1 Department of Obstetrics and Gynecology, Nanning Second Maternal and Child Health Hospital, 530001 Nanning, Guangxi, China
2 Department of Gynecology, Guangxi University of Chinese Medicine, 530001 Nanning, Guangxi, China
3 Department of Obstetrics and Gynecology, Songzi Maternal and Child Health Hospital, 434200 Jinzhou, Hubei, China
4 Department of Gynecology, Guangxi International Zhuang Medical Hospital Affiliated to Guangxi University of Chinese Medicine, 530001 Nanning, Guangxi, China
†These authors contributed equally.
Abstract
Background: Endometritis is a common gynecological disease
characterized by inflammation of the endometrium. The gynecological Tiaoqi Jiedu
formula has been widely used to treat endometritis with dampness and heat.
However, the mechanism of action remains unclear. Methods: A mouse model
of endometritis with dampness and heat was established. The pathological changes
of the uterus and tongue were detected by hematoxylin and eosin (HE) staining.
Gastric aquaporin 3 (AQP3) and uterine cluster of differentiation 14 (CD14)
protein were detected by immunohistochemistry. Concentrations of serum
inflammatory factors interleukin-6 (IL-6), IL-1
Keywords
- endometritis
- gynecology
- Tiaoqi Jiedu formula
- lipopolysaccharide
- inflammatory factors
- animal studies
Chronic endometritis (CE) is a persistent inflammatory disease that is characterized by mucosal interstitial edema, focal or diffuse congestion, endometrial polyps, and interstitial plasma cell infiltration [1]. The prevalence of CE in infertile women varies between 2.8% to 56.8%, and it is associated with adverse pregnancy outcomes and endometriosis [2, 3, 4, 5]. This disease has a significant impact on the health and quality of life of women and imposes a substantial economic burden on society. Currently, there are no universally accepted standardized diagnostic guidelines or treatment protocols for CE [6, 7]. Therefore, there is a pressing need to investigate the pathogenesis of endometritis to develop effective treatment options that can improve pregnancy rates.
Endometritis is a persistent inflammatory disease with a prevalence of 2.8–56.8% in infertile women and is related to adverse pregnancy outcomes [3, 8]. Traditional Chinese medicine (TCM) classifies CE as a dampness and heat syndrome and emphasizes the importance of tonifying Qi and nourishing blood, clearing away heat and detoxification, promoting water, and seeping dampness [9, 10]. Gynecological Tiaoqi Jiedu formula has been used for many years in gynecological clinics to treat endometritis with certain effects in improving menstruation and pregnancy rate. However, there is a lack of in-depth research on its biological basis and molecular mechanism. This study established a mouse model of endometritis with dampness and heat by simulating the TCM pathogenesis, and observed the molecular mechanism of gynecological Tiaoqi Jiedu formula, providing a theoretical basis for clinical medication. The results showed that the Tiaoqi Jiedu formula demonstrated anti-inflammatory, anti-pyroptosis, and protective effects in endometritis, indicating its potential as a therapeutic option for the treatment of CE.
Experimental animals were treated in accordance with the Animal Quality Management Measures, issued by the State Science and Technology Commission. Specific pathogen free (SPF) grade Kunming female mice of 8 weeks, with an average weight of 30–35 g, were purchased from Changsha Tianqin Biotechnology Co., Ltd., with animal certificate number 43072620100069835, and production license number SCXK (Xiang) 2019-0014.
The animal experimental center of Guangxi University of Traditional Chinese Medicine (Guangxi, China) processed a high-fat and high-sugar diet in accordance with standardized diagnostic guidelines or treatment protocols. The formula ratio consisted of 60% standard chow, 12% palm oil, 18% sucrose, 3% cholesterol, and 7% egg yolk powder. Additionally, the animal experiment center provided a common feed standard diet.
The formula for gynecological Tiaoqi Jiedu is composed of: 15 g Huanghua Ddaoshuilian, 15 g Wuzhi Maotao, 30 g Baizhu fried with bran, 30 g Shanyao, 15 g Jiubiying, 15 g Tufuling, 15 g Buzhaye, 10 g Bijie, 15 g Cangzhu fried with bran, 6 g Baishao, 9 g Cheqianzi (bagged by cloth), 3 g Chaihu, 3 g Jingjiesui, 6 g Gancao, and 5 g Chenpi. The Chinese herbs should be decocted into three concentrations: 50 mL, 100 mL, and 200 mL, which are provided by the preparation department of the international Zhuang Medical Hospital, affiliated with Guangxi University of Traditional Chinese Medicine. Levofloxacin hydrochloride (HCl) tablets (100 mg) were supplied by Zhejiang Jingxin Pharmaceutical Co., Ltd. (Shaoxing, Zhejiang, China) (Approval number SFDAH1990060).
100 mg lipopolysaccharide (LPS, L2630, Bioswamp, Wuhan, Hubei, China), human
myeloperoxidase (MPO) enzyme-linked immunosorbent assay (ELISA) Kit (batch No.:
MU30238, Bioswamp, Wuhan, Hubei, China), interleukin 6 (IL-6) ELISA Kit (batch
No.: MU30044, Bioswamp, Wuhan, Hubei, China), IL-1
The instruments used in this study were a high-speed tissue grinder with low temperature (Wuhan Servicebio Biotechnology Co., Ltd., Wuhan, Heibei, China), a SIM-F140AY65-PC ice maker machine (Panasonic, Osaka, Japan), a PowerPac™ Basic Power Supply (Bio-Rad company, Hercules, CA, USA), a FluorChem E system (Protein Simple Co., Ltd., Tokyo, Japan), a 5430R high-speed freezing centrifuge (Eppendorf China Ltd., Beijing, China), a DW-86-L290-80oC pathological slicer (Shanghai Leica Instrument Co., Ltd., Shanghai, China), a film spreading machine (Zhejiang Kedi Instrument Equipment Co., Ltd., KD-P, Jinhua, Zhejiang, China), a microscope (Nikon E100, Tokyo, Japan), an imaging system (Nikon DS-U3, Tokyo, Japan), a Light Cycler 96 automatic real-time fluorescent quantitative PCR instrument (Roche, Basel, Switzerland), and an Infinite m200 Pro multi-functional microplate detector and microplate reader (TECAN Trading Co., Ltd., Shanghai, China).
After a week of adaptive feeding, ten mice were randomly selected for the control group, and the remaining fifty mice were used for the model of endometritis (with dampness and heat). The mice were evaluated according to references and were randomly divided into the model group, low-dose group, medium-dose group, high-dose group, and levofloxacin hydrochloride (HCl) group.
2.2.2.1 Syndrome Model Establishment
In the control group, the animals were kept in a SPF grade laboratory room with
standard diet and water at 20–25 °C and 50–60% humidity for 28 days. In the
dampness and heat model group, the animals were kept in an artificial climate box
at 30
2.2.2.2 Drug Intervention
On day 28, the mice in the control and model groups were administered normal saline daily. The low-, medium-, and high high-dose groups of gynecological Tiaoqi Jiedu Formula formula were given 0.37 mL each time, according to body weight, twice a day. In the western medicine group, the levofloxacin hydrochloride HCl tablets were crushed and diluted to 18 mg/mL with normal saline. According to body weight, 3.6 mg (0.2 mL) was used each time, once a day. All drugs were administered intragastrically, continuously for 5 days.
