTNR1A in rats [11]. The role of melatonin and MTNR1A in perimenopausal sleep disorders remains unknown and needs further investigation.
Cellular experiments showed that melatonin/MTNR1A could activate extracellular signal-regulated kinase 1/2 (ERK1/2) in HEK-293 cells through the Gs/cAMP/protein kinase A (PKA) pathway [12]. Melatonin increased the phosphorylation of PKA (Thr197) and ERK (Thr202/Tyr204) in Neura-2A cells [13]. Melatonin has been found to promote the phosphorylation of MEK1/2 and ERK1/2 in a dose-dependent manner in MT1-CHO cells [14]. An in vitro model verified that PKA-ERK1/2 can mediate the shift of ventricular choroid plexus clock and regulate circadian rhythm [15]. Melatonin [16] and melatonin receptor agonists [17] have been widely used in the treatment of perimenopausal sleep disorders, but their mode of action remains unknown. Studies have attempted to explore the relationship between melatonin and perimenopausal sleep disorders from the perspective of lipid peroxidation and antioxidants [18], but the peroxidation-oxidation defense mechanism has not been adequately clarified. We speculate that melatonin may change the circadian rhythm through MTNR1A-mediated regulation of PKA-ERK1/2, thereby being a useful agent to treat perimenopausal sleep disorders.
Poor sleep quality and chronic sleep disorders are common in perimenopausal patients [19]. The hypothalamic-pituitary-ovarian axis and sleep-wake regulatory system are neuro-endo analytic systems that regulate the changes of the hormone environment and sleep quality in perimenopausal women [20]. The role of melatonin in gonadal steroid production may be mediated by multiple transduction pathways supporting cell physiology and spermatogenesis, as well as the production of estrogen and progesterone [21]. In this study, we attempted to determine the role of melatonin as a single agent for perimenopausal sleep disorders.
