1 Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, McGill University, Montreal, QC H2L 4S8, Canada
2 MUHC Reproductive Center, McGill University, Montreal, QC H2L 4S8, Canada
Abstract
Background: Although the number of follicles at intrauterine
insemination (IUI) is associated with the pregnancy rates and multiple pregnancy
rates. Multiple pregnancy rates are low in older women. Therefore, this study was
undertaken to determine the clinical pregnancy rate of IUI in women 38–43 years
of age based on the number of stimulated mature follicles. Methods: A
retrospective cohort study was performed including all the first to third
stimulated IUI cycles conducted after the age of 38 years in a single academic
fertility center between January 2011 and March 2018. Results: A total of 1574 IUI cycles were included in the study. The patients were divided
according to the number of mature follicles (
Keywords
- controlled ovarian hyperstimulation (COH)
- intrauterine insemination (IUI)
- older patients
- infertility
Controlled ovarian hyperstimulation (COH) with or without intrauterine insemination (IUI) is often used as a treatment for unexplained infertility, early-stage endometriosis, and borderline male-factor infertility [1]. COH combined with IUI is an important tool in infertility therapy, increasing the number of available oocytes thus enhancing the probability of conception.
Female age is an important predictor of both natural and treatment-related live
birth rates (LBR), which decrease rapidly after 35 years of age [2]. Predictive
factors for ongoing pregnancy with IUI other than woman age
As women age, rates of aneuploidy increase with aneuploidy rates in human
blastocysts rising from a 30% baseline in women younger than 35 years to
Although COH/IUI is widely used, there is limited evidence to understand the role of the number of mature follicles and suggest the optimal protocol to maximize the likelihood of conception and LBR in older women while minimizing the risk of multiple-pregnancy. We aimed this study to determine the pregnancy-rate in IUI for women 38–43 years of age based on the number of mature follicles stimulated.
We performed a retrospective cohort study of women 38–43 years of age at the time of IUI in a single academic fertility center between January 2011 and March 2018. Institutional review board approval was obtained (Approval number 2019-5254).
In our location, between 2011–2015, IVF was government funded. However, there were often waiting lists for IVF, so patients elected to perform COH/IUI which were more available, while in anticipation for IVF. In 2015 government coverage for IVF ceased but was continued for IUI (up to 9 cycles). Therefore, patients requested IUI despite the indication for care, due to the lack of cost of this treatment. It was felt that this would provide a rather unbiased population to study.
The primary outcome the was clinical pregnancy rate per IUI cycle. Secondary
outcomes were pregnancy rates and multiple-pregnancy rates. Pregnancy was defined
as a serum
Inclusion and exclusion criteria: all first to third COH/IUI cycles for women ages 38–43 years were analyzed. Natural (unstimulated) cycles were excluded from the study.
All subjects had at least one patent fallopian tube. None of the women were known to have stage 3/4 endometriosis, submucosal fibroids, or polyps in situ.
Doses used were Clomiphene citrate 50 or 100 mg (Sanofi Canada, Laval, QC,
Canada or EMD Serono, Montreal, QC, Canada), Letrozole 5 mg (Novartis, East
Hanover, NJ, USA), and Gonadotrophins (GTs) as injectable recombinant follicle
stimulating hormone (FSH) (Gonal-F; EMD Serono, Montreal, QC, Canada or Puregon,
Organon/Merck Canada, Kirkland, QC, Canada), or human menopausal gonadotropin
(hMG) (Menopur, Ferring Canada, Longueuil, QC, Canada) 50–300 IU daily, based on
ovarian reserve. Patients using Clomiphene citrate or Letrozole ingested their
medication on days 2–6 of the cycle and had an ultrasound at day 10 to monitor
follicular growth. Patients in care would have a baseline ultrasound on cycle
day 2–3. If ultrasound revealed endometrial thickness
When the leading follicle was
For the sake of this study, mature follicles were defined
Both partner sperm and commercially available donor-sperm insemination cycles were included in the study. When partner sperm was used, the partner was instructed to abstain from ejaculation for 48-hours before IUI. Samples were produced on-site and processed within 30 minutes of production. A basic wash and a density gradient centrifugation were performed on all semen samples. The concentrated pellet was reconstituted to a volume of 0.5 mL with tubal media (Ferticult, Beemm, Belgium). All samples were inseminated into the uterus using a Cook catheter (Cook Corporation, Bloomington, IN, USA).