2.2.2.3 Endometritis Model Establishment
One hour after the last gavage, sodium pentobarbital (40–70 mg/kg) was injected intraperitoneally. The skin was prepared and disinfected, and the uterus was located by entering the abdomen from the position slightly below the renal area. 25 µL of LPS (2.5 µg/µL) was injected using a microsyringe into the uterine cavity. The control group was not specially treated. After 24 hours, blood was collected from the eyeballs, and the mice were sacrificed by cervical dislocation. The relevant samples were collected, and the indexes were determined [12, 13].
The tissues of the tongue root, stomach, and uterus of the mice were fixed with 4% formaldehyde, followed by paraffin embedding, sectioning, and hematoxylin and eosin (HE) staining. The pathological changes of each group were observed under the microscope [14, 15].
The expression of gastric aquaporin 3 (AQP3) and uterine cluster of differentiation 14 (CD14) was detected using the Strept Avidin-Biotin Complex (SABC) method [16]. The sections were dewaxed with xylene, rinsed with a gradient of ethanol to water, and washed with distilled water for 5 minutes. Antigen retrieval was conducted for 8 minutes and washed three times (5 minutes each). The sections were then incubated with 3% hydrogen peroxide (version: 10011208, Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China) for 25 minutes, washed three times, and incubated with 3% bovine serum albumin (BSA) (version: GC305010, Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China) for 30 minutes. The primary antibody was incubated at 4 °C overnight, followed by three washes. The sections were then incubated with the secondary antibody for 50 minutes, followed by color development with diaminobenzidine (DAB) and stopped with distilled water. After counterstaining with hematoxylin (version: 1212, Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China), the sections were dehydrated, made transparent, and neutral balsam was used to seal them. All tissues were observed under a microscope, and images were collected and analyzed.
Digital pathological image analysis software based on artificial intelligence learning. Artificial intelligence (AI) deep learning principle is used to train algorithms based on massive data and integrate them into automated image analysis software. The specific process is as follows:
(1) Tracking: automatically locate and delineate the area to be measured along the tissue to be measured, and manually locate according to specific requirements;
(2) Color selection: according to Hue Saturation Intensity (HSI) automatic positive judgment, can be manually corrected according to the specific situation;
(3) Calculation: According to the requirement, the nucleus was located automatically by the software and cytoplasm range was expanded. Different parameters of the positive cells such as the quantity which range from weak to strong, cell rate, tissue area and integrated optical density (IOD) were calculated.
(4) Analysis: Gradually calculate the area to be tested at high power. After completion, each project is calculated automatically according to the original basic data and the algorithm formula to obtain the analysis results, and generate a report.
Blood from the eyeballs was collected and stored at –80 °C after
centrifugation. The content of the serum inflammatory factors IL-6,
IL-1
The total RNA from the mice uterine tissue was extracted using Trizol reagent
(version: #DP424, Tiangen Biochemical Technology Co., Ltd., Beijing, China) and
reversed into cDNA. The primer sequence was designed by Beijing Qingke
Biotechnology Co., Ltd. (Beijing, China). The primers used are shown in Table 1. The primers for
NLRP3, GSDMD, Caspase-1, TLR4, p65, phosphorylation
of p65 (p-p65), and other reaction reagents were added
to the reaction system according to the requirements of the fluorescent
quantitative amplification kit. The reaction procedure consisted of
pre-denaturation at 95 °C for 30 seconds, denaturation at 95 °C
for 3–10 seconds, annealing extension at 60 °C for 10–30 seconds, and
40 cycles. Each sample was run in three wells. The data were statistically
analyzed by the maximum second derivative method (2
| Gene | Sequences | Length (5′→3′) |