Patients entered into this study between March 2019 and December 2019 from the
Obstetrics and Gynecology Department of the Second Affiliated Hospital of
Xinjiang Medical University. The study included 60 perimenopausal patients
without sleep disorder (Control), 30 female patients with postmenopausal sleep
disorder group (PSD), and 30 female patients with postmenopausal sleep disorder
who received melatonin treatment (PSD + MEL). Perimenopausal status was defined
by the ‘2011 Stages of Reproductive Aging Workshop criteria’ [22]. All patients
who met the diagnostic criteria, based on Stages of Reproductive Aging Workshop
[23] and a Pittsburgh Sleep Quality index (PSQI) Score
Blood samples were centrifuged at 1000 g at 2–8 ℃ for 15 min after being stored at room temperature for 2 hours. The supernatant was taken after centrifugation for evaluation. Melatonin (CSB-E08132H, Cusabio, Wuhan, Hubei province, China), MTNR1A (ABIN6957741, antibodies-online GmbH, Germany), PKA (ABIN771168, antibodies-online GmbH, Germany), ERK1/2 (ML060672, Mlbio, Shanghai, China), p-ERK1/2 (ML060183, Mlbio, Shanghai, China) detection kit and multi-functional microplate analyzer (MB-530, HEALES, Hertford, UK) were used to detect melatonin, MTNR1A, PKA, ERK1/2 and p-ERK1 levels.
Statistical analysis was performed using GraphPad Prism 8.0 software (GraphPad,
San Diego, CA, USA). The measurement data were expressed as mean
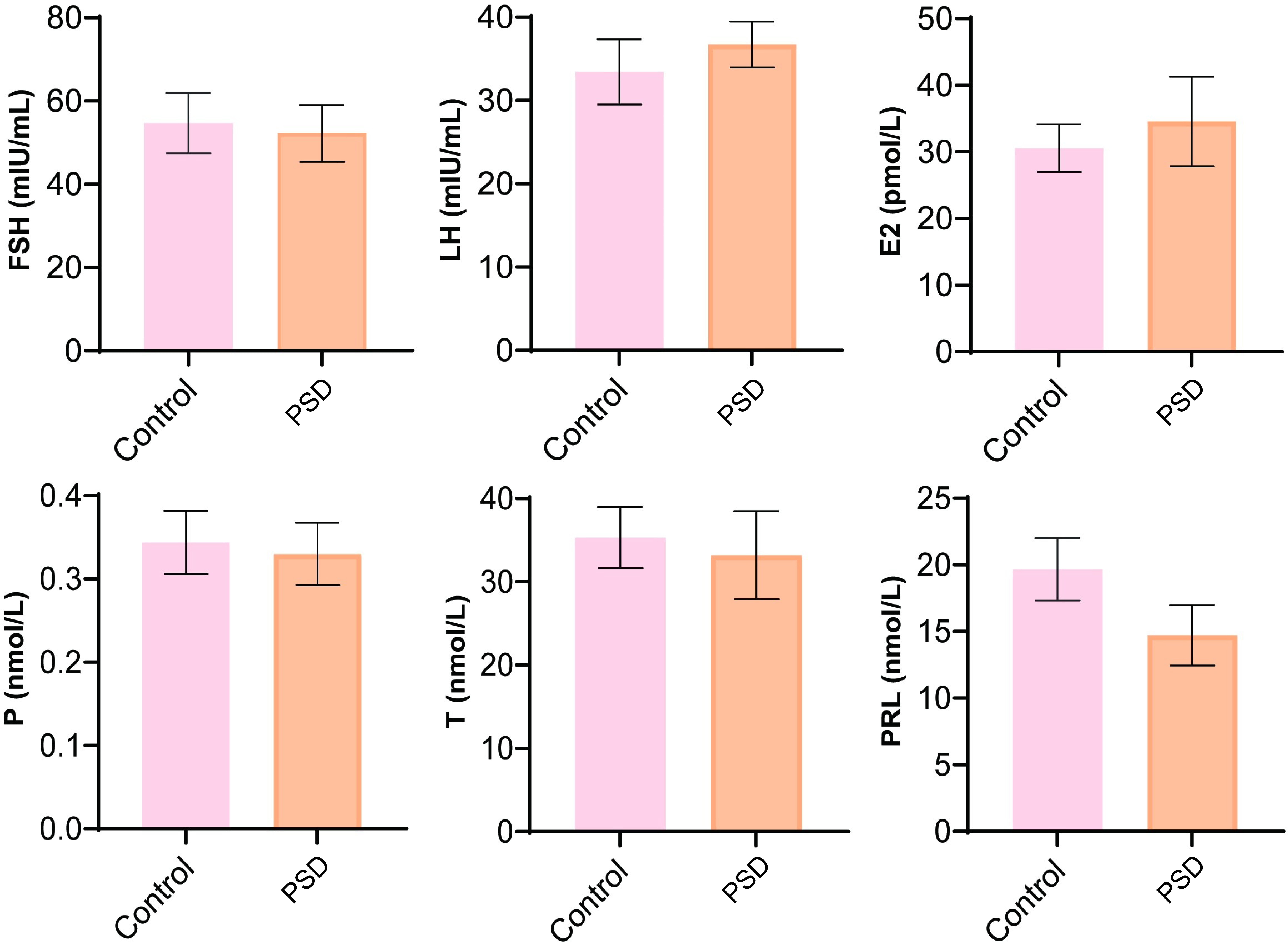
We analyzed the changes in the sex hormone levels and found that FSH, LH, E2, P, T, and PRL demonstrated no significant changes between the control and perimenopausal sleep disorder groups (Fig. 1).
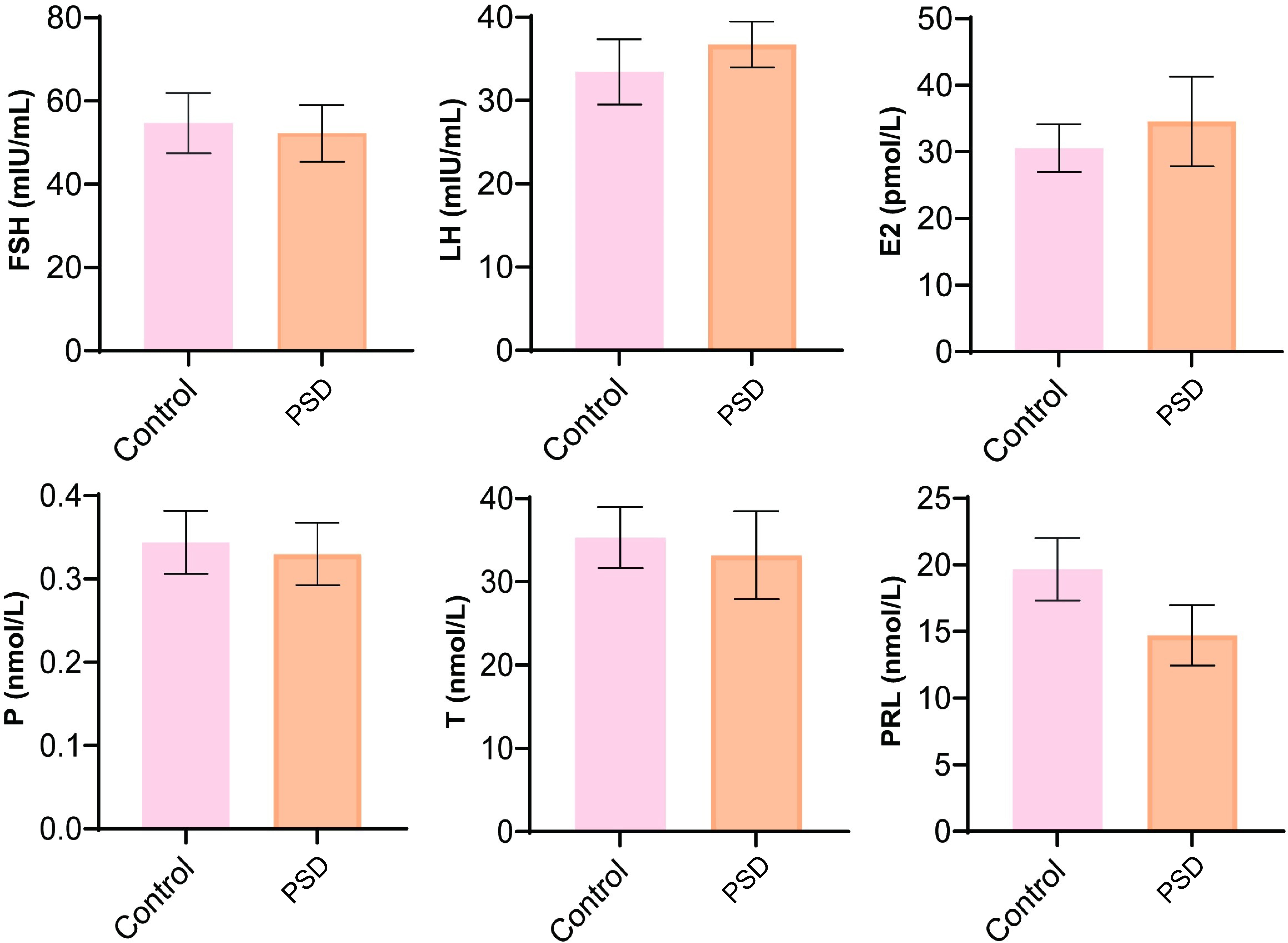 Fig. 1.
