1 Department of Medicine and Surgery, University of Parma, 43126 Parma, Italy
2 Unit of Paediatrics, University Hospital of Parma, 43126 Parma, Italy
Abstract
Objectives: This narrative review analyzes current knowledge on the pathophysiology of dysmenorrhea and the different therapeutic options currently available for adolescents and young women. Mechanism: Dysmenorrhea is the most common gynecological disorder among adolescents and young adult women. This condition can have a strong negative impact on the quality of life involving both physical and mental health. Although physiopathological mechanisms have been hypothesised there is still a poor understanding of this condition. Findings in Brief: The prevalence of dysmenorhea is quite variable depending on different studies but overall high. Nonsteroidal anti-inflammatory drugs are the preferred initial treatment; hormonal therapy, alone or in combination with non-hormonal treatments, is generally the next treatment option. There are evidences of the efficacy of non-pharmacological treatment, thus, these must be considered. Grading the intensity of pain would be of importance to address therapeutic choices and treatment options. Conclusions: To date there are yet many gaps in the understanding of dysmenorrhea that to do not allow any real personalized treatment. These gaps need to be filled in order to improve and target future treatment.
Keywords
- dysmenorrhea
- adolescence
- pelvic pain
- treatment
- NSAIDs
- non-hormonal treatment
- pathophysiology
- etiology
Dysmenorrhea is the most common gynecological disorder among adolescent and young women and is a leading cause of recurrent short-term missing from school or work. Interestingly, it was not considered a medical problem until the 1970s [1]. Thus, primary dysmenorrhea is not only a gynecological problem, but also a significant problem with regard to public health, work/school performance, and family concerns, as it negatively impacts quality of life and economy [2]. Dysmenorrhea can have negative effects on social relationships, academic and work performance, psychological well-being: it is responsible for limitations in daily activities, sleep disturbances, difficulty in concentration, loss of self-confidence, and social withdrawal; however, most adolescent and young women do not seek medical attention, resorting to self-medication with suboptimal management of this disease [3, 4]. In student academic life, pain related with dysmenorrhea has been shown to reduce concentration, and the amount of information learned [5, 6]. In particular, one study conducted on 1720 Romanian medical students from five university centres throughout the country, reported that 63.4% believed that intensity and duration of dysmenorrhea affected their quality of life, family relationships, and friendships. In academic activities, the intensity of pain was affecting individual study, ability to concentrate on courses, and volume of information accumulated [7] confirming the data of a previous study [5].
According to the different definitions used, the rates and impact of dysmenorrhea can vary significantly. In some studies, even mild and intermediate menstrual pain has been considered sufficient to define dysmenorrhea. Others have considered dysmenorrhea as a menstrual pain associated with “the need for medication and the inability to function normally”. Furthermore, adolescent girls and young women aged 14 to 20 years have been reported to miss school and working days monthly, due to symptoms associated with dysmenorrhea, and 1 in 4 of interviewed women has reported self-administered pain medications without consulting a doctor [8]. A more recent survey based on a population of women with primary dysmenorrhea over the age of 18 years reported that more than half of the young women described symptoms as limiting daily activities, and subsequently 17% (1 in 8 girls), missed hours of school and work [9]. In another survey-based study, the overall prevalence of experiencing menstrual pain was found to be very high, since 84.1% of participants reported experiencing pain at some time. About 43.1% of the participants reported having painful menstruation in every menstrual period, and 41% reported having pain at some time. These two definitions are very different, and the same study found that the intensity of menstrual pain did not coincide with the need for medication or the inability to function normally. About 55% of the women in this study reported having menstrual pain and needing medication, while poor social functioning or absenteeism because of menstrual pain ranged from 32% to 40%. Considering the more complete picture of dysmenorrhea, characterized by menstrual pain, absenteeism, and need for medication, the prevalence was reported to be about 25% [10]. A systematic review conducted in 1996 on the prevalence of chronic pelvic pain, which summarized community and hospital surveys conducted in developed countries, estimated a prevalence ranging from 45 to 95 percent. A further systematic review of studies also conducted in developing countries reported that 25%–50% of adult women and about 75% of adolescent girls experienced pain during menstruation, with 5%–20% reporting severe dysmenorrhea or pain that prevented them from participating in activities of daily living. A third systematic review and meta-analysis on prevalence rates concluded that the prevalence of dysmenorrhea was 59% [11]. The World Health Organization assessed the worldwide prevalence of dysmenorrhea in 124,259 nonpregnant women with or without endometriosis reporting a prevalence ranging from 8.8% in hospitalized women (aged 19–41 years) to 94% in girls aged 10–20 years [1]. In the United States, 15% of adolescent girls have report severe dysmenorrhea with prevalence rate estimates for absenteeism ranging from 20% to 30% [12]. If we focus on Europe the prevalence of dysmenorrhea reported in the literature varies from an estimated 56% in Italy to 86.6% in Switzerland. Thus, the prevalence of dysmenorrhea reported ranges from 16% to 91% in women of reproductive age, with severe pain being reported in 2% to 29% of women [11]. An additional longitudinal study on a cohort of Swedish women reported a prevalence of dysmenorrhea of 90% at age 19 and 67% at age 24, with 10% of 24-year-olds reporting pain that interfered with daily functions [12].
One cross-sectional study gave for dysmenorrhea a clear definition of a painful menstruation with cramping sensation in the lower abdomen or lower back, occurring with menstrual flow or the day before, and assessed severity based on pain intensity, as measured by a numerical rating scale, possibly associated with systemic symptoms, impact on daily activities, and need for analgesic treatment using the Verbal Multidimensional Scoring System (VMSS), and concluded that dysmenorrhea was moderate in 65.2% and severe for 8.9% of women. In addition, a strong impact on daily life was clearly shown, as 43.3% of the girls reported missing from school, 74.9% had difficulties in attending classes, and 77.2% had difficulties in practicing sport activities [13]. Variables contributing to differences in prevalence rates include demographics, symptom severity, ethnic, sociocultural, or biological factors in the populations studied [14].
As to treatment, the majority (98%) of adolescent girls relies on non-pharmacological methods such as heat, rest, or distraction with a perceived efficacy of 40% or less [3]. From 30% to 70% of girls refer self-medication at least occasionally with over-the-counter pain medications, and 57% of these reported to have used subtherapeutic doses. Up to 54% of adolescent girls are reported to know that some medications can relieve menstrual cramps, but 27% of these are unable to recognize the main nonsteroidal anti-inflammatory drugs (NSAIDs) listed as possible treatments for dysmenorrhea [3].