| NLRP3 | Forward primer | ATTACCCGCCCGAGAAAGG |
| Reverse primer | TCGCAGCAAAGATCCACACAG | |
| GSDMD | Forward primer | CCATCGGCCTTTGAGAAAGTG |
| Reverse primer | ACACATGAATAACGGGGTTTCC | |
| Caspase-1 | Forward primer | ACAAGGCACGGGACCTATG |
| Reverse primer | TCCCAGTCAGTCCTGGAAATG | |
| TLR4 | Forward primer | ATGGCATGGCTTACACCACC |
| Reverse primer | GAGGCCAATTTTGTCTCCACA | |
| p65 | Forward primer | GGAGGCATGTTCGGTAGTGG |
| Reverse primer | CCCTGCGTTGGATTTCGTG | |
| p-p65 | Forward primer | AGGCTTCTGGGCCTTATGTG |
| Reverse primer | TGCTTCTCTCGCCAGGAATAC | |
| GAPDH | Forward primer | CTGGGCTACACTGAGCACC |
| Reverse primer | AAGTGGTCGTTGAGGGCAATG |
NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; GSDMD, gasdermin D; TLR4, toll-like receptor 4; p-p65, phosphorylation of p65; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NOD, anti-nucleotide binding oligomerization domain; LRR, leucine-rich repeat.
The fresh uterine tissue was ground with steel beads using radio immuno
precipitation (RIPA) buffer (version: #R0010, Beijing Solaibao Technology Co.,
Ltd., Beijing, China) and phenylmethyl sulfonyl fluoride (PMDF) (Version: B0009,
Suzhou Yake Technology Co., Ltd., Suzhou, Jiangsu, China), followed by
centrifugation. After extracting the total proteins, the sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) (version: G2043-50T, Wuhan Cel
Technology Co., Ltd., Beijing, China) was boiled for 5 minutes. The bicinchoninic
acid (BCA) method was used to detect the protein concentration. The proteins were
loaded into the electrophoresis system, with the upper gel electrophoresed at 80
volts and the lower gel at 120 volts. After electrophoresis, the proteins were
transferred to the polyvinylidene fluoride (PVDF) membrane and washed with tris
buffered saline-tween (TBST) (versio: T1082, Beijing Solaibao Technology Co.,
Ltd., Beijing, China) for 10 minutes (3 times). The membranes were incubated in
primary antibodies (NLRP3, GSDMD, Caspase-1p20, TLR4, p65, p-p65 ratio of
1:1000; ratio 1:2000 for caspase-1,
Three biological replicates were conducted for all experiments. The figures and
the data analysis were performed with the software GraphPad Prism 8.0 (GraphPad
Software, Inc., San Diego, CA, USA) and SPSS 17.0 statistical analysis software
(IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) and Tukey’s post hoc test was
performed after confirmation of normality test. Independent paired
t-test was used for the comparison between the groups. The results were
expressed as mean
The mice in the control group showed good mental state, sensitive reactions,
normal diet and drinking water, good hair luster, normal urination, and normal
vaginal secretions (Fig. 1). Conversely, the mice in the induced model group
(dampness heat syndrome model) displayed less activity, were listless with slow
reactions, had dull hair, low dietary and drinking water intake, slow weight
gain, dark purple lips, yellow and foul-smelling urine, loose stool, dirty around
the anus, and increased vaginal secretions. After drug intervention in the mice
with low- and medium-doses of gynecological Tiaoqi Jiedu formula, the body weight
and appearance did not show significant changes (p
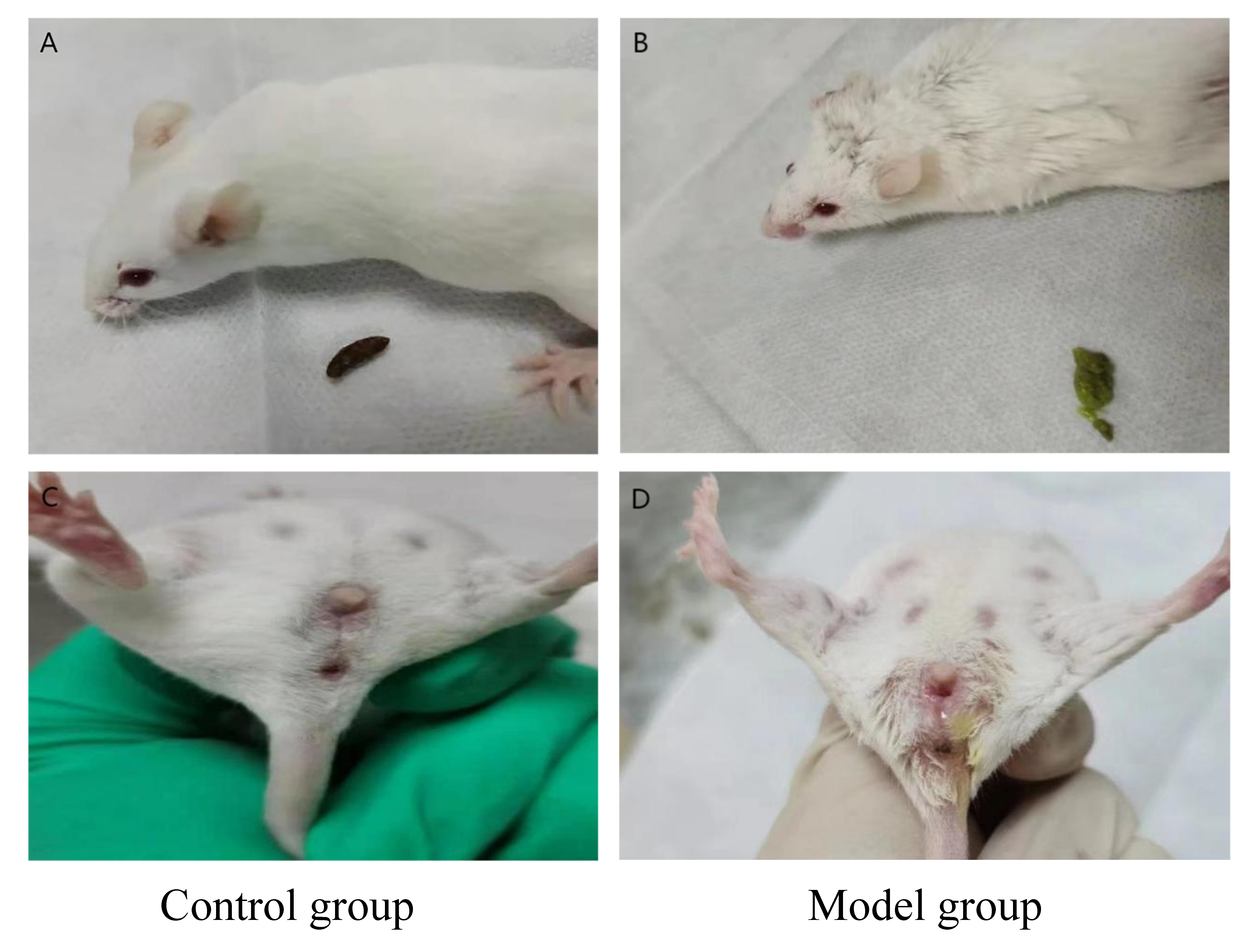 Fig. 1.
Fig. 1.Appearance of dampness heat syndrome in model group mice (B,D) compared to control group mice (A,C).
| Group | 0 day (g) | 7 days (g) | 14 days (g) | 21 days (g) | 28 days (g) | 33 days (g) |
| Control | 32.6 |
34.1 |
35.6 |
37.2 |
38.5 |
39.5 |
| Model | 32.5 |
33.9 |
35.2 |
36.0 |
36.8 |
37.4 |
| Low-dose | 32.6 |
34.1 |
35.2 |
36.9 |
36.9 |
37.4 |
| Medium-dose | 32.7 |
33.8 |
35.1 |
35.9 |
36.8 |
37.8 |
| High-dose | 32.5 |
33.8 |
35.1 |
36.1 |
36.8 |
38.6 |
| Western medicine | 32.7 |
33.9 |
35.2 |
36.1 |
36.8 |
38.6 |
n, number. Compared with control group (
The histopathology of the tongue mucosal tissues is shown in Fig. 2. The tongue
mucosa of the control group was clear and distinct, with even and moderately
arranged papillae density, and clear and orderly arranged muscle bundles of
lamina propria (Fig. 2A). In comparison with the control group (Table 3), the
model group showed increased thickness of the lingual papillae (p
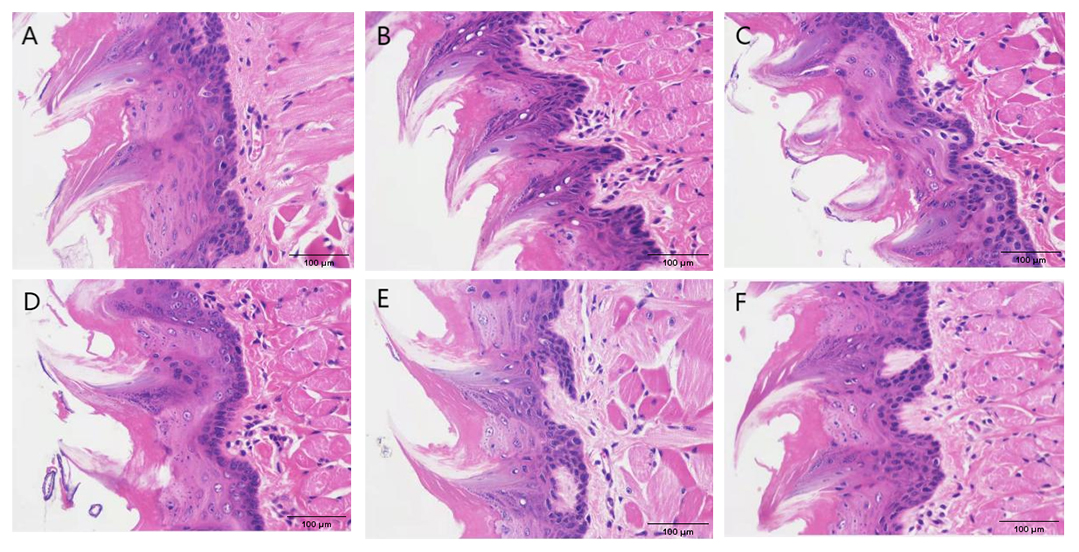 Fig. 2.
