- Academic Editor
Background: Intrahepatic cholestasis of pregnancy (ICP) is a
common liver disorder specific to pregnancy. Taurocholic acid (TCA) has been
implicated in the pathogenesis of ICP. This study aimed to investigate the
association between serum TCA levels and adverse maternal and infant outcomes in
women with ICP. Methods: Pregnant women diagnosed with ICP were
categorized into normal or adverse groups based on their pregnancy outcomes.
Baseline data, including age, pre-pregnancy body mass index (BMI), systolic blood
pressure (SBP), diastolic blood pressure (DBP), and fasting blood sample (5 mL),
were collected at 28 weeks of gestation. Serum levels of total bile acid (TBA),
alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TBIL),
and TCA were measured using a fully automatic biochemical analyzer. The
predictive value of serum TCA levels for adverse outcomes in ICP was analyzed
using receiver operating characteristic (ROC)
curve analysis. Subsequently, ICP patients were divided into high and low TCA
expression groups, and the changes in baseline data and adverse outcomes were
compared between the groups. The relationship between serum TCA levels and
adverse outcomes was evaluated using adverse maternal and infant outcome curves.
Logistic regression analysis was performed to identify independent risk factors
for adverse outcomes in ICP patients. Results: The
adverse outcome group showed significant differences in
gestational age at delivery (median value of 37 years old,
p = 0.0001), levels of TBA (mean
During pregnancy, the body undergoes various anatomical and physiological changes to support the development of fetus [1]. One organ that adapts its metabolism during pregnancy is the liver, which plays a crucial role in transporting bile, and regulating bile acid levels in the blood [1]. While moderate increases in total bile acid (TBA) levels are normal, excessive elevation can lead to intrahepatic cholestasis of pregnancy (ICP) and increase the risk of adverse perinatal outcomes [2]. ICP is associated with adverse maternal and infant outcomes, including meconium staining of the amniotic fluid, spontaneous preterm delivery, and stillbirth [3, 4, 5]. The pathogenesis of ICP involves hormonal, genetic, environmental, and immunological factors, with the estrogen-bile acid axis playing a significant role [5]. However, the precise etiology of ICP remains poorly understood, and its management remains challenging due to limited data on diagnosis, treatment, and associated adverse outcomes [6]. Early diagnosis and timely management of ICP can significantly reduce the risk of complications, including unexpected intrauterine death [3]. Therefore, there is an urgent need to identify reliable biomarkers for ICP.
In ICP, taurochenodeoxycholic acid (TCDCA), glycocholic acid (GA), tauroursodeoxycholic acid (TUDCA), taurocholic acid (TCA), and glycochenodeoxycholic acid (GDA) are the predominantly affected bile acids [7]. Previous studies have reported a correlation between elevated serum TCA levels and the severity and clinical prognosis of drug-induced liver injury [8]. Among the bile acids, TCA and GA are the most prominent types detected in the serum of severe ICP patients, with levels significantly higher than those in mild ICP or normal pregnant women, among which TCA has been identified as a promising biomarker for predicting the risk of fetal complications [9]. However, research on TCA in the context of ICP patients remains limited, with most studies focusing on overall bile acid metabolism [7]. Therefore, this study aimed to investigate the serum levels of TCA in pregnant women with ICP and explore the relationship between TCA levels and adverse maternal and infant outcomes, with the goal of improving perinatal outcomes.
The present study was reviewed and approved by the Academic Ethics Committee of the Third Affiliated Hospital of Zhengzhou University, and conducted in accordance with the principles outlined in the Declaration of Helsinki (approval number: 2023-168-01). Informed consent was obtained from all participants after providing a detailed explanation of the study objectives and procedures.
This prospective analysis included a total of 372 pregnant women diagnosed with
ICP who were admitted to the Third Affiliated Hospital of Zhengzhou University
between January 2020 and December 2022 due to skin itching. Among them, 85
patients did not meet the inclusion criteria, 36 refused to participate, 11
withdrew from the study, and 10 had incomplete data. Ultimately, 230 pregnant
women with ICP were included in the study. Based on their maternal and infant
outcomes, the participants were allocated to two groups: the normal group (with
good perinatal outcomes, n = 156) and the adverse group (with poor perinatal
outcomes, n = 74). Mild ICP was defined as TBA levels ranging from 10 to 40
µmol/L, while severe ICP was defined as TBA
 Fig. 1.