A serum
Statistical analysis was performed with SPSS 23.0 (IBM Corp, Chicago, IL, USA).
All data are presented as percentages or mean
A total of 1574 IUI cycles were included in the study. All subjects were
stimulated with either Clomiphene Citrate (n = 240), Letrozole (n = 176) or GTs
(n = 1158). We divided the patients according to the number of mature follicles
(
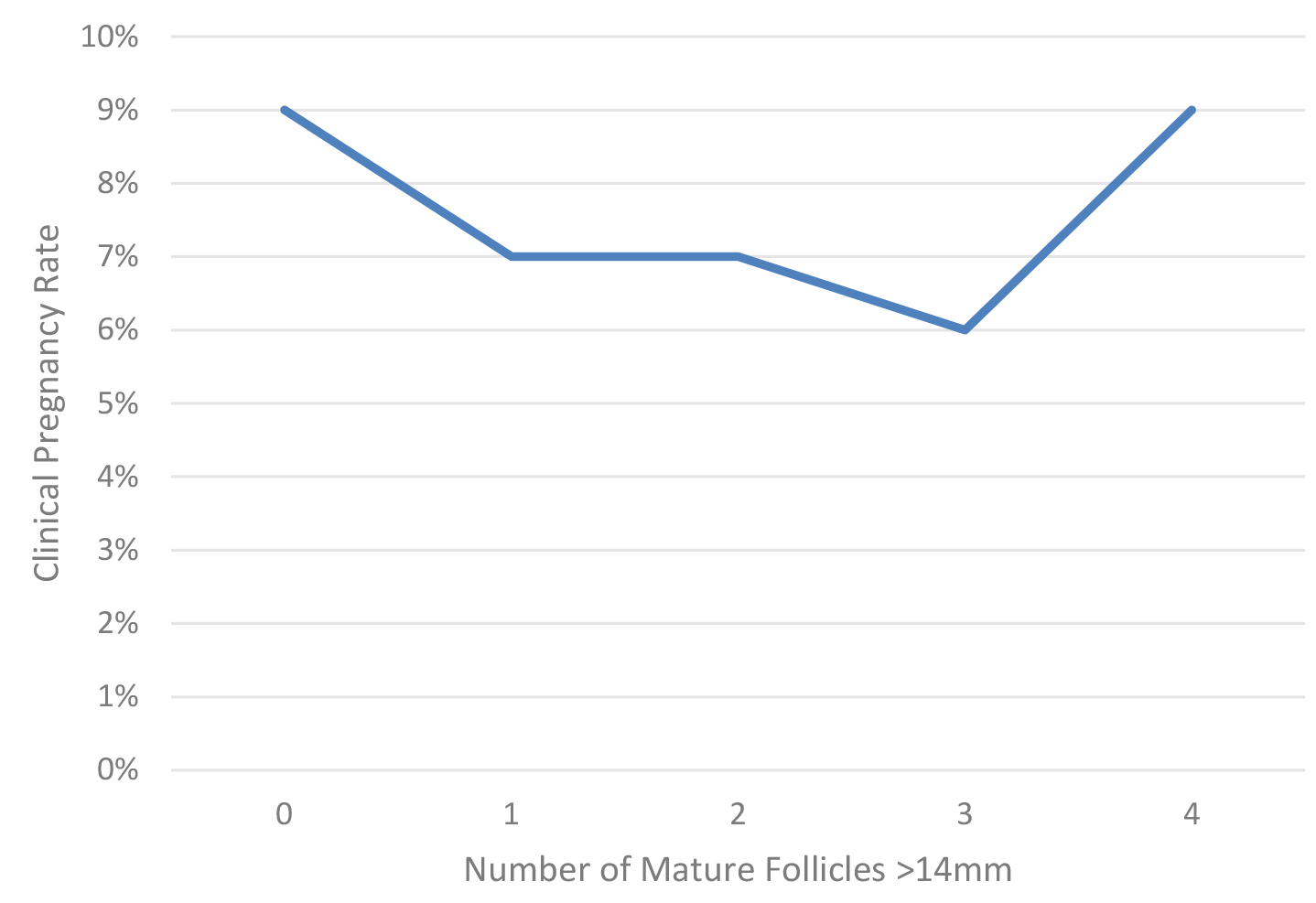
Table 1 presents the demographics and IUI outcomes by the number of mature follicles. The parity (P) (p = 0.049), the number of follicles 10–14 mm in diameter (p = 0.002) and the endometrial thickness (p = 0.003) were significantly different between the groups. There was no significant difference between the groups in terms of male age, TMS post processing, gravidity (G), AFC, basal estradiol, prolactin and thyroid stimulating hormon (TSH) levels. No statistical difference was observed between the groups regarding pregnancy rates and clinical pregnancy rates. Fig. 1 presents the clinical pregnancy rate as a function of the number of mature follicles. Multivariate stepwise-logistic regression controlling for confounders (Female age, TMS, AFC and endometrial thickness) comparing clinical pregnancy rates with the standard as 1 follicle 14 mm or greater as the benchmark is also presented in Table 1. After controlling for confounders effects, no statistical difference was observed in the clinical pregnancy rate for all the groups with one mature follicle stimulated being the gold standard selected: 0 follicles (adjusted odds ratio (OR) = 3.5, 95% confidence interval (CI) 0.33–37.6, p = 0.298); 2 follicles (adjusted OR = 1.8, 95% CI 0.53–6.5, p = 0.339); 3 follicles (adjusted OR = 2.5, 95% CI 0.43–13.9, p = 0.309); 4 follicles (adjusted OR = 0.72, 95% CI 0.16–3.2, p = 0.667); 5 follicles (adjusted OR = N/A, 95% CI N/A, p = 0.996).