Fig. 2.Histopathological images of tongue mucosal tissues. (A) Control group. (B) Model group. (C) Low-dose group. (D) Medium -dose group. (E) High-dose group. (F) Levofloxacin hydrochloride (HCl) group.
| Group | Number of lingual papillae of lamina propria | Thickness of lingual papillae (mm) | Number of lingual papillae per unit length (/mm) |
| Control | 2.50 |
0.44 |
4.37 |
| Model | 7.67 |
0.51 |
13.84 |
| Low-dose | 7.63 |
0.50 |
13.49 |
| Medium-dose | 5.00 |
0.47 |
10.76 |
| High-dose | 3.83 |
0.46 |
6.48 |
| Western medicine | 3.50 |
0.47 |
6.55 |
Compared with control group (
In comparison to the control group (Fig. 3A), the uterine serous surface of the mice in the model group was congested and edematous (Fig. 3B). The color was dark red, the uterine diameter was about twice that of the control group, and the lumen was filled with pus (Fig. 3B). However, the swelling, congestion, and size of the uterus were altered to different levels after treatment with gynecological Tiaoqi Jiedu formula (Fig. 3C–E), and western medicine group (Fig. 3F).
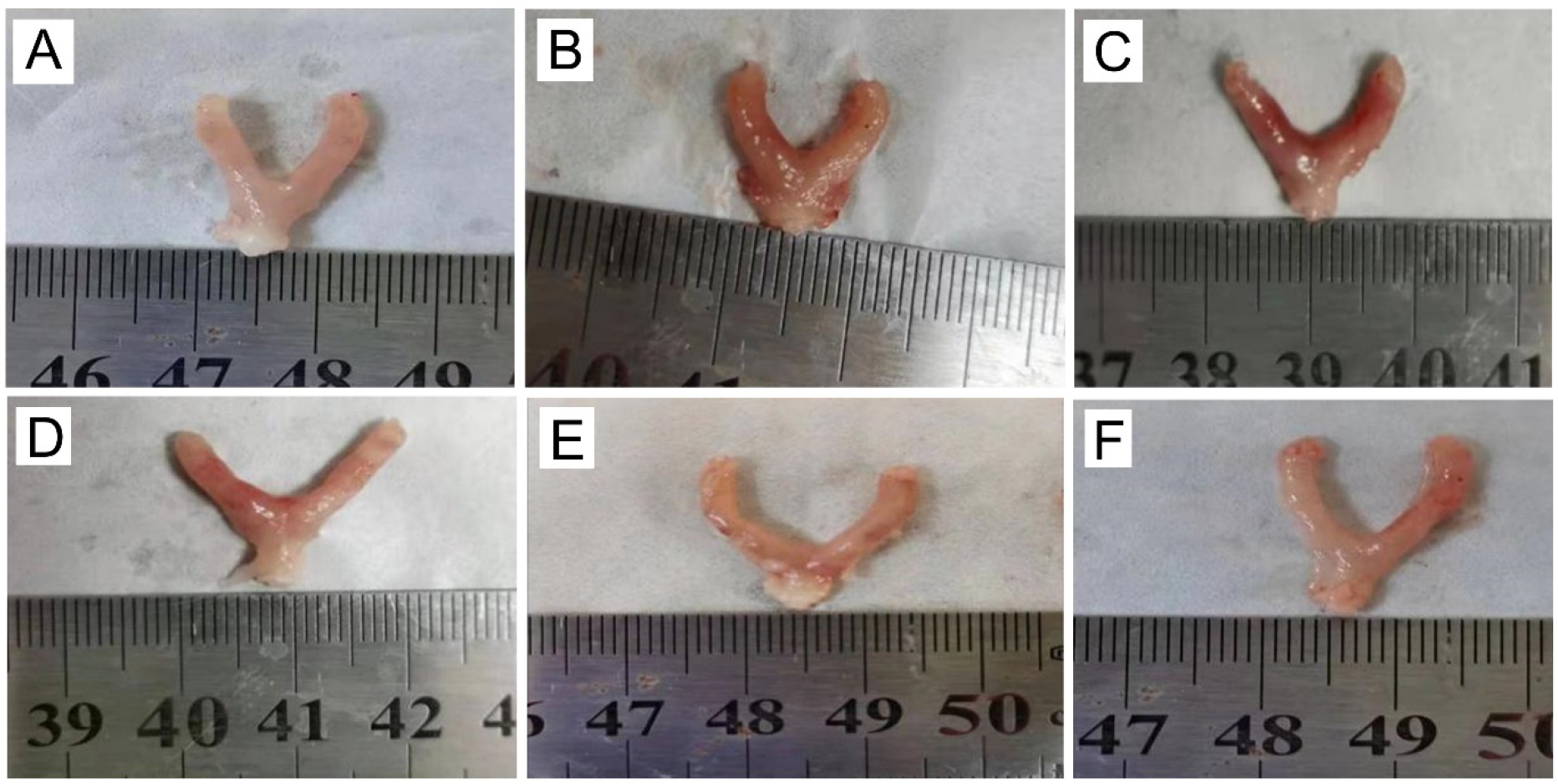 Fig. 3.
Fig. 3.Appearance of uterus in mice. (A) Control group. (B) Model group. (C) Low dose group. (D) Medium group. (E) High dose group. (F) Levofloxacin HCl group.
The mice uterus of the control group showed clear and complete layers of uterine endometrial epithelium and normal cell morphology. The number of uterine glands in the lamina propria was abundant and evenly distributed, and the myocytes in the myometrium were arranged regularly without obvious abnormalities (Fig. 4A). The mice uterus in the model group showed an uneven, thick endometrium, with a large number of epithelial cells, as a sign of necrosis. The nuclei were pyknosis, characterized by deep staining, fragmentation, or dissolution (black arrow). A small number of epithelial cells of uterine glands in the lamina propria were necrotic, and the nuclei were pyknotic, hyperchromatic, fragmented, or dissolved (red arrows). A large amount of granulocyte infiltration could be seen in the lamina propria and muscularis of the intima (blue arrow) (Fig. 4B). The mice treated with a low- to high-doses of gynecological Tiaoqi Jiedu formula and levofloxacin HCl showed neutrophil infiltration, cytolysis, and fragmentation, which varied in different degrees in the lamina propria and muscularis of the inner membrane (Fig. 4C–F).