Underestimation of dysmenorrhea results in diagnostic delay. Theological and traditional attitudes regarding menstruation, as well as the common belief in some patients that pain is an expected and unavoidable part of menstruation, could underlie the dual failures of omission or lack of communication [2, 9]. Dysmenorrhea affects up to 80% of reproductive-age women, often causing significant pain that can deeply impact social and occupational roles. Prevalence varies among ethnic groups, partly reflecting different cultural attitudes of women with respect to menstruation. Clearly, there is variation across cultures, as for example whereas most women in Western cultures report some degree of dysmenorrhea, only a quarter of rural Maya women do so [15]. The Islamic law may forbid menstruating women from praying, fasting during Ramadan, or engaging in sexual intercourse. Similarly, some Hindu followers may consider the touch of a menstruating woman as impure. This stands true also for Israeli Arab minorities [16]. Socio-cultural factors can not only influence the perception of clinical symptoms but may also themselves be the cause of dysmenorrhea. In fact, while menstruation can bring about positive changes in the social role of Israeli Arab girls, it can also lead to conflicting attitudes towards menstruation, which may present as a negativity because of dysmenorrhea. The interface between cultural and ethnic influences on the symptoms is not well-defined. In a patriarchal society, menstruation, pregnancy, and childbirth are considered significant events in the female developmental process. From the perspective of this cultural outlook, menstruation means a girl’s entry into her expected social role as a mature woman. From the onset of menarche, the family may impose stricter rules regarding social behavior upon her [17, 18]. In some countries like Palestine, there is a lack of published studies on women’s health in general and on gynecological problems in particular [19]. Taboos in general, can lead to underestimation of pain, under-reporting of dysmenorrhea prevalence, and ultimately, inadequate treatment. This aspect has been observed not only in Arab cultures but also in Pakistani, Malaysian, and Hindu cultures. As an example, some Hindu followers may consider the touch of a menstruating woman impure [20]. In conclusion, many girls accept dysmenorrhea as a normal part of menstruation and believe it cannot be alleviated. These misconceptions have been reported to be a result of the lack of information on the causes of dysmenorrhea, absence of medical guidance, and cultural background. Enhancing girls’ knowledge could therefore potentially influence their healthcare-seeking behavior, even in countries where female health information is currently limited [21].
This narrative review analyzes current knowledge on the pathophysiology of dysmenorrhea and the different therapeutic options currently available for adolescents and young women. We have focused on the definitions. The authors performed a literature search in PubMed, using selected key words (‘dysmenorrhea and primary dysmenorrhea’) AND (‘adolescent or adult’) AND (‘diagnosis or definition’) AND (‘pathophysiology’) and (‘treatment’ or ‘drugs’ or ‘alternative treatment’). The authors used both automated search and manual search for additional relevant publications in the bibliographies of the papers automatically identified, and they independently identified the most relevant papers published in English in the past 15 years, including original papers, metanalysis, reviews, and randomized controlled trials (RCT). Case reports, series, and letters were excluded. All the contributions were critically reviewed by all the authors.
Dysmenorrhea is a condition characterized by recurrent, cramp-like pain located predominantly in the lower abdomen during menstruation. It can be classified as primary (or functional) in the absence of pelvic pathology, or secondary if the pain can be attributed to an underlying pelvic pathology.
The pain may radiate to the inside of the abdomen, lower back, or along the thigh. Feelings tend to begin a few hours before or after the onset of menstrual flow and peak at the time when blood flow is most intense and tend to resolve on the second/third day of the cycle. The pain associated with primary dysmenorrhea does not change its characteristics from one cycle to the next but remains constant in all its manifestations. In case of reported worsening of pain, or pain that lingers at the end of menstruation, or unilateral pain, it is important to investigate secondary causes of dysmenorrhea [9]. This condition presents a wide variability of clinical manifestations, and therefore a thorough history and evaluation of symptoms is essential. Among these are nausea, vomiting, diarrhea and dyschezia, poor appetite, headache, and muscle cramps. In addition, severe dysmenorrhea affects sleep quality including insomnia, dizziness, depression, irritability, and nervousness (Table 1) [8, 14]. This condition seems to be associated with an increased risk of developing chronic pain [22]. Patients report physical and psychosocial scores comparable to those of other chronic diseases such as cystic fibrosis, diabetes mellitus [23]. Therefore, dysmenorrhea has to be approached as a chronic condition, considering the entire life course of a patient and identifying the individual aspects that determine the cumulative impact of the chronic condition. Thus, the evaluation of an adolescent/young adult with dysmenorrhea must begin with a detailed history obtained privately and confidentially because the doctor-patient relationship is crucial to obtain all information. It is essential to collect a complete menstrual history that takes into consideration age at menarche, duration and extent of bleeding, and features of cycles [9]. Iron deficiency can be a predictor of multiple pain disorders if associated with dysmenorrhea: the relationship between primary dysmenorrhea and iron deficiency may have therapeutic implications. A routine martial profile analysis and possible supplementation is indicated, with resolution of the deficiency and possible benefit on dysmenorrhea as well [24]. A recent study demonstrated maternal transmission of primary dysmenorrhea in adolescent and young women, with an important gene influence on dysmenorrhea. Thus, genetic and environmental influences contribute to the multifactorial etiology of dysmenorrhea [25].
| Risk factors | Symptoms | |
| More Frequent | Less Frequent | |
BMI, body mass index.
Risk factors and major symptoms associated with dysmenorrhea that should be investigated during medical history are reported in Table 1.
A history of pain and associated symptoms is useful to define onset, duration, severity, aggravating and attenuating factors, and its relationship with menstruation. Gastrointestinal, urinary, musculoskeletal, and psychological symptoms should be investigated to understand how they affect daily activity or impair work or school function. Sexual history, if adequate, should include dyspareunia, previous sexually transmitted or pelvic infections. Previous treatments by reporting the medication taken, dosage and timing, and effectiveness must be carefully recorded [26]. Physical examination and possibly ultrasound evaluation are important, the last one being very useful in the workup of secondary dysmenorrhea. Biochemical tests are never conclusive, although Complete Blood Count (CBC) and vaginal swabs are suggested if a picture of secondary dysmenorrhea is suspected. Menstrual symptoms have been evaluated on several tools developed over the years, although presenting weaknesses and many problems related to their development or validation. Several scales became part of clinical practice allowing a sort of objective assessment of pain.
The Andersch and Milsom scale [27] correlates pain intensity with the presence of systemic symptoms, limitation of daily activities, and use or not of analgesics. Together with a visual analog scale also the Cox Menstrual Symptoms Scale can be used to assess the frequency and severity of dysmenorrhea symptoms. This latter scale is reported to have high reliability, validity, and sensitivity [4]. Table 2 (Ref. [27]) reports a modified Andersch and Milsom scale.
| Grade | Ability to work | Systemic symptoms | Nonsteroidal anti-inflammatory drugs |
| GRADE 0: painless menstrual flow; preserved everyday life. | Intact | No symptoms | Not needed |
| GRADE 1: painful menstrual flow; lively pains; rare limits to daily living; rare drug requirement. | Seldom restricted | No symptoms | Rarely needed |
| GRADE 2: limited daily living; moderate pain; analgesics required and effective; rare absences from school. | Moderately restricted | Few symptoms | Seldom needed |
| GRADE 3: very limited daily activities; severe pain; ineffective analgesics. | Highly compromised | Clear symptoms | Poorly effective |
In addition, multidimensional scales such as the Menstrual Distress Questionnaire (MEDI-Q) and the Menstrual Symptom Questionnaire are available as new instruments to assess menstrual-related distress and provide a representative score of perceived stress. Both can be added to women’s routine health care to help identify and adequately monitor menstrual cycle-related discomfort and its effect on well-being in a timely manner [28]. This questionnaire analyses a total of 25 items, which include pain, discomfort, psychological/cognitive changes, gastrointestinal symptoms, and changes in physiological function; all areas potentially affected by menstrual discomfort, including mood, cognitive function, energy, nutrition, sleep, and sexuality, are investigated in addition to pain and bleeding [28].