| Number of mature follicles |
0 | 1 | 2 | 3 | 4 | 5 | Total | p-value |
|---|---|---|---|---|---|---|---|---|
| N = 86 | N = 983 | N = 385 | N = 94 | N = 22 | N = 4 | |||
| Compared by ANOVA | ||||||||
| Female age (years) | 39.8 |
39.8 |
39.9 |
40.1 |
40.5 |
41.0 |
39.8 |
0.033 |
| Paternal age (years) | 40.9 |
41.5 |
41.4 |
42.2 |
42.9 |
41.8 |
41.5 |
0.649 |
| TMS (post processing) | 60.3 |
49.9 |
43.8 |
47.2 |
26.8 |
24.1 |
48.4 |
0.224 |
| G | 1.1 |
1.1 |
1.1 |
1.4 |
1.0 |
2.8 |
1.1 |
0.107 |
| P | 0.2 |
0.3 |
0.4 |
0.3 |
0.5 |
0.3 |
0.3 |
0.049 |
| Basal FSH (IU) | 9.5 |
8.4 |
8.8 |
9.1 |
7.8 |
9.2 |
8.6 |
0.265 |
| Basal E2 (pmol/L) | 178 |
198 |
189 |
165 |
237 |
174 |
193 |
0.202 |
| AFC | 15.5 |
14.0 |
13.3 |
12.3 |
10.3 |
14.8 |
13.7 |
0.169 |
| Prolactin (ng/mL) | 12.1 |
10.4 |
10.6 |
10.8 |
12.7 |
8.3 |
10.6 |
0.393 |
| TSH (mU/L) | 1.9 |
1.7 |
1.8 |
1.8 |
1.6 |
1.6 |
1.8 |
0.910 |
| Number of follicles 10–14 | 1.6 |
1.1 |
1.1 |
1.0 |
1.7 |
2.0 |
1.1 |
0.002 |
| Endometrial thickness | 7.6 |
8.6 |
8.9 |
8.6 |
8.6 |
10.8 |
8.6 |
0.003 |
| Pregnancy rate | 8 (9.3%) | 87 (8.9%) | 35 (9.1%) | 12 (12.8%) | 2 (9.1%) | 0 | 144/1574 | 0.933 |
| Compared by multivariate logistic regression | ||||||||
| Clinical pregnancy rate | 8 (9.3%) | 67 (6.8%) | 28 (7.3%) | 6 (6.4%) | 2 (9.1%) | 0 | 103/1574 | 0.208 |
| Multiple-pregnancy | 0 | 1 | 2 | 2 | 0 | 0 | 5/1574 | |
| 95% CI | 0.33–37.6 | N/A | 0.53–6.5 | 0.43–13.9 | 0.16–3.2 | N/A | ||
| OR | 3.5 | N/A | 1.8 | 2.5 | 0.72 | N/A | ||
| p value | 0.298 | N/A | 0.339 | 0.309 | 0.667 | 0.996 | ||
Data presented as mean
Clinical pregnancy rates; CI, OR and p value when controlling for the confounding effects (Female age, TMS, AFC and endometrial thickness) compared to 1 follicle as benchmark.