Fig. 1.Analysis of sex hormone levels included follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), progesterone (P), testosterone (T), and prolactin (PRL). Control represented perimenopausal women without sleep disorder, PSD represented the female patients with postmenopausal sleep disorder group.
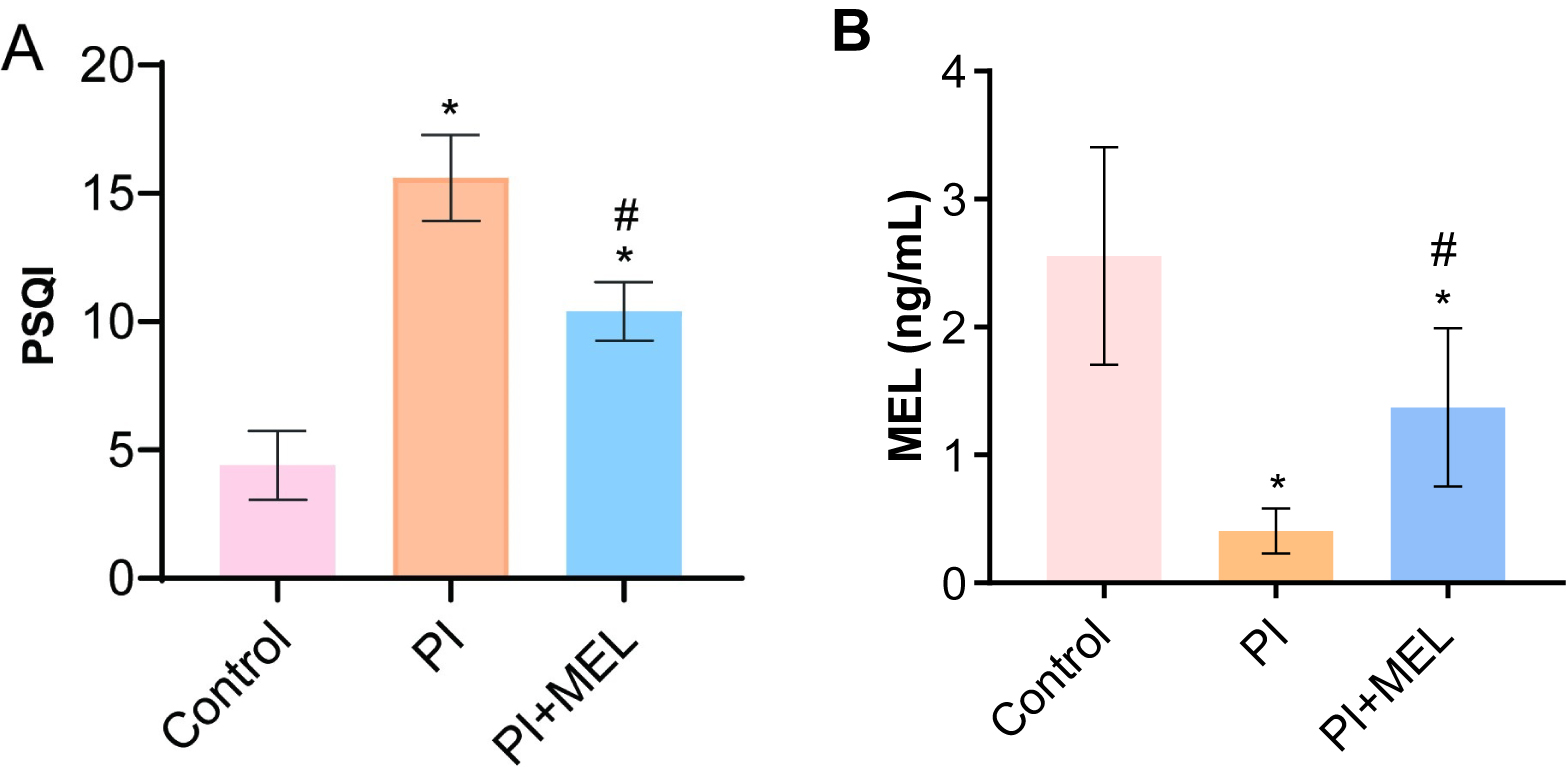
We compared the melatonin level within the control group and discovered that the PSQI scores in the PSD group significantly increased while a decrease was noted in the melatonin group (Fig. 2A). Compared with the control group, melatonin levels in the perimenopausal sleep disorder group were significantly decreased (Fig. 2B). When compared to the perimenopausal sleep disorder group, peripheral blood melatonin levels were significantly increased in patients in the melatonin intervention group (Fig. 2B).