Primary dysmenorrhea (PD) is linked to anovulatory cycles and usually occurs 6 months after menarche [29, 30]. It is well recognized that symptoms are usually more marked during adolescence, and multiparity women tend to have less dysmenorrhea [31]. There is some controversial evidence on the association of age at menarche and menstrual pain, since both a very early [10] or late [32] menarche have been considered risk factors for menstrual pain in women. While smoking is a well-known risk factor, exposure to environmental tobacco smoke can also aggravate menstrual pain, possibly because nicotine stimulates vasoconstriction, especially myometrium vasoconstriction [4]. The effect of a lower body mass index (BMI), longer menstrual flow, a history of abortions, gynecological pathologies, clinical suspicion of pelvic inflammatory disease, premenstrual syndrome and psychological symptoms are controversial also risk factors for dysmenorrhea [10, 13]. Another potentially modifiable risk factor is poor mental health: depression, anxiety, frequent life changes, fewer social or stressful relationships have been associated with menstrual pain [33]. Moreover, a lower socioeconomic status and mood disorders can worsen symptoms [31]. Finally, low intake of fish [34] and presence of intrauterine contraceptive devices may also be associated with dysmenorrhea nevertheless a further review reported that use of oral contraceptives was negatively associated with pain suggesting an important role of diet and hormonal contraception in this condition [10]. Overall the origin of dysmenorrhea seems to be multifactorial.
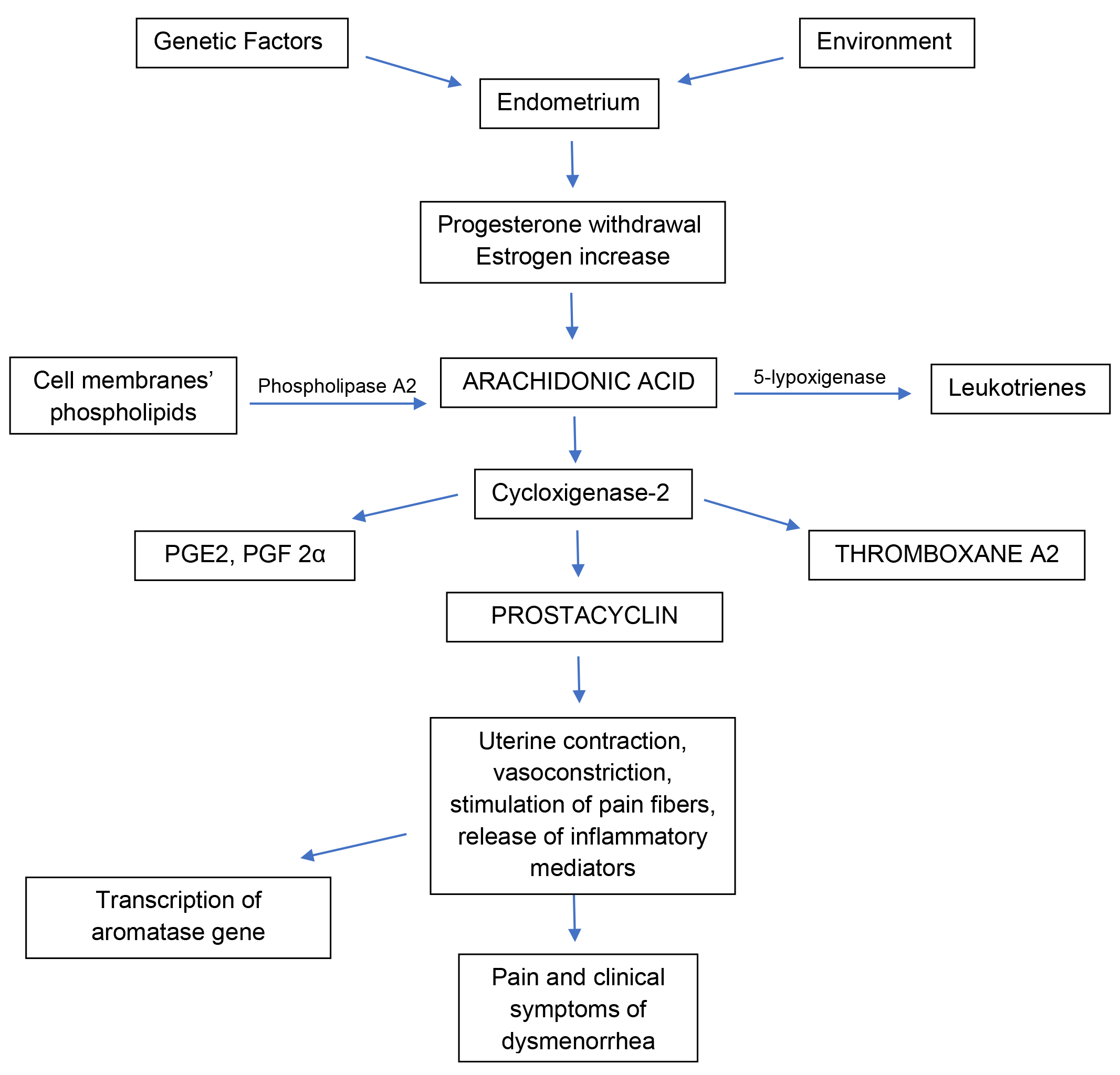
Primary dysmenorrhea, also defined as functional, is generally more frequent than secondary dysmenorrhea among adolescents and young adults and is associated with normal ovulatory cycles and no pelvic pathology [1]. After ovulation and before menstruation, progesterone withdrawal stimulates a huge release of arachidonic acid, an omega-6 fatty acid located among the phospholipids of cell membranes, that promotes the activation of prostaglandins and leukotrienes in the uterus. Progesterone inhibits this mechanism while estrogens have a stimulatory effect [4]. Thus, increased estrogen production, after progesterone withdrawal, increases prostaglandin (PG) production in the endometrium through the activation of cyclooxygenase-2 (COX-2) and nuclear factor kappa B (NF-kB), a nuclear transcription factor responsible for the activation of genes involved in the inflammatory cascade [35]. In detail, NF-kB activates COX-2 gene transcription, which is responsible for a huge PG cascade synthesis, triggering inflammation in the endometrium [35]. This strong inflammatory response is thought to cause dysmenorrhea symptoms, such as nausea, vomiting, bloating, headache, and affects the intensity of dysmenorrhea and bleeding. This repetitive inflammation in the endometrium is also able to stimulate the transcription of the aromatase gene in susceptible patients (absent in women without dysmenorrhea), which would cause in turn an increase in estrogens locally, maintenaning inflammation [35].
Two factors are considered to be involved in PD pathophysiology: the first is
uterine contraction and vasoconstriction, the second is release of inflammatory
mediators and stimulation of pain fibers. Prostaglandins are involved in the
regulation of ovulation and in endometrial physiology, including endometrial
proliferation and endometrial changes during menses [36]. High levels of
prostaglandins cause increased myometrial tone, uterine contraction and thereby
pain. PG, in particular prostaglandin F2
The mechanisms described above are showed in Fig. 1.