Multivariate logistic regression could not be performed for the group with 5 mature follicles since no pregnancies occulted in this group and numbers were small.
ANOVA, Analysis of Variance; TMS, total motile sperm count; G, gravidity; P, parity; E2, estradiol; FSH, follicle stimulating hormone; IUI, intra uterine insemination; IU, international units; AFC, antral follicle count; TSH, thyroid stimulating hormone; CI, confidence interval; OR, odds ratio; N/A, not available; N, number; SD, standard deviation.
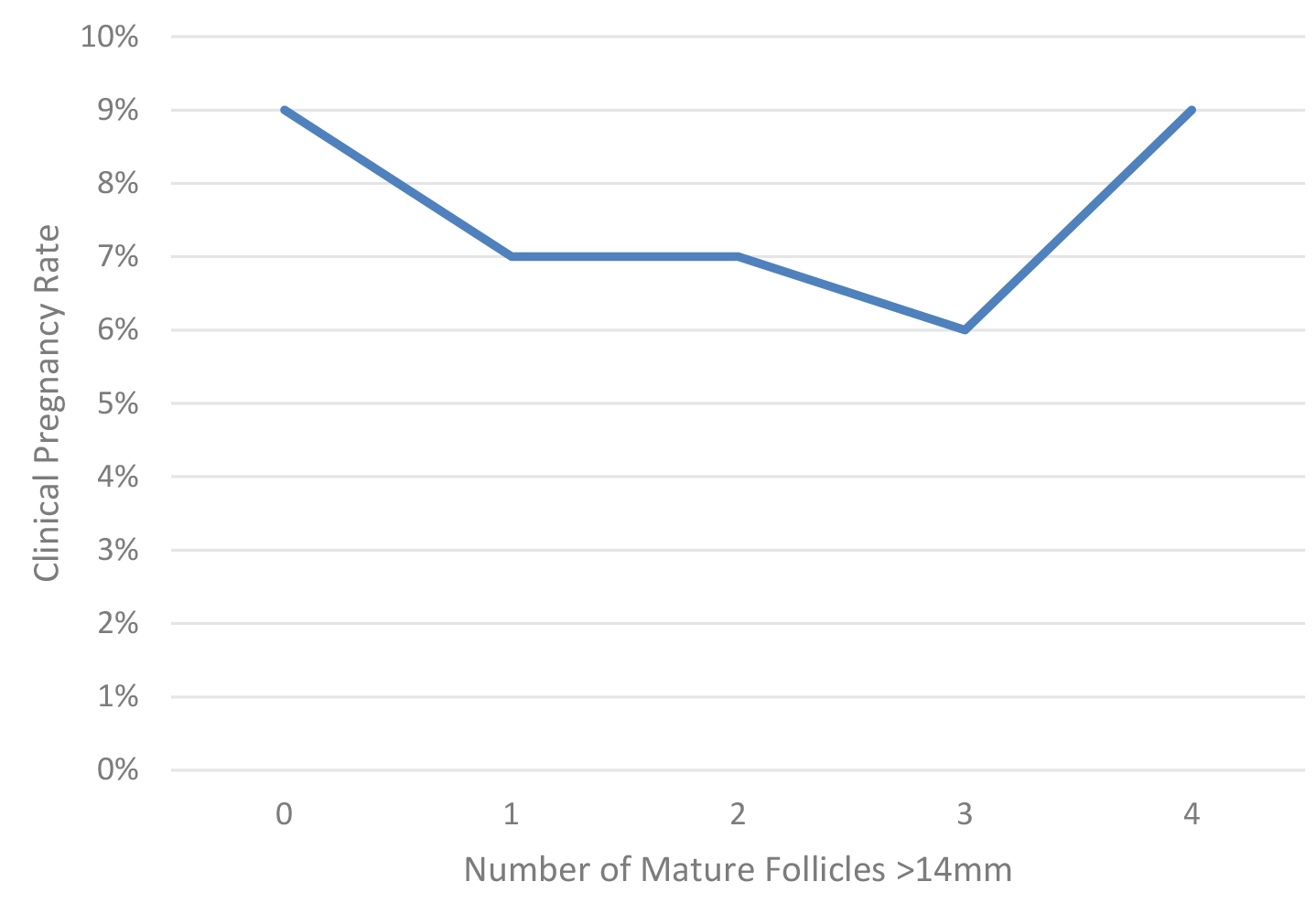 Fig. 1.