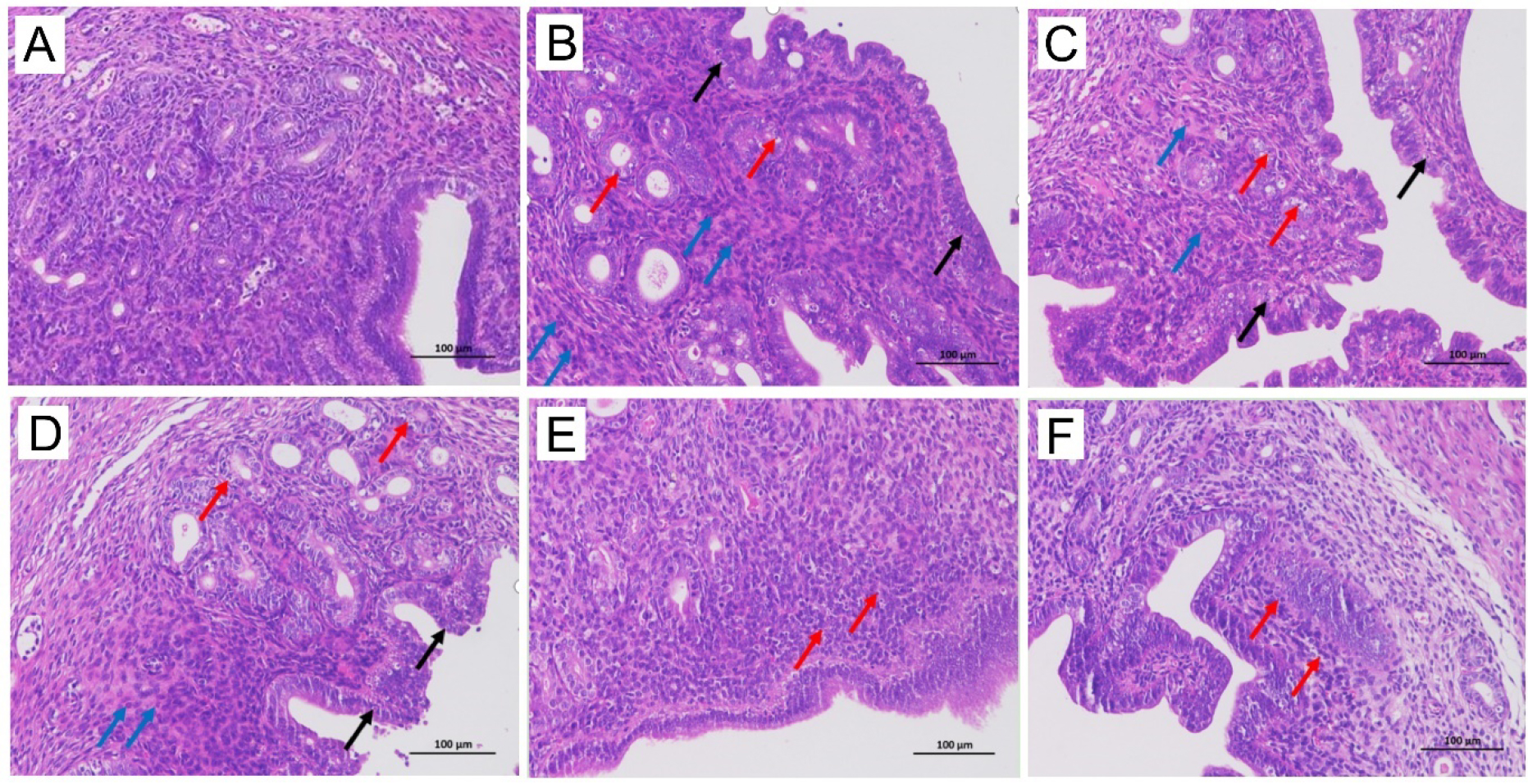 Fig. 4.
Fig. 4.Histopathological images (200
In regards to the immunohistochemical results, the brown staining of CD14 in the
model group was deeper compared to the control group, indicating higher
expression of CD14 (Fig. 5A,B). Next, in comparison to the model group (except
for the low-dose group), the positive staining of CD14 in the treatment groups
was reduced to varying degrees, as shown in Fig. 5C–F. The gray value of CD14 in
the model group was significantly higher (p
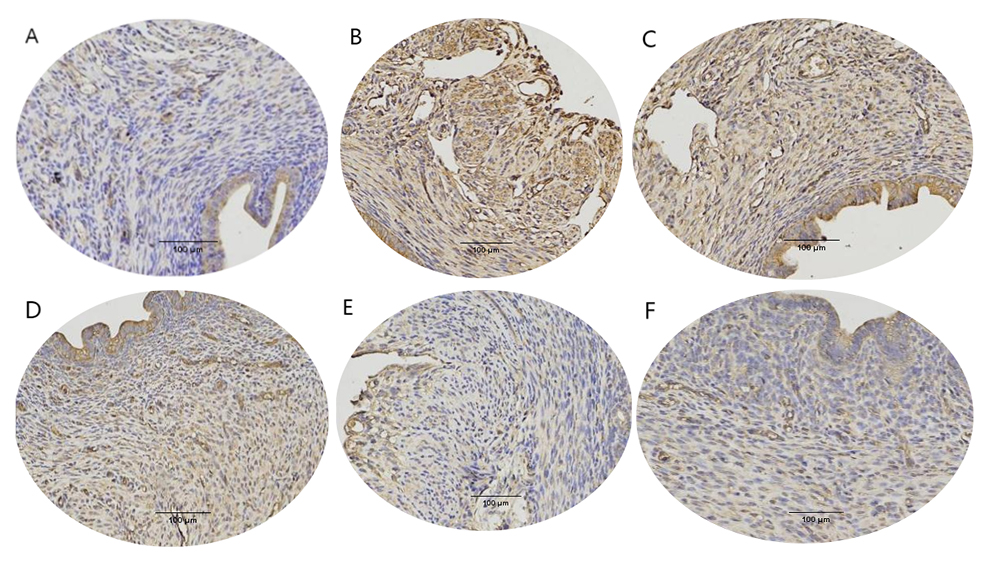 Fig. 5.
Fig. 5. Cluster of differentiation 14 (CD14) expression on mice uterus
(200
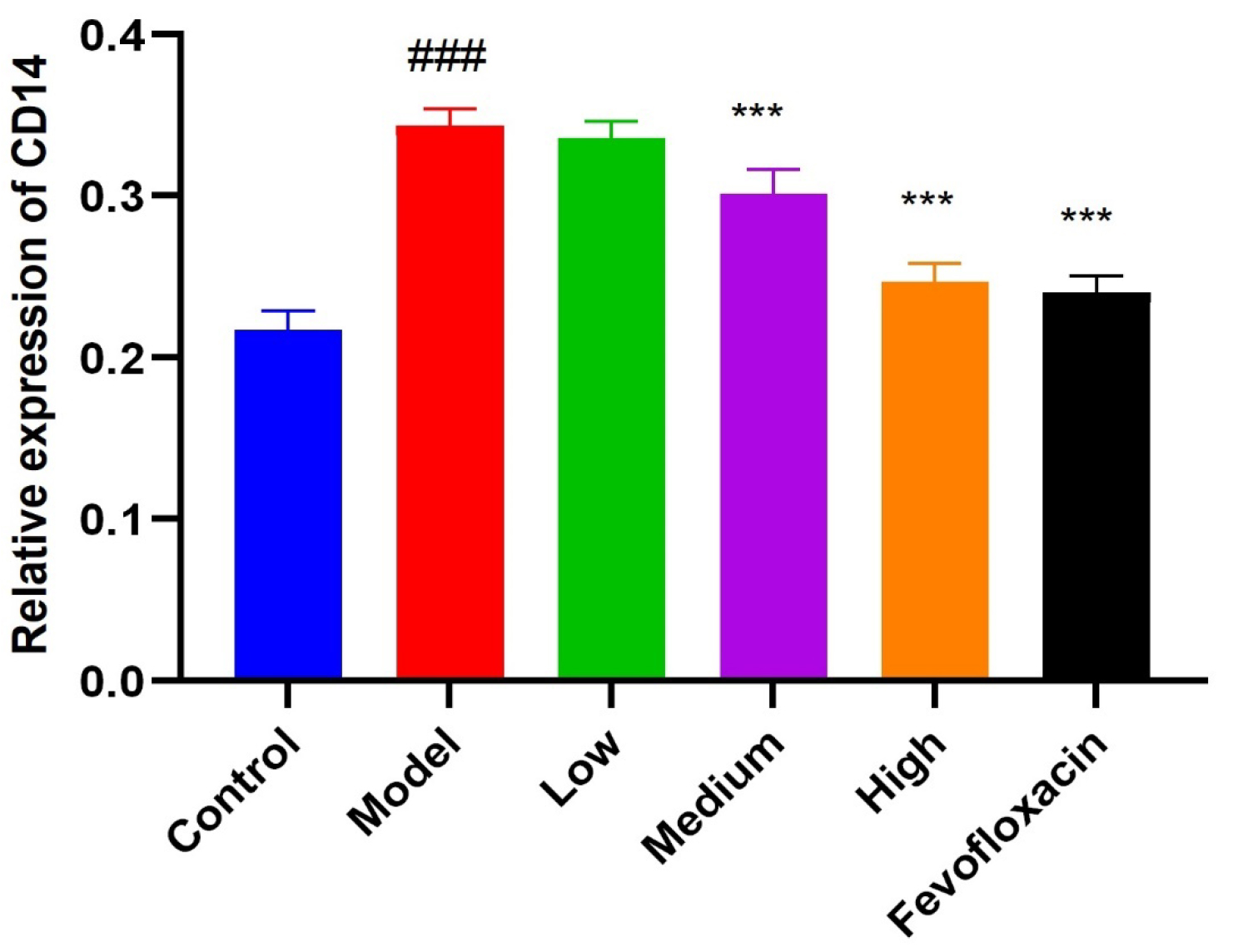 Fig. 6.