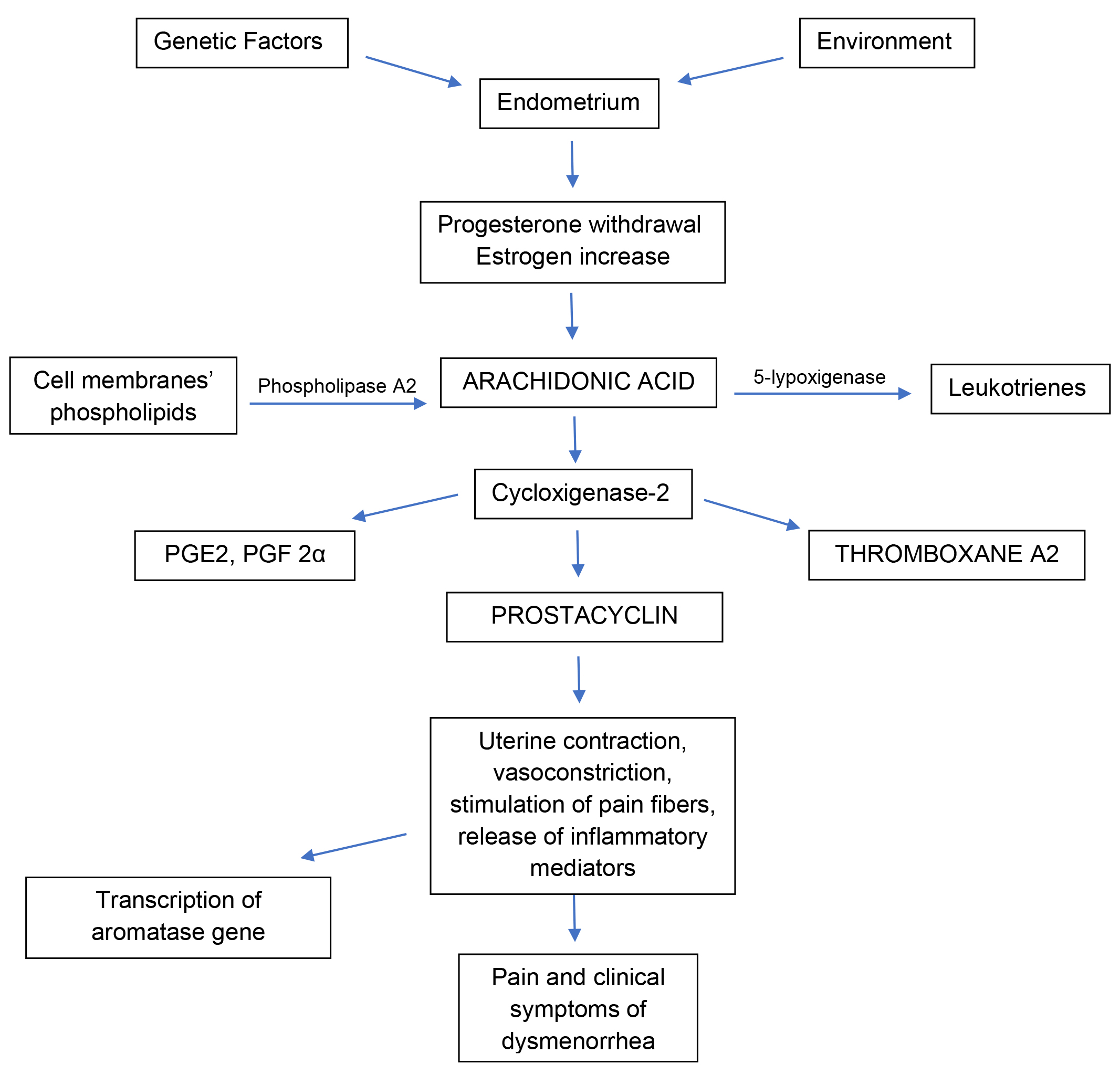 Fig. 1.
Fig. 1.Schematic representation of the main mechanisms occurring in the uterus which dysregulation is shown and/or thought to be associated with primary dysmenorrhea. PGE2, prostaglandin E2; PGF2α, prostaglandin F2α.
Finally, genetic component is also thought to have an impact on PD pathophysiology. A recent study, comparing correlation of pain, in primary dysmenorrhea among twins, showed a much stronger correlation for chronic pain in monozygotic twins compared with dizigotic twins, suggesting that there is a genetic influence on dysmenorrhea in young woman [25]. A genome-wide association study showed that common variants in the ZMIZ1 gene (rs76518691 locus) and in the NGF gene (rs7523831) conferred a risk of primary dysmenorrhea [39]. NGF played a fundamental role in production of pain and hyperalgesia while ZMIZI was involved with autoimmune conditions [25]. Another study, performed among Taiwanese people with PD, showed an association with a polymorphism in a brain-derived neurotrophic factor gene (BDNF) Val66Met [40]; this polymorphism was reported to have an effect on the descending pain modulatory systems [41] and to be a possible regulator of menstrual pain [42].
Painful menstruations due to recognized medical conditions, in particular pelvic pathology, is necessary to define secondary dysmenorrhea. The main cause of this disease is endometriosis [43], however, other conditions can be involved also, such as adenomyosis, infection, myomas, mullerian or obstructive reproductive tract anomalies or ovarian cysts [4].
Chronic pelvic pain is the main clinical manifestation, defined as a progressively worsening pain that lasts from at least 6 months, associated with irregular or heavy menstrual bleeding; the pain can be constant, intermittent, cyclic or acyclic [44].
Dysmenorrhea of recent onset, associated with pelvic tenderness and abnormal vaginal secretions should suggest an infectious etiology [45].
Endometriosis is the main cause of chronic pelvic pain and secondary dysmenorrhea in young women. It remains a surgical diagnosis in adolescents, and diagnosis requires the presence of glands and stroma outside of the endometrial cavity. The pathogenesis of this disease is not well-known, and some controversies remain limiting efficacy of long-term treatment. Laparoscopy for chronic pelvic pain and/or for dysmenorrhea was performed in 62%–75% of adolescents and in 70% of adolescents with pelvic pain that did not improve with on nonsteroidal anti-inflammatory drugs and/or combined oral contraceptive pill [46]. The main sites where endometriosis implants can be found are in the pelvis and in the ovaries [47]. Endometriosis is associated with a complex interaction among hereditary predisposition, environmental factors and epigenetic changes. Studies in monozygotic twins have shown a heritability in 51%–75% of cases [47] while having a first-degree relative with dysmenorrhea involves a higher risk of dysmenorrhea with respect to the general population [48]. In addition to this, inheritance is likely polygenic and multifactorial with an important interaction with environmental factors [48]. The most recognized and accepted theory about the development of endometriosis is Sampson’s theory of retrograde menstruation which shows how menstrual fluid containing endometrial mesenchymal stem cells, epithelial progenitor cells and stromal fibroblasts, leaks back through fallopian tubes and implants into the peritoneum [49]. This theory, however, does not explain why most of the women who present this menstrual reflux do not develop endometriosis as only 5%–10% shows this complication [50]. Other factors most be implicated, as immunological abnormalities and local inflammation, causing impaired cleaning of endometriotic cells from aberrant locations in the peritoneal cavity, outside the endometrium [51]. Other theories include the transformation of coelomic mesothelial cells into endometrial cells through the process of metaplasia (Meyer’s theory) and the possibility of hematogenous or lymphatic dissemination of endometrial cells (Halban’s theory) [52]. Finally, women with endometriosis could have altered immunologic functions and increased levels of pro-inflammatory cytokines and growth factors which can stimulate altered proliferation of the endometrium [53]. It is hypothesized, in addition, that endometrial mesenchymal stem cells show epigenetic changes that are present in the eutopic endometrium of endometriosis patients but not in disease-free endometrium [54]. Epigenetic changes present in this tissue would affect the transcription of several genes, in particularof the aromatase gene, giving to the endometrium the ability to synthesize estrogens. As previously explained this would upregulate COX-2 expression and the synthesis of PG resulting in local inflammation [54, 55]. This local inflammation would sustain epigenetically modified endometrial cells, capable of producing estrogens locally, that would be transported by menstrual retrograde flow into the pelvis, where new foci of endometriosis can start.