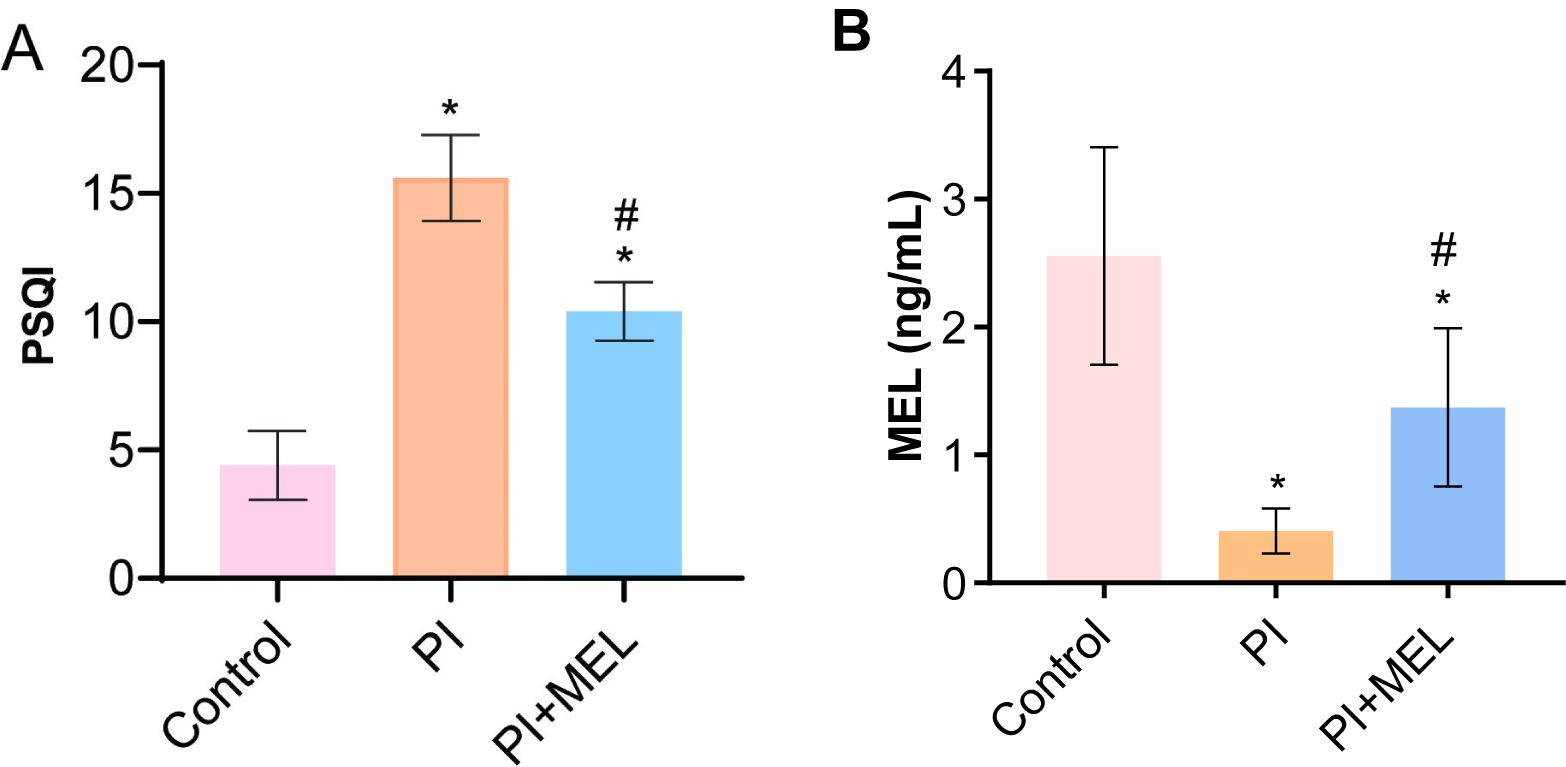 Fig. 2.
Fig. 2.Melatonin treatment affected sleep quality and hormone levels.
(A) The PSQI score. (B) Melatonin levels were detected by ELISA. *p
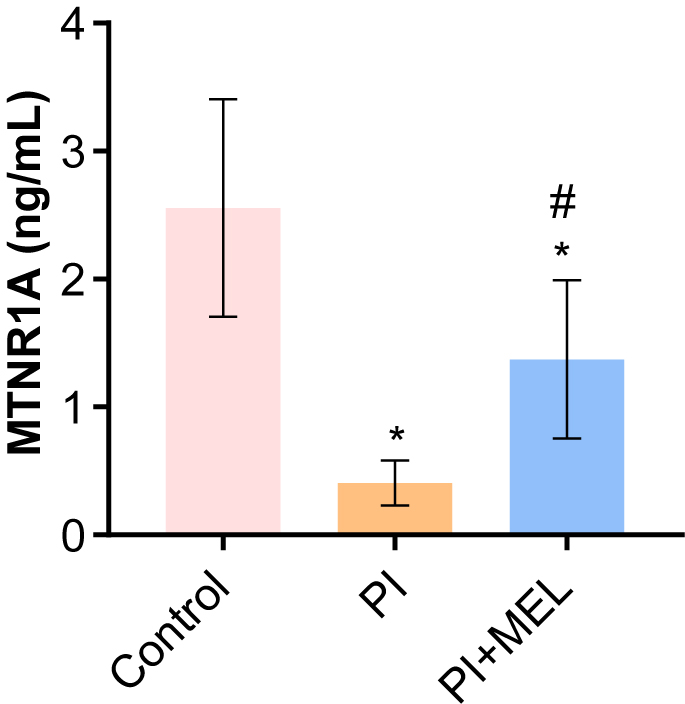
We investigated the effect of melatonin intervention on MTNR1A expression in perimenopausal sleep disorders. The results demonstrated that compared to the control group, the expression levels of MTNR1A protein in blood of the perimenopausal sleep disorder group were significantly decreased (Fig. 3). The expression levels of MTNR1A protein were increased in blood of the melatonin intervention group (Fig. 3). The results demonstrated that melatonin intervention could increase the expression level of MTNR1A in blood of female patients with postmenopausal sleep disorder.