Fig. 1.Research on the relationship between serum TCA levels and adverse maternal and infant outcomes in pregnant women with ICP. ICP, intrahepatic cholestasis of pregnancy; n, number; TC, total cholesterol; TG, triglyceride; GLU, fasting blood glucose; TBA, total bile acid; ALT, alanine transaminase; AST, aspartate transaminase; TBIL, total bilirubin; TCA, taurocholic acid; ROC, receiver operating characteristic; KM, Kaplan-Meier.
The following inclusion criteria were applied: (1) diagnosed with ICP based on
elevated serum TBA levels (TBA
The following exclusion criteria were applied: (1) twin or multiple pregnancies; (2) multiparous women; (3) history of hepatobiliary system diseases; (4) use of hormone drugs to induce ovulation or assisted reproductive technologies; (5) deranged liver function tests (LFT) with low TBA; (6) skin itching caused by other etiologies; (7) presence of pregnancy complications such as gestational diabetes mellitus, hypertension, and anemia; (8) concurrent dysfunction of organs such as heart or kidneys; (9) missing diagnostic records; (10) unclear ICP diagnosis.
Baseline data of pregnant women with ICP were collected at 28 weeks of gestation, including age, pre-pregnancy body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), and a 5 mL fasting blood sample obtained from the elbow vein. The gestational age at the time of delivery in ICP was also recorded. The fasting blood sample from the elbow vein was centrifuged at 2000 r/min for 20 minutes, and the upper serum was collected in sterile Eppendorf tubes and stored at –80 °C. The levels of serum total cholesterol (TC), triglyceride (TG), fasting blood glucose (GLU), TBA, alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TBIL), and TCA were measured using an automatic biochemical analyzer (Perlong Technology, Beijing, China). TC, TG, TBA, ALT, AST, TBIL, and TCA kits were all purchased from Biolab Technology (Beijing, China), and the GLU kit was obtained from Maccura Biotechnology (Chengdu, Sichuan, China). The biochemical analyzer was calibrated at regular intervals to ensure the accuracy of biochemical test results.
The sample size was estimated using G-Power version 3.1.9.2
(Franz Faul, Kiel, Germany). The sample size of the independent sample
t-test for each group was determined to be at least 64, and the total
sample size was set to be at least 128. For the Chi-square test, the total sample
size was determined to be at least 220 (Supplementary Fig. 1). Data
analyses and plotting were performed using the GraphPad Prism 6.0 software
(GraphPad Software, San Diego, CA, USA). The Shapiro-Wilk test
was used to assess the normal distribution of data. Normally distributed
measurement data were presented as mean
A total of 230 pregnant women with (ICP were included in this study, with 156
assigned to the normal group, and 74 to the adverse group, based on pregnancy
outcomes. Statistical analyses of clinical baseline characteristics (Table 1)
revealed no significant differences between the normal group and the adverse
group in terms of age, pre-pregnancy BMI, blood pressure (SBP, DBP), blood lipids
(TC, TG), and GLU (p
| Feature | Normal group (n = 156) | Adverse group (n = 74) | p-value | |
| Age (years) | 29 (23, 35) | 29 (23, 34) | 0.239 | |
| Pre-pregnancy BMI (kg/m |
22.47 (20.23, 23.96) | 22.26 (19.94, 23.85) | 0.142 | |
| Gestational age at delivery (weeks) | 38 (37, 40) | 37 (35, 39) | 0.0001 | |
| SBP (mmHg) | 116.60 |
118.80 |
0.1342 | |
| DBP (mmHg) | 75.00 (60.00, 89.00) | 76.50 (61.00, 89.00) | 0.3183 | |
| TC (mmol/L) | 5.89 |
5.85 |
0.7633 | |
| TG (mmol/L) | 3.37 |
3.48 |
0.2234 | |
| GLU (mmol/L) | 4.72 |
4.78 |
0.5366 | |
| TBA (µmol/L) | 32.75 |
47.05 |
||
| ICP disease severity | ||||
| Mild ICP (10–40 µmol/L), % | 141 (90.38%) | 11 (14.86%) | ||
| Severe ICP ( |
15 (9.62%) | 63 (85.14%) | ||
| ALT (U/L) | 71.35 |
82.59 |
||
| AST (U/L) | 52.33 (41.37, 60.14) | 67.50 (40.73, 77.82) | ||
| TBIL (µmol/L) | 32.70 |
47.05 |
||
Note: BMI, body mass index; n, number; SBP, systolic blood pressure; DBP,
diastolic blood pressure; TC, total cholesterol; TG, triglyceride; GLU, fasting
blood glucose; TBA, total bile acid; ICP, intrahepatic cholestasis of pregnancy;
ALT, alanine transaminase; AST, aspartate transaminase; TBIL, total bilirubin.