While the beginning of endometriosis would be ascribed to epigenetic alterations, progression would be determined mostly by the intensity of aromatase activity regulated in turn by the degree of inflammation. PG are a potent inducer of aromatase expression and indirectly they stimulate estrogen production, therefore, resulting in further expression and activity of aromatase and increase of estrogens [4]. Aromatase expression is high also in the endometrium of patients who develop adenomyosis, endometrial polyps and myomas besides endometriosis, and absent in the endometrium of disease-free women [56]. In addition, a defective immunological system fails to destroy these altered cells transported by retrograde menstrual flow [57]. Estrogens play an inhibiting role in immune surveillance: the presence of estrogen receptors in the macrophages, capable of inactivating their scavenger function, prevents the destruction of endometrial cells in the peritoneum, arrived through retrograde menstruation [58].
Treatment options for primary dysmenorrhea aim to reduce the associated pain and symptoms that often cause discomfort and impair patients’ quality of life. Different treatment target different mechanisms; some interfere with prostaglandin production, others reduce uterine tone or inhibit the perception of pain through a direct analgesic effect. Regarding this latter, there are several pharmacological and non-pharmacological treatment options that can be used alone or in combination, and can be tailored to individual characteristics. For optimal treatment and adequate compliance, it is important to discuss the choice of the therapeutic strategy and share with the patient, parents, and any other health care professional. All necessary information on the available options, their effectiveness, potential side effects, and manageability should be provided. Indeed, it must be considered that primary dysmenorrhea may appear in adolescence but often persists into adulthood, so different treatment options may be appropriate for a given patient over time depending on the stage of life [59].
Pharmacological therapies include both hormonal and non- hormonal therapies.
The mainstay of treatment for primary dysmenorrhea are prostaglandin synthetase inhibitors and the most commonly used are the nonsteroidal anti-inflammatory drugs.
NSAIDs work by interrupting the action of cyclooxygenase (COX), an enzyme responsible for prostaglandin synthesis. Lower levels of endometrial prostaglandins lead to less vigorous contractions of the uterus, and, therefore, to less discomfort. In general, approximately 70% of women experience moderate to complete relief of painful cramping with the use of prostaglandin inhibitors, although single trials show wide variations in outcomes. Overall studies have shown that NSAIDs are significantly better than placebo in providing pain relief in PD [60]. They are most effective when started 1 to 2 days before the onset of menses and taken at weight-specific doses on a routine schedule through the first 2 to 3 days of bleeding. Adolescents and young adults who cannot predict the beginning of their periods should be instructed to start NSAIDs as soon as menstrual bleeding begins, or as soon as they have any menstruation-associated symptoms. The more frequent reasons why adolescents do not respond to NSAIDs is delayed use and subtherapeutic dosing. Some patients do not respond to NSAIDs despite adequate adherence due either to the presence of underlying secondary causes of dysmenorrhea or molecular etiologies [14].
Traditional NSAIDs are commonly known as non-selective because they inhibit both COX-1, the constitutive isoform of cyclooxygenase, responsible for the synthesis of prostaglandins in the gastric mucosa, platelets, and kidney, which inhibition is thought to be responsible for gastric mucosal damage and inhibition of platelet aggregation, and COX-2, the inducible isoform, activated upon tissue damage that is thought to act primarily on the synthesis of prostaglandins involved in inflammation. Keeping this in mind the anti-inflammatory and pain-relieving effects depend on COX-2 inhibition, while their side effects (as indigestion, headaches, and lethargy considered to be the most concerning adverse effects) are related to their action on COX-1. However, as dysmenorrhea typically resolves by day 2 to 3 of the menstrual period, the short course of treatment limits the development of conventional NSAIDs-associated side effects. Taking the medication with food and increasing fluid intake may mitigate gastrointestinal and renal adverse effects [61]. Treatment with one of the conventional NSAIDs in a therapeutic dose is the preferred initial treatment and should be tried for at least three menstrual periods. Adolescents and young adults should receive specific instructions on the dose and maximum daily frequency of the recommended NSAIDs. If one preparation does not provide relief, a second NSAID preparation should be tried [4]. Sufficient randomized, placebo-controlled trials support the efficacy of NSAIDs in the management of primary dysmenorrhea and their greater efficacy compared with paracetamol for pain relief.
Paracetamol can be used in addition to NSAIDs because of its fewer gastrointestinal side effects [6]. A network meta-analysis, including 73 randomized control trials on women with primary dysmenorrhea with the purpose of comparing NSAIDs and placebo and NSAIDs with each other to evaluate their efficacy and safety, showed that all drugs except aspirin were more effective than placebo; Marjoribanks et al. [62] concluded that short term administration was recommended, and when compared with each other, NSAIDs did not show large differences with regard to the pain-relieving effect. Many randomized, placebo-controlled studies have shown that several NSAID preparations, including naproxen sodium, mefenamic acid, ketoprofen, ibuprofen, and diclofenac, are effective treatments for primary dysmenorrhea. Naproxen was used widely and showed significant relief of pain in PD in early times. However, with the development of NSAIDs drugs, several other drugs have proved to have similar efficacy. There is limited evidence to recommend one specific NSAIDs as more effective or safer than another in the treatment of dysmenorrhea, so cost considerations, side-effect profiles, and dosing regimen are reasonable considerations when deciding which medication to use. It is possible to change the drug with another one if it does not provide adequate relief [8]. As for pain relief, all drugs except nimesulide are superior to aspirin. Ketoprofen, naproxen and ibuprofen are scored significantly higher than placebo. As for the safety outcome, tiaprofenic acid and mefenamic acid were indicated as the safest NSAIDs drugs, while indomethacin was the worst and more likely to cause mild gastrointestinal discomfort. Flurbiprofen was considered the best among all for efficacy, and aspirin was worse than most of the others [63].
Highly selective COX-2-specific inhibitors (coxibs) were developed and first
launched in 1999 in order to improve tolerability of NSAIDs. These specific COX-2
inhibitors spare prostaglandins produced by COX-1 and essential for the integrity
of the gastric mucosa. However, concerns regarding the risk of cardiovascular
and/or dermatological adverse events associated with the long-term use of some
coxibs has arised, and some have been withdrawn by the manufacturers [4]. In
those with a history of impaired renal function, heart failure, gastrointestinal
bleeding or ulcer disease, liver dysfunction, for those taking diuretics,
angiotensin converting enzyme (ACE)-inhibitors, or angiotensin II antagonists, Celecoxib (Celebrex) should be
used with caution and considered only when high dose conventional NSAID is
uneffective, and in the case of coagulation deficiencies. Moreover, during
treatment with Celecoxib (Celebrex), blood pressure should be monitored closely.