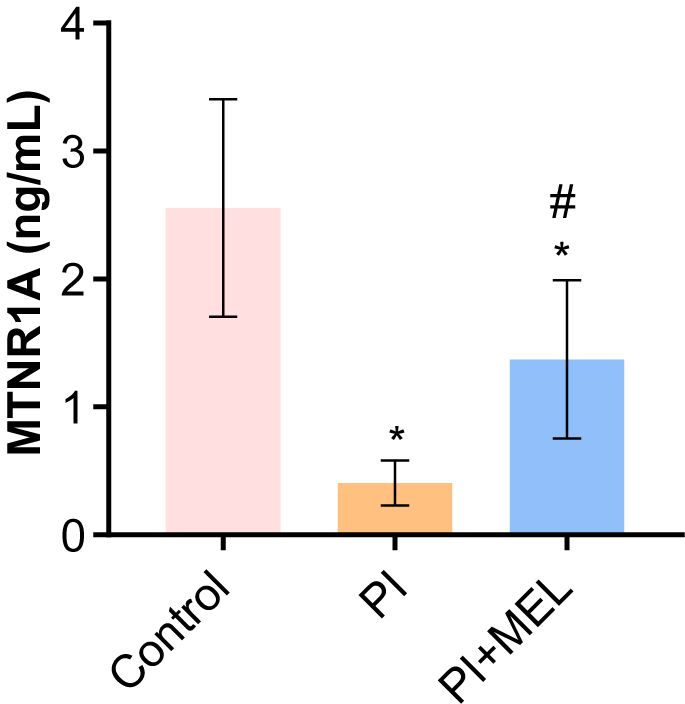 Fig. 3.
Fig. 3.The level of MTNR1A in peripheral blood. *p
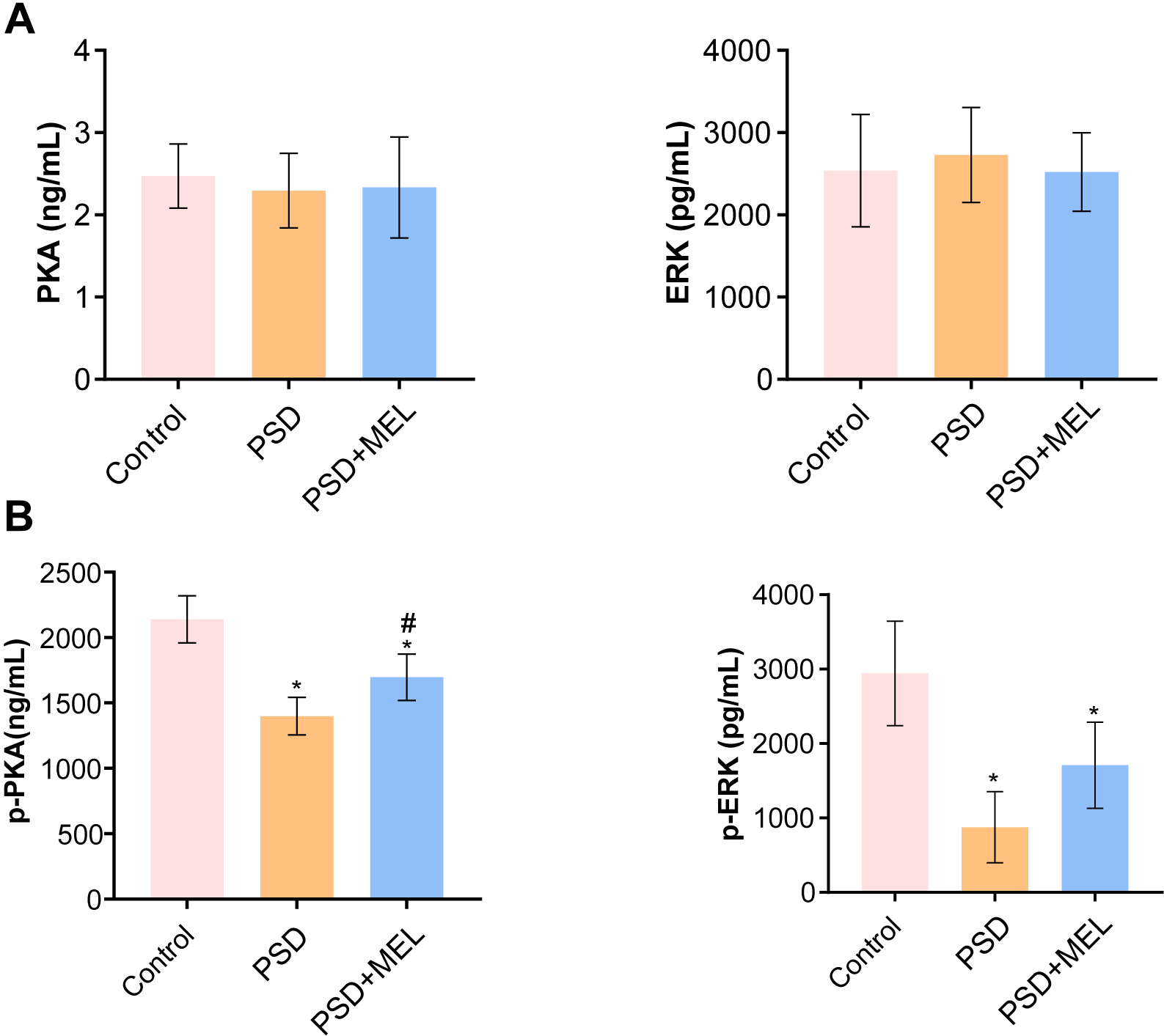
We explored the effects of melatonin intervention on PKA-ERK1/2 pathways in perimenopausal sleep disorder patients. Compared with the control group, the expressions of PKA and ERK1/2 in blood of the perimenopausal sleep disorder group and the perimenopausal sleep disorder + Melatonin group were not significantly changed (Fig. 4A). Protein phosphorylation analysis showed that phosphorylation–PKA (p-PKA) and p-ERK1/2 protein expressions in blood of the perimenopausal sleep disorder group were decreased compared to the control group (Fig. 4B). Melatonin intervention promoted the expression of p-PKA and p-ERK1/2 proteins in blood of perimenopausal patients with sleep disorders (Fig. 4B). These results suggest that melatonin can alleviate perimenopausal sleep disorders by promoting the expression of PKA-ERK1/2.