Data conforming to normal distribution were depicted as mean
The serum TCA levels of ICP pregnant women were measured in
the normal group (11.72
 Fig. 2.
Fig. 2.Differential expression levels of serum TCA in ICP pregnant
women. Data conforming to normal distribution were expressed
as mean
ROC curves were generated to assess the predictive value of serum TCA levels for adverse maternal and fetal outcomes in ICP pregnant women. The area under the curve (AUC) for serum TCA levels predicting adverse perinatal outcomes in ICP pregnant women was 0.8430. The optimal cut-off value was determined to be 16.17, with a sensitivity of 66.22%, and specificity of 91.03% (Fig. 3). These findings indicate that TCA has predictive value for adverse maternal and infant outcomes in ICP pregnant women.
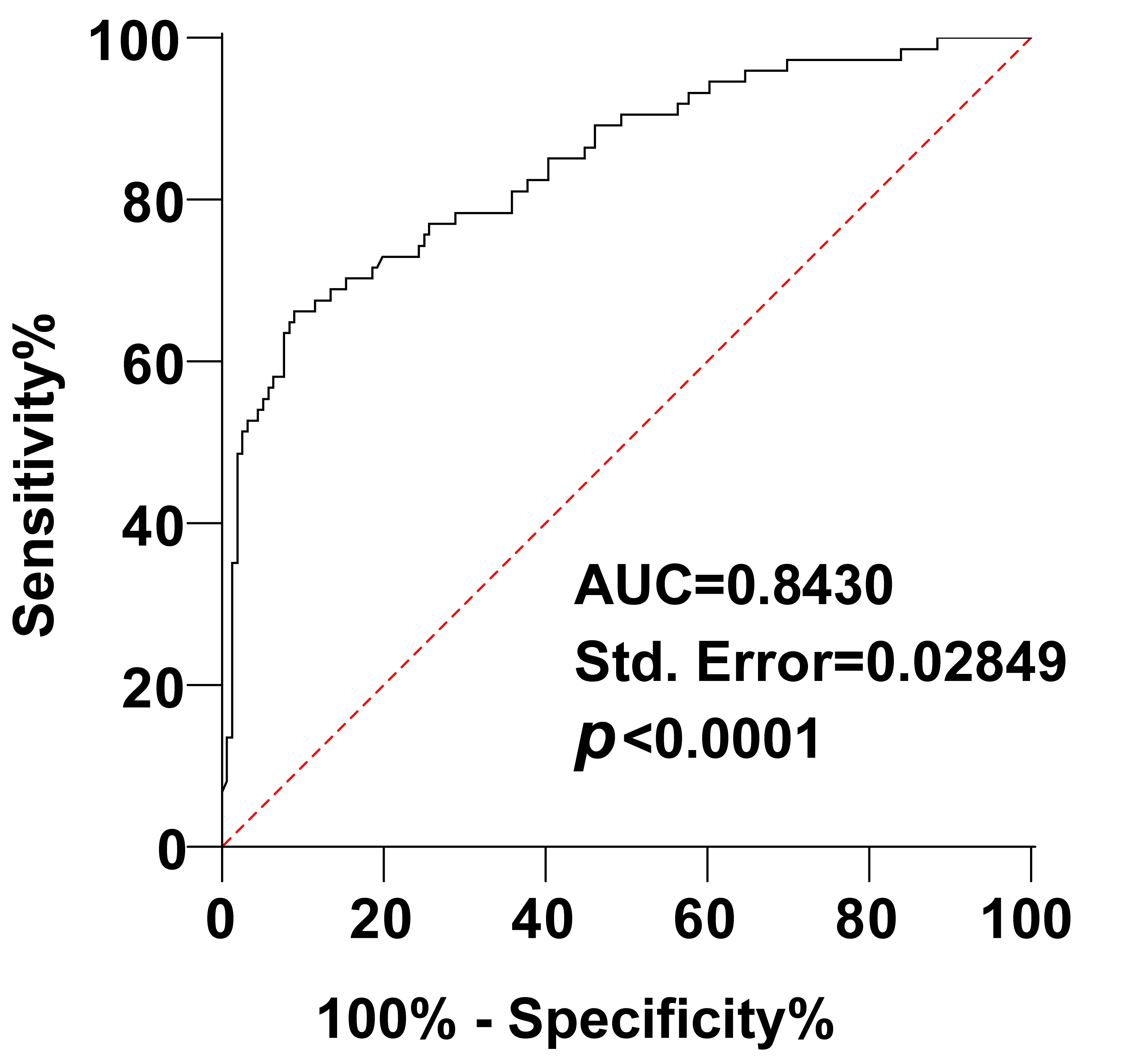 Fig. 3.
Fig. 3.The predictive value of TCA levels for adverse maternal and infant outcomes in ICP pregnant women was analyzed using the ROC curve. AUC, area under the curve.
Based on the ROC cut-off value of TCA (16.17), the ICP pregnant women were
divided into the low TCA expression group (n = 167) and the high TCA expression
group (n = 63). The changes in baseline data were compared and analyzed. There
were no significant differences between the two groups in terms of age,
pre-pregnancy BMI, blood pressure (SBP, DBP), blood lipids (TC,
TG), and GLU (p
| Feature | Low TCA (n = 167) | High TCA (n = 63) | p-value | |
| Age (years) | 29 (23, 35) | 29 (23, 34) | 0.1145 | |