Fig. 1.Clinical pregnancy rate as a function of the number of mature follicles in women at least 38 years of age.
One might postulate that the group contains a wide range of ages, hence we also
investigated the group of women ages 38–39 separately. Multivariate logistic
regression controlling for the confounding effects comparing clinical pregnancy
rate with the standard as one follicle
| Number of mature follicles |
0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| N = 39 | N = 476 | N = 183 | N = 35 | N = 7 | N = 1 | |
| Clinical pregnancy rate | ||||||
| 95% CI | N/A | N/A | 0.43–1.34 | 0.58–18.13 | N/A | N/A |
| OR | 0.001 | N/A | 0.241 | 1.023 | N/A | N/A |
| p value | 1.000 | N/A | 0.105 | 0.988 | N/A | N/A |
| Pregnancy rate | ||||||
| 95% CI | 0.53–31.9 | N/A | 0.517–1.79 | 0.279–3.43 | 0.048–4.9 | N/A |
| OR | 4.125 | N/A | 0.963 | 0.760 | 0.480 | N/A |
| p value | 0.175 | N/A | 0.906 | 0.970 | 0.536 | N/A |
TMS, total motile sperm count; FSH, follicle stimulating hormone; AFC, antral follicle count; CI, confidence interval; OR, odds ratio; N/A, not available; N, number.
All baseline demographics with p
The main finding of this study is that there were no association between the number of mature follicles and the pregnancy and clinical pregnancy rates in women aged 38–43 undergoing
COH/IUI. One mature follicle yielded similar pregnancy and clinical pregnancy rates compared to multiple follicles. These results remained unchanged when controlling for confounding effects. Interestingly, in subjects requiring triggering prior to development of any mature follicles, pregnancy rates remained robust (9%). Overall pregnancy rates in this older group were acceptable at 7% per-IUI cycle.
Multiple follicular growth is thought to increase the chances of pregnancy while
increasing the risk of multiple-pregnancies, which in turn increases maternal
risks, preterm delivery and perinatal morbidity and mortality [14]. However, this
data primarily is derived from younger patients. Today, the risks of
multiple-pregnancies are well known and therefore, clinics attempt to maintain
multiple-pregnancies to a minimum. Therefore, a balance between the acceptable
pregnancy rate and strict limitations of the number of mature follicles is
practiced. In order to prevent high rates of multiple-gestations with IUI, Cohlen
B. et al. [13] have suggested in a review and systematic assessment of
the evidence that IUI should be withheld when more than two dominant follicles
Although we had 86 patients with no follicles
The strength of our study design is a relatively large number of IUI cycles, done in almost all patients seen at the center prior to undergoing IVF, due to the funding climate and waiting times at the time of funding. It gives us a unique possibility to analyze and compare different groups of women 38–43 years old with good statistical power. The limitation of this study is that it is a retrospective study, which could be masking possible undetected bias. The inability to track LBR is another limitation; IUI patients are not followed after clinical pregnancy for outcomes at our center. In lieu of this data, we used clinical pregnancy with a fetal heartbeat as our main outcome. Few subjects in our study had 4 or 5 mature follicles at the time of hCG and results of resultant comparisons should be taken with caution and confirmed in other studies. The role of vitamin D levels on the outcomes was not assessed and may have contributed to the results seen [20].
In women 38–43 years of age undergoing COH/IUI, the clinical pregnancy rate is not affected by the number of mature follicles in the range of 0 to 3 which were stimulated. One mature follicle yielded similar pregnancy and clinical pregnancy rates compared to three follicles and zero mature follicles when follicles were stimulated between 10–13.9 mm in average diameter.
The data will be available on request for a period of up to 7 years from the time of IRB acceptance, per the McGill University Health Center protocols.
MAS, RF and JRL collected the data. MHD conceived of the study. NS wrote the article. KRO and MHD edited the article. All authors read and approved the final manuscript.
Institutional review board approval was obtained (Approval number 2019-5254). Being a retrospective study patient consent was not required and approval was obtained for such from the Director of Services Professional of the hospital.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Michael H. Dahan is serving as Editor-in-Chief and Guest editors of this journal. We declare that Michael H. Dahan had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Paolo Ivo Cavoretto.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.