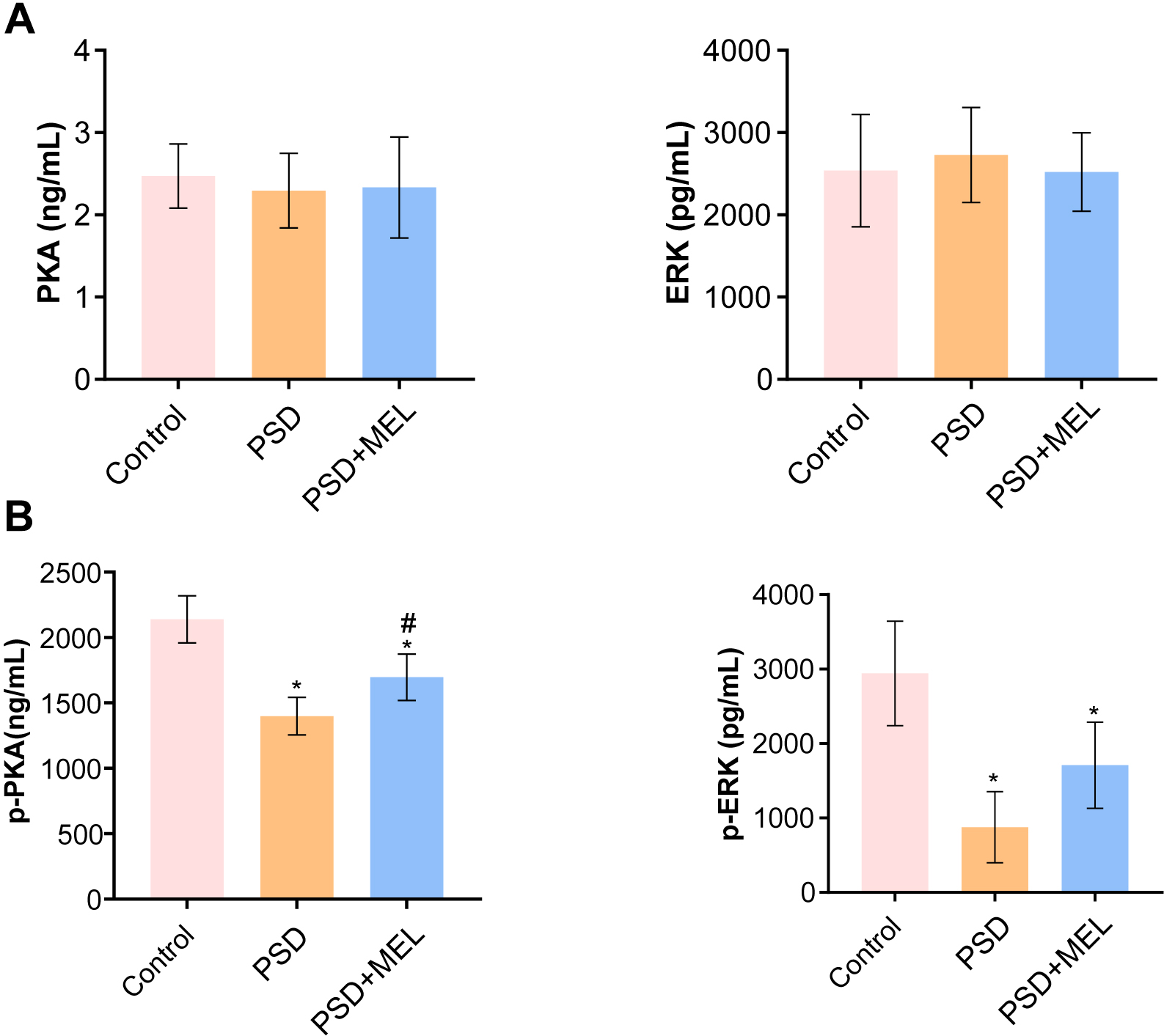 Fig. 4.
Fig. 4.The expression of PKA- ERK1/2 in peripheral blood. (A) The PKA
and ERK1/2 protein levels in peripheral blood were measured by ELISA.
(B) The levels of p-PKA and p-ERK1/2 in peripheral blood were measured by ELISA.