Celecoxib provides relief from menstrual pain within 1 h of administration and is
the only available COX-2 inhibitor approved by the US Food and Drug
Administration (FDA) for the treatment of PD in patients
Non-hormonal treatments for dysmenorrhea and their efficacy are reported in Table 3 (Ref. [63]).
| NSAIDs | |||
| Initial Dose | Subsequent Dose, as Needed | Maximum Dose Per Day in Short-Term Use | |
| Naproxen sodium | 550 mg | 1375 mg | |
| Ibuprofen | 400–600 mg | 2400 mg | |
| Ketoprofen | 50 mg | 300 mg | |
| Mefenamic acid | 500 mg | 1000 mg | |
| Diclofenac | 75–100 mg | 1800 mg | |
| COX-2 INHIBITORS | |||
| Celecoxib | 400 mg | 200 mg every 12 hours | 400 mg |
NSAIDs, nonsteroidal anti-inflammatory drugs; COX-2, cyclooxygenase-2; FDA, Food and Drug Administration.
Hormonal therapy, alone or in combination with non-hormonal medications or alternative and complementary agents, is another treatment option for primary dysmenorrhea. Hormonal contraceptives are considered effective in the treatment of dysmenorrhea by reducing the production of prostaglandins and leukotrienes due to limiting endometrial proliferation and/or ovulation.
Hormonal contraceptives are subdivided into Short Acting Reversible Contraceptives (SARCs), such as estroprogestin oral contraceptives (COC), progestin oral contraceptives (POP), estroprogestin vaginal ring and estroprogestin transdermal patch, and Long Acting Reversible Contraceptives (LARCs) methods, such as progesterone-medicated intrauterine devices (which may be considered in case of established contraindications to the use of the other therapeutic aids, such as mental disability) and subcutaneous implants containing etonogestrel (ETN) lasting three years (indicated for women aged 18 to 40 years) and medroxyprogesterone acetate injections every 12 weeks, off-label in some countries, as Italy. The combination of the estrogen and progestin contained in the COC pill, as well as the different dosages and routes of administration, must be considered when tailoring treatment, to provide the therapy best suited to needs and complaints, and according to specific contraindications [14].
When effective, COC are considered by some an ideal treatment for adolescent dysmenorrhea for safety reasons, and for the association with several non-contraceptive health benefits important to adolescents, in particular when there is a high rate of unplanned sexual activity, risk of pregnancies, and abortions. Observational studies have supported an association between COC use and decreased dysmenorrhea [64]. Combined hormonal contraceptives are reported to be effective for the treatment of dysmenorrhea in approximately 70%–80% of women using an oral contraceptive pill (OCP), or contraceptive intravaginal ring, or the patch [65]. The studies have shown also reduced absenteeism in the progesterone-dominant COC group compared to non-COC and non-intra uterine device users. No differences were noted between the low-progesterone-activity group and the non-COC and non-intra uterine device groups [66].
Cyclic OCP (cyclic 21 days active pills and 7 days placebo pills administration) induces withdrawal bleeding and allows the production of prostaglandins and persistent pain; continuous OCP results in more pain relief and recent studies show its superiority, even if cyclic use is as beneficial long term [67].
The major concern with OCPs is the risk of deep vein thrombosis (DVT). In addition to the known thrombotic effects of estrogens, the progestin may also affect DVT risk [68].
The risk may be lower with a second-generation progestin (i.e., norgestrel or levonorgestrel) compared with a third-generation progestin [69].
In the case of dysmenorrhea secondary to endometriosis, it is important to make an individualized, long-term treatment plan, based on symptoms, age, and contraceptive need to control disease progression and reduce pain while trying to postpone surgery until closer to the time of desire for conception. Indeed, it has been shown that even after surgical removal of all obvious foci of endometriosis by experienced surgeons, the recurrence rate of symptoms and lesions varies from 10% to 55% within 12 months. In addition, all surgeries on the ovary, but especially those for endometrioma, inevitably reduce follicular reserve and thus impact future fertility. As medical therapies, in addition to the use of NSAIDs as needed, endometriotic foci have been documented to respond to progestins, either taken alone or in combination with estrogen.
The choice is therefore between low-dose estrogenic estroprogestins, either by os or vaginal route preferably on an extended regimen, especially if the girl also needs contraception, and progestins which are the most suitable option in cases of deep infiltrating endometriosis and symptomatic adenomyosis. These can give spotting, sometimes changes in mood and there is, in very young patients, the doubt of a slight impact on peak bone mass attainment. Progestin-only contraceptive preparations are under study [70].
The role of non-pharmacological therapies for the management of dysmenorrhea has been established by numerous studies. Systematic reviews and trials have stated that there is poor evidence on the efficacy of these remedies. Still, some evidence exists on interventions such as acupressure, psychotherapy, herbs and nutrients, transcutaneous electrical nerve stimulation, heat, etc. More studies are however needed to assess the real efficacy of these methods.
The following paragraphs provide an overview of current non-pharmacological remedies for dysmenorrhea [71, 72].
Smith et al. [73] summarised the knowledge on acupuncture in the management of dysmenorrhea, including the results from 42 RCTs, and concluded that there is no sufficient evidence in favour of acupuncture; the evidence was considered overall of low quality due to the presence of bias, poor information and inconsistency [74]. One more recent study considered 60 women aged 17–23 years randomly divided into a study group and a control group; the study group received acupuncture for 20 minutes/day for 15 day/month for 3 months, the control group, no treatment. The study group showed an improvement of menstrual cramps and other symptoms (dizziness and headache, fainting, diarrhea, psychological symptoms, tiredness, nausea and vomiting) suggesting that acupuncture could be an effective remedy in the treatment of dysmenorrhea [73].
Exercise is considered among the possible remedies for the treatment of dysmenorrhea.
Kannan included 70 women a study where a group practiced aerobic type physical activity for 7 months (the first month of which was under supervision), and these showed a significant improvement in pain quality and intensity at month 1, 4 and 6, strengthening the evidence that aerobic exercise may improve dysmenorrhea [75].
The practice of yoga has attracted a lot of attention [76]. In a Thailandese RCT including 34 volunteers, 17 were asked to practice yoga twice a week for 12 weeks. At the end, menstrual pain wes evaluated together with physical fitness outcomes (flexibility and muscle strength). The regular practice of yoga showed a positive effect on menstrual pain and also on physical fitness and quality of life, confirming yoga among the effective non-pharmacological remedies for dysmenorrhea [76, 77].
Among vitamins, vitamin E has been studied, alone or in combination with fish oil (omega-3), as a possible treatment for the management of dysmenorrhea. In one study, 100 subjects were divided into 4 groups receiving respectively omega-3, vitamin E, omega-3 plus vitamin E or placebo, daily. Both vitamin E and omega 3 relieved menstrual pain. However, the greatest effect was achieved when the two remedies are administered in combination [78].
Vitamin D supplementation has also been experimented, in one study vitamin D was administered for 9 weeks to 897 adolescent girls referring premenstrual syndrome (PMS) and/or menstrual pain; the authors report a reduction of both menstrual pain and psychological symptoms [79].
4.3.2.1 Gegen Decoction
A double-blinded RCT conducted by Chai compared the GeGen Decoction (a well-known Chinese herbal formula) with placebo in managing the menstrual pain. The decoction was created following the recipe by Professor Bo-yang Yu [80]. Gegen was given seven days before the beginning of the menstrual cycle for three months. They studied the differences in pain intensity and the serum levels of arginine vasopressin and estrogens. GeGen decoction showed an analgesic effect, that might be related with the regulation of pituitary, hypothalamic and ovarian hormones and would interfere with metabolic change, while blood samples did not find appreciable toxic side effects [81].