Fig. 6.Cluster of differentiation 14 (CD14) expression on mice uterus. Compared with control group
(
Regarding immunohistochemical results, a brownish yellow dot (positive) and sepia (strongly positive) represents AQP3 expression. Compared to the control group (Fig. 7A), the brown staining in the model group was deeper, which suggests higher expression of AQP3 (Fig. 7B). AQP3 expression in the low-dose treatment group (Fig. 7C) showed no change compared to the model group (Fig. 7B). The positive staining of AQP3 in other treatment groups was reduced to varying degrees, as shown in Fig. 7D–F.
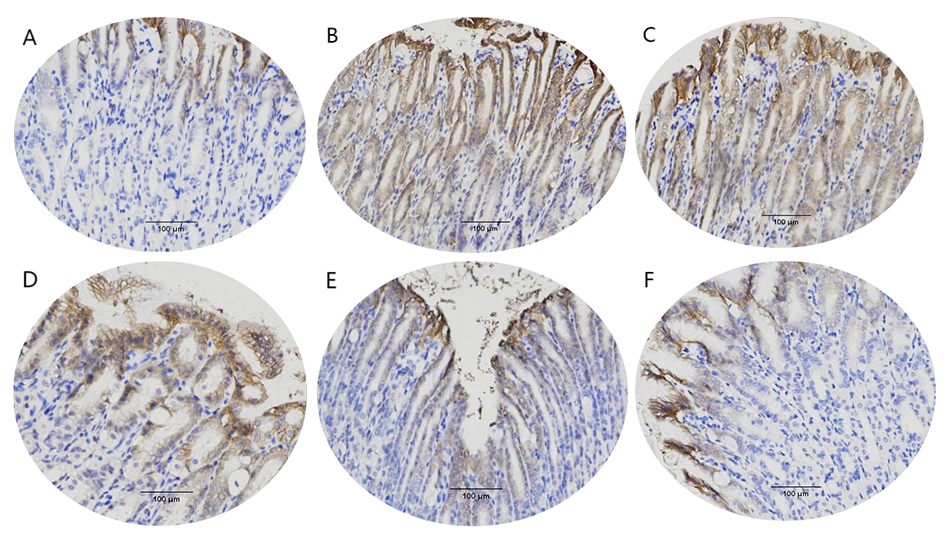 Fig. 7.
Fig. 7. Aquaporin 3 (AQP3) expression in mice stomach
(200
The gray value of AQP3 in the model group was significantly (p
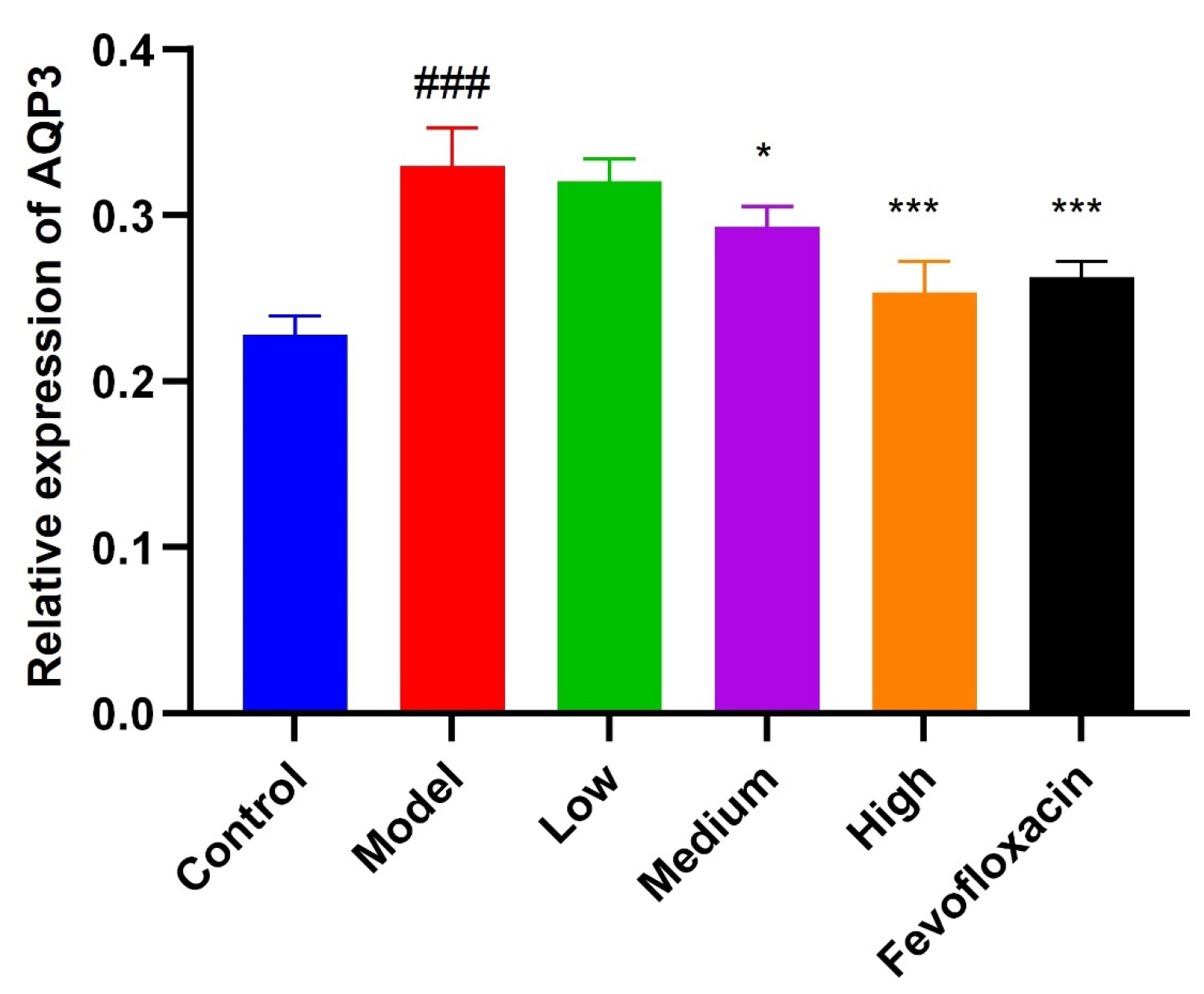 Fig. 8.