*p
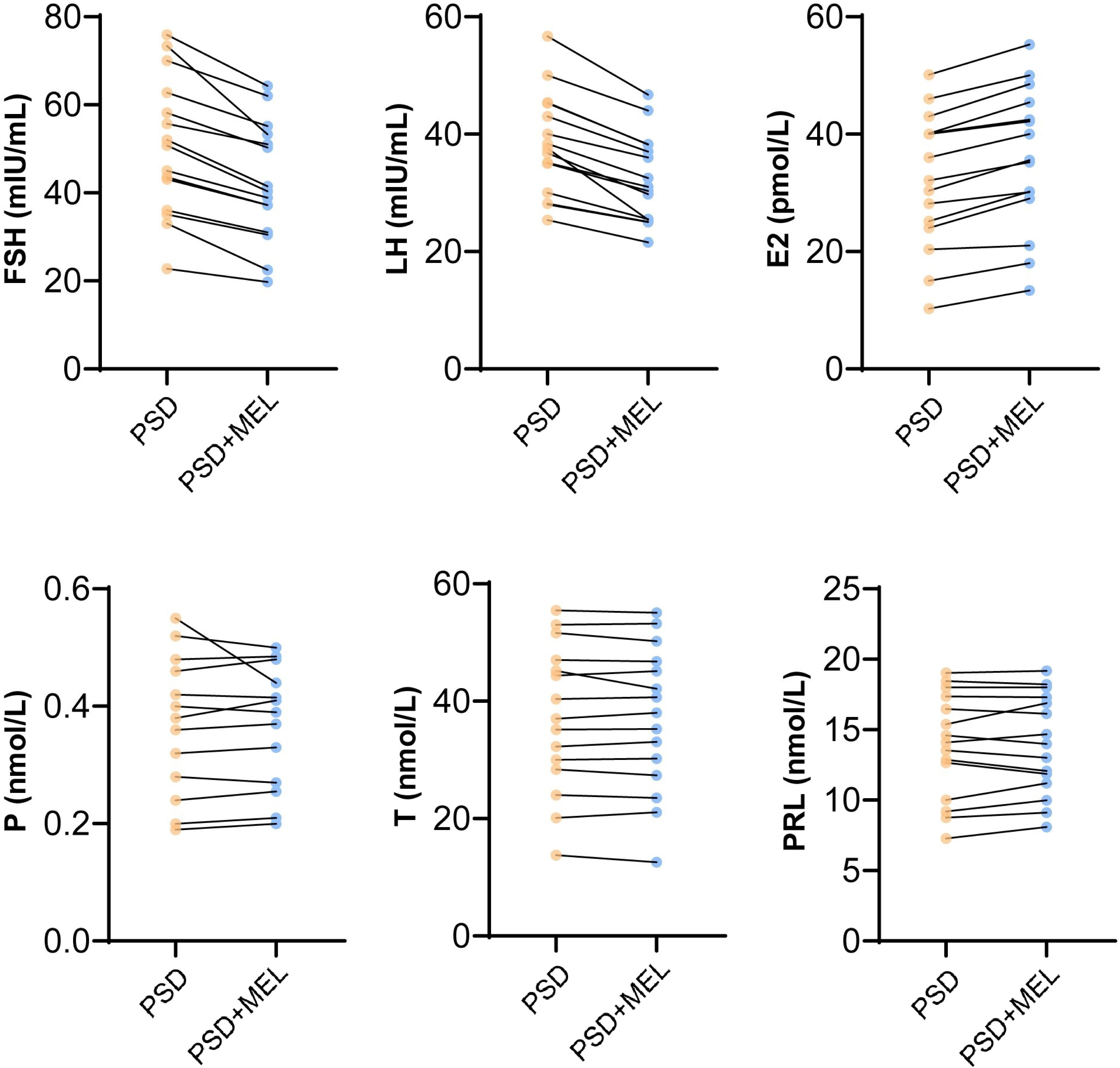
Our results revealed that melatonin intervention improved PSQI and melatonin level, as well as the expression of PKA, p-ERK and MTNR1A in peripheral blood of perimenopausal sleep disorder patients. Clinical follow-up studies demonstrated that melatonin significantly improved the FSH, LH and E2 levels in patients with perimenopausal sleep disorders, but had no significant effect on P, T and PRL (Fig. 5). The results demonstrated that melatonin intervention improved FSH, LH and E2 levels in female patients with postmenopausal sleep disorder.
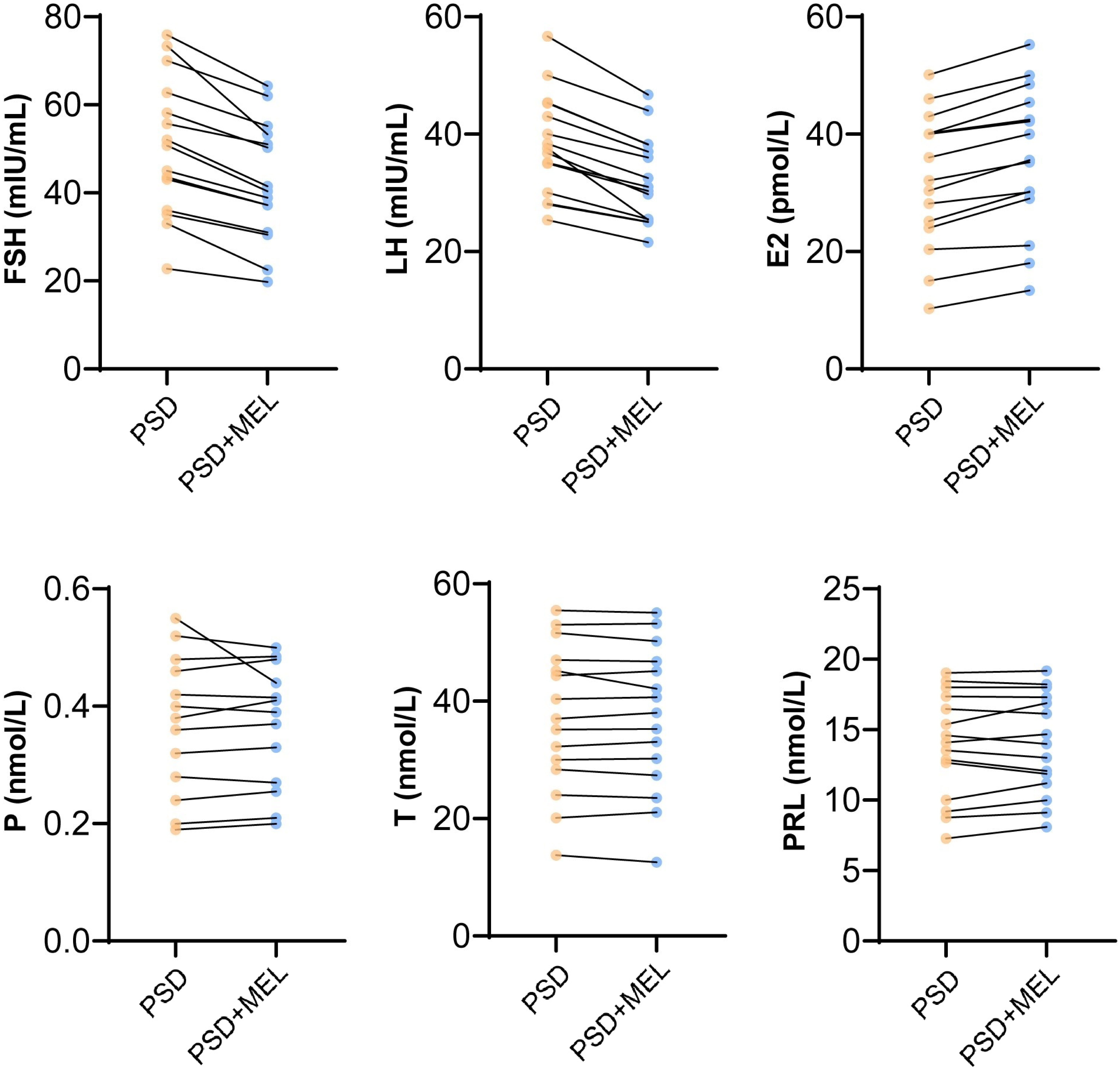 Fig. 5.
Fig. 5.Melatonin affecting sex hormone levels. FSH, Follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; P, progesterone; T, testosterone; PRL, prolactin; MEL, Melatonin. Control represented perimenopausal women without sleep disorder, PSD represented the female patients with postmenopausal sleep disorder group.
Sleep disturbances were associated with reduced melatonin levels only in
perimenopausal women [26]. As one of the components of the antioxidant defense
system in white and Asian female patients with postmenopausal sleep disorder,
melatonin contributed more than 60% of the total component of the antioxidant
protection system in those with sleep disorders [18]. Melatonin is a natural
hormone that promotes sleep in people with sleep disorders and has been widely
used to treat sleep disorders, jet lag and fibromyalgia [27]. Melatonin
containing products are one of the main treatments for age-related
estrogen-deficient sleep disorders [25]. In our study, patients were treated with
3 mg of melatonin for 3 months was able to restore peripheral melatonin levels.
The effects of melatonin are mainly mediated by MTNR1A and subsequent regulation
of downstream signaling pathways including cAMP/PKA, ERK/MAPK, P38 and Ca
PSQI is a useful tool for assessing subjective sleep quality in non-clinical and clinical settings [29]. Women have poorer sleep quality during the menopausal transition and into post-menopause [30]. The fluctuation of the PSQI score, habitual sleep efficiency, and latency was most pronounced in perimenopausal women [31]. According to PSQI and multivariate logistic regression analysis, perimenopausal and postmenopausal women were 1.50 and 1.73 times more likely to have poor sleep hygiene than premenopausal women [32]. Our study found that melatonin therapy significantly decreased the PSQI scores in perimenopausal sleep disorder patients. Based on previous clinical trials combined with the results from our study, melatonin may be an important treatment for women with sleep disorders.