4.3.2.2 Melissa Officinalis
Lemon balm (Melissa Officinalis) is traditionally used for dysmenorrhea management, as it is thought to release smooth muscle, diminishing cramps [82]. Some studies have been performed showing a good effect of Melissa in ameliorating energy levels and psychological symptoms; though no statistical significance was found on the effect of Melissa in improving menstrual pain [83].
4.3.2.3 Ginger
In their meta-analysis, Daily et al. [84] proved that ginger can be effective in alleviating dysmenorrhea. In fact, all the considered studies showed a positive effect of ginger powder in easing pain being reported to be as effective as analgesic drugs but without the adverse effects of NSAIDs [83].
4.3.2.4 Others
A systematic review from 2016, studied the efficacy of dietary supplements for the treatment of primary dysmenorrhea found no evidence for any efficacy of dill, guava, or fennel and only limited evidence for an efficacy of fenugreek, fish oil, fish oil plus vitamin B1, ginger, valerian, zataria, and zinc sulphate. When supplements were compared with NSAIDs, no difference was found between dill, fennel, guava, rhubarb, valerian, and NSAIDs, but very limited evidence was found for chamomile. When supplements were compared with each other, no difference in effectiveness was shown between ginger and zinc sulphate, but vitamin B1 might be more effective than fish oil [84].
Phytoestrogen is the generic name to refer to a group of nonsteroidal phenolic
plant compounds such as isoflavones, lignans, coumestans that are found in a
variety of vegetables, fruits, grains, and especially soybeans and related
products such as bean curds/tofu and soy milk. According to data from the
European Prospective Investigation into Cancer and Nutrition (EPIC), a
multi-center cohort study focusing on diet, environment, and lifestyle in
European countries, the total intake, subcategories of phytoestrogens as well as
their food origins vary widely among different countries/regions [85]. Natural
hormonal support options are not as effective as synthetic ones but consuming
moderatelyfoods that contain decent levels of phytoestrogens may help to regulate
feminine hormonal health and minor symptoms of dysmenorrhea. By their structural
similarity to estrogens, these compounds can bind to both estrogen receptors (ER)
Zinc has also been studied as a remedy for menstrual pain and bloating, however, other studies are needed to establish the effectiveness. It probably works on cyclooxygenase-2, lowering prostaglandins and subsequently inflammation [88].
Although the pharmacological mechanism is not well known, the literature reports some data on the efficacy of magnesium in the prevention and treatment of premenstrual syndrome, dysmenorrhea and post-menopausal symptoms [89].
Low calcium levels increase uterine contractions, decreasing blood flow and consequently causing pain.
Based on this assumption, in their systematic review Abdi et al. [90] evidenced that calcium intake can be effective in reducing the severity of dysmenorrhea and the use of analgesic drugs [91, 92].
In their RCT, Machado et al. [93] reported that TENS, combined with thermotherapy, immediately reduces pain, with an effect lasting for the subsequent 24 hours. When compared, heat alone showed major effectiveness on pain reduction than TENS alone [90, 93].
Elastic therapeutic taping has been studied as a remedy to improve pain intensity and anxiety, even if with moderate quality evidence. However, it should be noted that this strategy also has side effects, such as possible skin allergies and dizziness, even if no worse than the side effects depending on NSAID use [61, 94, 95].
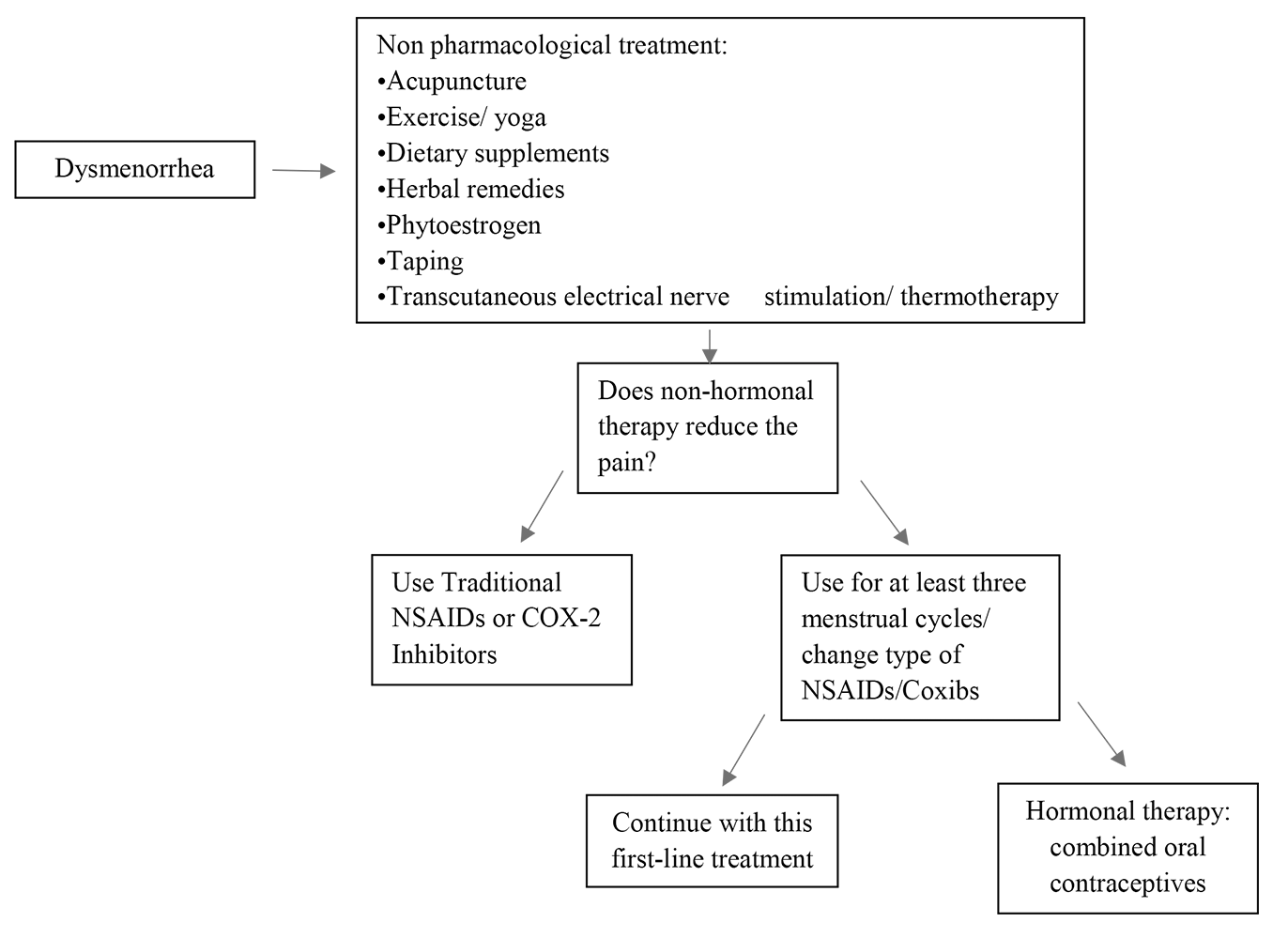
Fig. 2 summarizes the main therapeutic options for PD, and a decision-making diagram.