Fig. 8.AQP3 expression in mice stomach. Compared with control group
(
In the model mice group, the serum inflammatory factors (TNF-
| Group | IL-10 | TNF- |
IL-8 | IL-6 | IL-1 |
| Control group | 187.2 |
105.5 |
129.7 |
23.5 |
58.3 |
| Model group | 132.8 |
199.3 |
208.9 |
66.1 |
152.0 |
| Low-dose group | 134.6 |
197.7 |
204.0 |
63.3 |
146.0 |
| Medium-dose group | 141.9 |
164.3 |
184.2 |
48.4 |
129.5 |
| High-dose group | 167.9 |
122.4 |
158.8 |
32.3 |
63.2 |
| Western medicine | 169.7 |
126.3 |
155.6 |
32.5 |
64.4 |
Compared with control group (
In comparison to the control mice (Fig. 9), the relative mRNA expression of key
genes related to pyroptosis, such as GSDMD, NLRP3, Caspase-1 mRNA, together with
p-p65, and TLR4 mRNA in the TLR4 signaling pathway in the model group (with damp
heat endometritis induced by multiple factors) were significantly increased
(p
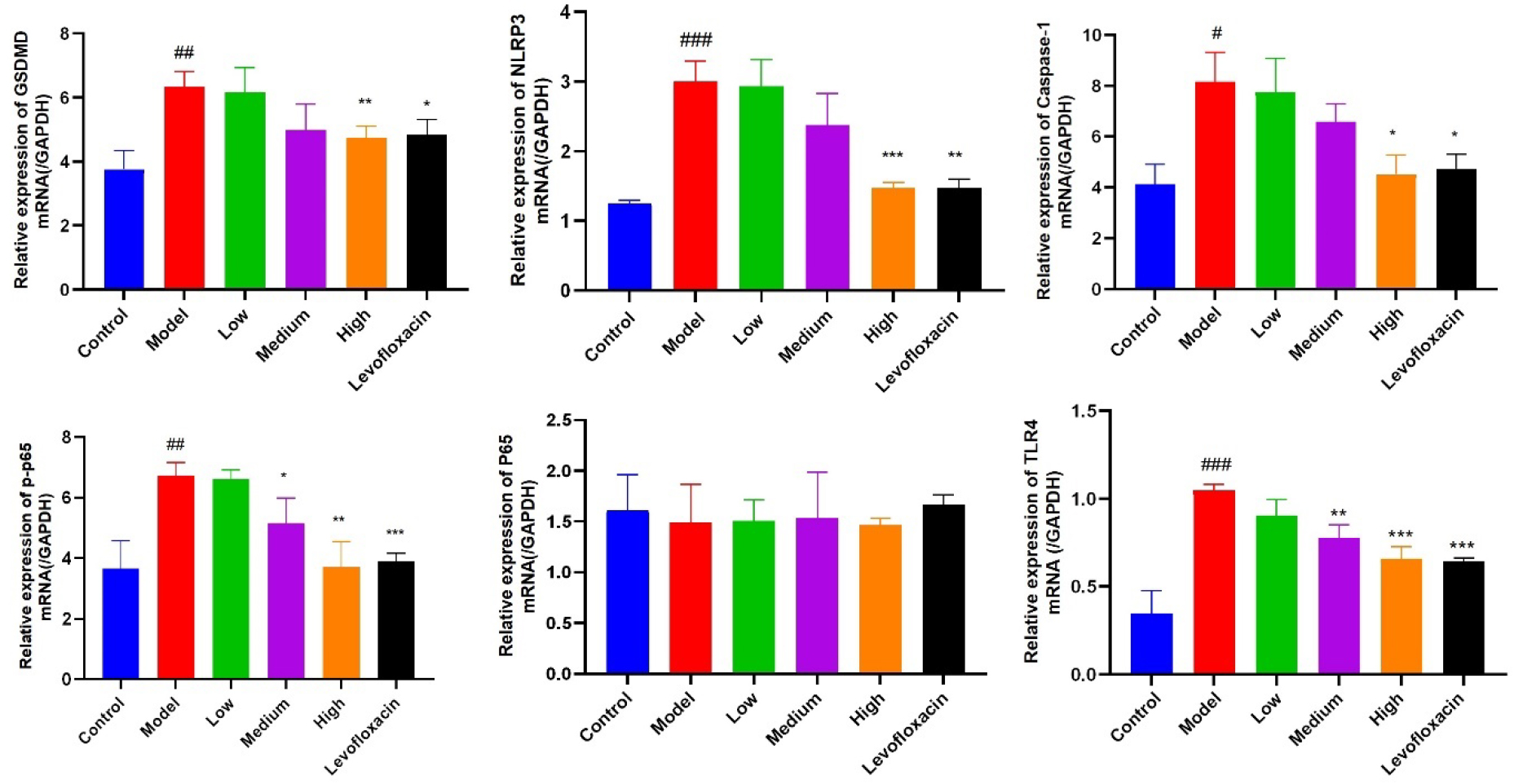 Fig. 9.