Correlation analysis of reproductive hormone levels with sleep indicators assessed objectively and subjectively showed that the faster the FSH changed, the longer the sleep duration, and the poorer the sleep quality all were associated with poorer self-reported PSQI [33]. Estradiol valerate has been shown to improve serum sex hormone levels, sleep disturbances, negative mood and quality of life in patients with perimenopausal syndrome [34]. In this study, melatonin treatment significantly improved the FSH, LH and E2 levels in patients with perimenopausal sleep disorders, but had no significant effect on P, T and PRL. Our data supports that melatonin treatment improves sex hormone levels and sleep disorders in women with perimenopausal sleep disorders, which may be related to the expression of MTNR1A and PKA/ERK pathway. This study provides new insights into the treatment of perimenopausal sleep disorders and further supports the use of melatonin.
Although our study was limited due to a short duration and single center, we demonstrated that melatonin regulated perimenopausal sleep disorders by upregulation of MTNR1A and downstream PKA/ERK pathway, which may suggest a new approach for the treatment of sleep disorder related conditions.
All data generated or analyzed during this study were included in this manuscript.
PC was responsible for conception and design. PC, QZ and TZ were responsible for data collection and data analysis. PC drafted initial manuscript. All authors critized the first draft and finally approved the version to be published.
This research was approved by the Medical Ethics Committee of Xinjiang Medical University. The reference number was No.20181207-05. Because this was a retrospective study and many patients were discharged without follow-up, intervention, or personal real-name information, the ethics committee waived informed consent.
We thank the anonymous reviewers for their constructive comments and suggestions.
The study was supported by the Natural Foundation of Xinjiang Uygur Autonomous Region (No.2018D01C225).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.