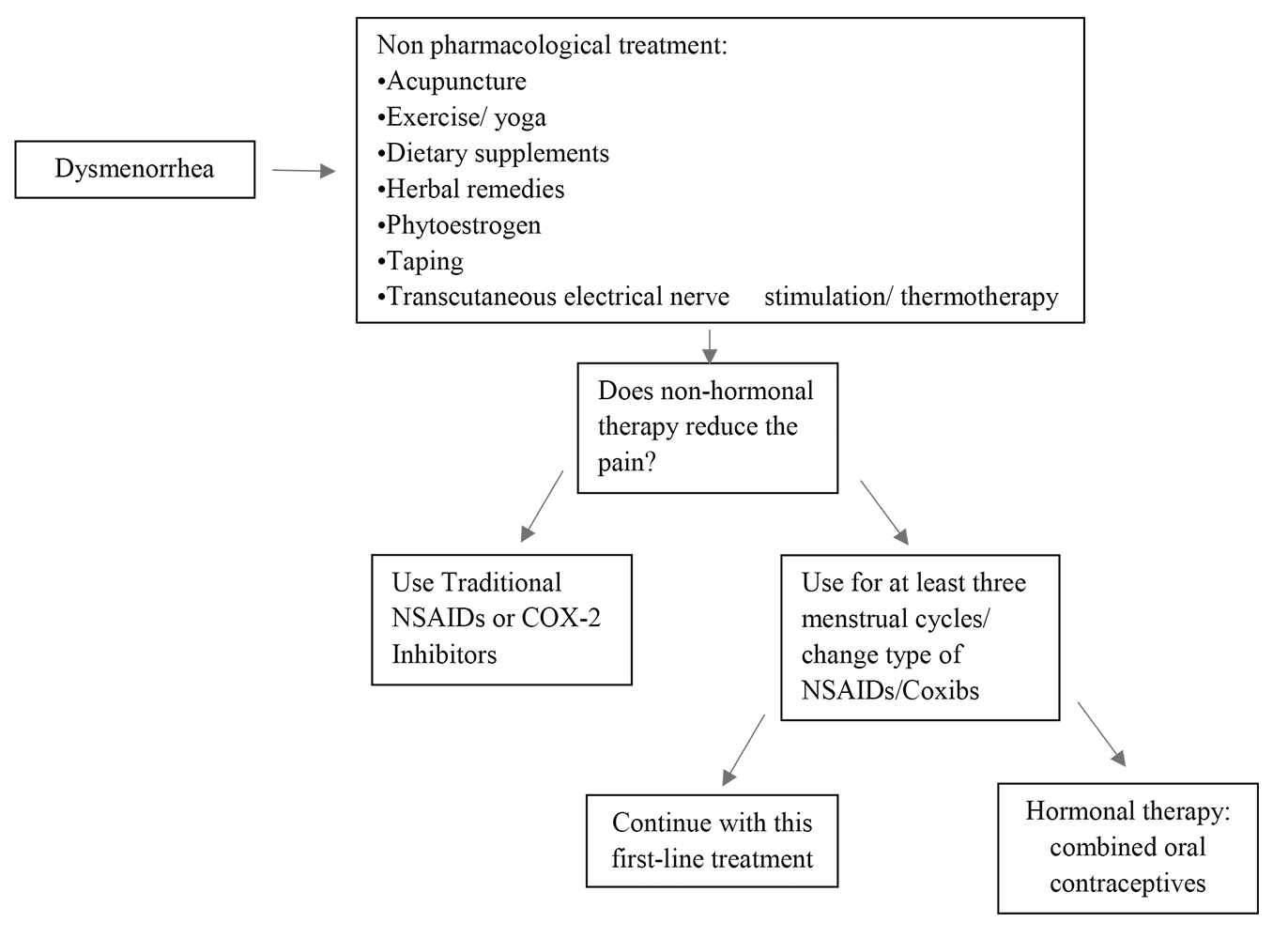 Fig. 2.
Fig. 2.Decision-making scheme on different therapeutic strategies. Coxibs, COX-2-specific inhibitors.
An important association between mental health and primary dysmenorrhoea is described in the literature which evidences conditions as depression, stress and anxiety as risk factors. In light of this, psychotherapy can have a beneficial effect in the multidisciplinary approach to dysmenorrhea. Rogers et al. [96], in their meta-analysis, analysed also the effect of psychological intervention on the severity of pain and its impact on quality of life, howeveralthough they confirmed that pharmacological intervention improved both the severity of pain and its interference in daily activities, they evidences a lack data to support fully the usefulness of psychological intervention [96, 97]. Non-pharmacological therapy is mainly used in primary dysmenorrhoea; there is currently a lack of data in the literature quantifying its efficacy in the treatment of secondary dysmenorrhoea, whereas the diagnosis and the treatment of the underlying cause remains essential.
Dysmenorrhea can have a strong negative impact on quality of life, involving not only physical health but also mental health, affecting social life and relationships. Patients suffering from dysmenorrhea can be affected by depression, anxiety and stress-related disorders; in turn, these conditions can further exacerbate social and working functions.
Adolescence is the fundamental period of life when social networks are established, life goals are identified, and specific educational pathways begin to be undertaken; this is why a proper and accurate diagnosis is important for effective intervention.
Public health measures would need to be implemented to provide health education and information to improve the management of dysmenorrhea. It is generally recognized that this can be achieved by making use of the media through large-scale information campaigns and collective actions in schools as this would bring more awareness to young adolescent girls [13]. The inclusion of questions on menstrual symptoms in routine medical visits could be a useful tool to improve the identification of truly symptomatic patients and to monitor therapeutic response [14].
The key role of inflammatory mediators, in particular of PG and LT, in the pathogenesis of PD, has been long recognized, however, there are no new studies in recent years. Further understanding of the pathophysiologic mechanisms is warranted, in order to generate targeted preventive interventions and seek for new therapeutic options. The genetic component of primary dysmenorrhea needs also further understanding. Increasing the use of new instruments to classify and grade the intensity of pain would also be of importance in clinical practice for tailored treatment. Currently, treatment with one of the conventional NSAIDs is the preferred initial treatment and should be tried for at least three menstrual periods; If one preparation does not provide relief, a second NSAID can be tried. With the aim of improving the tolerability of NSAIDs, highly selective COX-2-specific inhibitors were developed, but if compared with NSAIDs they don’t appear more effective or tolerable. Hormonal therapy, alone or in combination with non-hormonal medications or alternative and complementary agents, is another first-line treatment option. Combined oral contraceptives, the contraceptive intravaginal ring, and the patch are effective for the treatment of dysmenorrhea in approximately 70%–80% of women. When treating dysmenorrhea, non-pharmacological therapies must be considered also as the literature reports some efficacy for some both alone or in combination with pharmacological remedies. Evidence exists for acupressure, psychotherapy, herbs and some nutrients, transcutaneous electrical nerve stimulation, and heat [98]. More studies are needed, however, to assess the effective functioning of these methods.
RF, MP, GM, AMB and BS contributed to the search of the literature, drafting and writing the manuscript, BS gave substantial contribution to preparing the figures, SMRE gave substantial contribution to the interpretation of findings, preparation of the manuscript and its revision, and MES was responsible for conceptualization of the manuscript, tables and figures, contributed to the writing and substantially revised the manuscript. All authors read and approved the final manuscript.
Not applicable.
Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest. Maria E. Street is serving as one of the Editorial Board members of this journal. We declare that Maria E. Street had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Shigeki Matsubara.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.