Fig. 9.mRNA expression of pyroptosis related factor GSDMD, NLRP3,
Caspase-1 and key factor of TLR4/nuclear factor-
The expression of GSDMD, NLRP3, Caspase-1, Caspase-1p20, TLR4, and p-p65/p65,
which are key proteins in the pyrolytic signaling pathway and TLR4/NF-
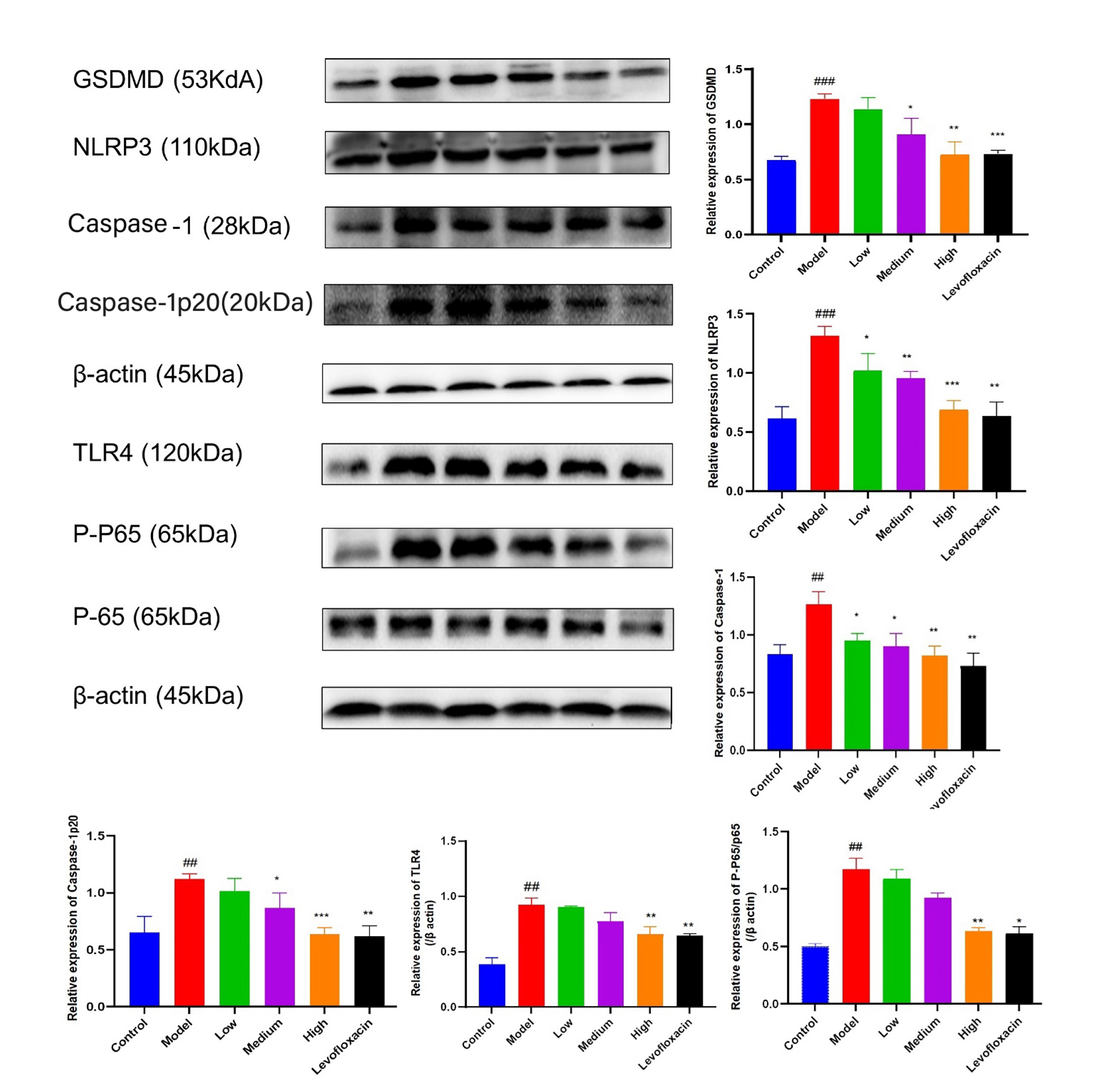 Fig. 10.
Fig. 10.Expression of key proteins in pyroptosis and
TLR4/NF-
A study has shown that the main cause of CE is a common bacterial infection,
and LPS is the main component [18]. Meanwhile, LPS is related to abortion and
premature birth, and its mechanism may be related to its ability to regulate the
release of pro-inflammatory factors, such as TNF-
Our research group has, for the first time, constructed for the first time, a mouse model of endometritis induced by dampness, heat, and multiple factors. This model simulates the pathogenic mechanisms of both TCM syndrome and Western medicine. As external reference indices of excess heat syndrome, we observed physical appearance and pathological sections. For the internal indices of the dampness heat syndrome model, we examined the expression of gastric AQP3 immunohistochemistry.
At the same time, we also established the model of endometritis with dampness heat, by combining it with an intrauterine injection of LPS. In terms of TCM syndromes, the appearance of the mice, such as mental malaise, little movement, loose stool, and tongue, was consistent with clinical observations. Meanwhile, the AQP3 in the model group was significantly increased, and there were changes after treatment. The expression of the LPS receptor CD14 in immunohistochemistry and HE staining of the uterus, jointly confirms that our model construction was successful, and the treatment of gynecological Qi regulating and detoxifying formula was effective.
The composition of the gynecological Tiaoqi Jiedu formula includes fifteen
traditional Chinese medicines and Zhuang medicines. It reduces heat, detoxifies,
and strengthens the spleen, and eliminates dampness, improves liver function
(with a soothing effect), and fixes astringency. Among them, the principal drugs
are Huanghua Daoshuilian and Wuzhi Maotao, which are medicinal materials,
homologous to drug food [27, 28]. Studies have shown that the main active
components of Huanghua Daoshuilian (also known as polygala) are saponins and flavonoids [29, 30, 31]. Saponins exhibit
anti-inflammatory and immune-regulating effects. By inhibiting the activation of
the NF-
In the 2015 edition of the Chinese Pharmacopoeia, there are three types of Chinese patent medicines containing Wuzhi Maotao, which are used to treat gynecological diseases: Gongyanping dropping pills, Fuyanjing capsules, and Gongyanping tablets. Numerous studies have shown that quercetin, an effective active ingredient in Wuzhi Maotao [37], can regulate inflammatory factors, growth factors, and signal pathways [38], leading to anti-inflammatory, anti-cancer, anti-oxidation, and immune-regulating effects [27, 39, 40, 41]. Quercetin has a similar structure to mammalian estrogen and can regulate estrogen receptors with high affinity. By regulating estrogen receptors [42], inhibiting the release of inflammatory factors, and regulating the endometrial microenvironment, quercetin can improve the pregnancy rate and protect the fetus. Thus, it explains the effect of gynecological Tiaoqi Jiedu formula in improving menstruation and increasing the probability of pregnancy, while treating endometritis with dampness and heat.
The study demonstrated that the Tiaoqi Jiedu formula was effective in treating endometritis with dampness and heat through multiple channels, and multiple targets. However, the reader should note the limitations of this study, which is the difference between animal and human bodies. In the future, the effect of Tiaoqi Jiedu formula on improving menstruation and pregnancy needs to be studied. The present study lays a solid foundation for future experiments involving human endometrial stromal cells. It also provides a fresh perspective and basis for protecting the endometrium and improving the pregnancy rate while treating CE.
AQP3, Aquaporin 3; CD14, Cluster of differentiation 14; IL, Interleukin; TNF,
Tumor necrosis factor; ELISA, Enzyme-Linked Immunosorbent Assay; NLRP3, NOD-,
LRR- and pyrin domain-containing protein 3; GSDMD, Gasdermin D; TLR4, Toll-like
receptor 4; NF-
Data will be available on request.
JC designed the research study; JC and CZ performed ELISA, Real-time PCR, Western Blot, Immunohistochemical staining, and HE staining experiments; WY and YL analyzed the data and performed research; NL were involved in formal analysis and funding. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was performed according to the guidelines of “Animal Quality Management Measures” issued by the State Science and Technology Commission. The animals were approved by the Ethics Committee of “The Guangxi International Zhuang Medicine Hospital” (approval number: DW2020127-200).
Thanks to Guangxi University of Traditional Chinese Medicine for the experimental equipment and venue. Thanks to Dr. Xiaoyun Zhang who gave guidance during the experiment. Thanks to the teachers who helped in the process of writing the paper. Thanks to all the peer reviewers for their opinions and suggestions.
This study was supported by Introduction of Talent Research Startup foundation of Guangxi International Zhuang Medicine Hospital (GZ2021RC012); Administration of Traditional Chinese Medicine of Guangxi Zhuang Autonomous Region in 2021 (GXZYZ20210172); “High-level Talent Cultivation and Innovation Team” Project of Guangxi University of Chinese Medicine (No:2022A008); The State Administration of Traditional Chinese Medicine “Twelfth Five-Year” Key Discipline of Traditional Chinese Medicine National Pharmacy (Zhuang Pharmacy); Guangxi Traditional Chinese Medicine Multidisciplinary Innovation Team Project.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.