| Pre-pregnancy BMI | 22.42 (20.10, 23.94) | 22.22 (19.94, 23.96) | 0.2081 | |
| Gestational age at delivery (weeks) | 38 (35, 40) | 37 (35, 40) | 0.0010 | |
| SBP (mmHg) | 116 (94.00, 140.00) | 119.0 (92.00, 137.00) | 0.1858 | |
| DBP (mmHg) | 76.00 (60.00, 89.00) | 76.00 (62.00, 89.00) | 0.3159 | |
| TC (mmol/L) | 5.91 |
5.79 |
0.3745 | |
| TG (mmol/L) | 3.38 |
3.48 |
0.3284 | |
| GLU (mmol/L) | 4.71 |
4.83 |
0.2035 | |
| TBA (µmol/L) | 35.01 (17.72, 59.54) | 43.40 (27.55, 58.95) | ||
| ICP disease severity | ||||
| Mild ICP (10–40 µmol/L), % | 134 (80.24%) | 18 (28.57%) | ||
| Severe ICP ( |
33 (19.76%) | 45 (71.43%) | ||
| ALT (U/L) | 73.11 (61.72, 94.63) | 79.89 (65.64, 93.88) | ||
| AST (U/L) | 54.39 (40.73, 74.66) | 63.87 (46.01, 77.82) | ||
| TBIL (µmol/L) | 34.87 (20.71, 59.54) | 43.79 (26.41, 58.95) | ||
Note: BMI, body mass index; n, number; SBP, systolic blood pressure; DBP,
diastolic blood pressure; TC, total cholesterol; TG, triglyceride; GLU, fasting
blood glucose; TBA, total bile acid; ALT, alanine transaminase; AST, aspartate
transaminase; TBIL, total bilirubin. Data conforming to normal distribution were
depicted as mean
The relationship between serum TCA levels in ICP pregnant women and adverse
pregnancy outcomes was further examined by grouping the patients into high and
low TCA expression groups. The high TCA expression group had a significantly
higher number of adverse maternal and fetal outcomes (postpartum hemorrhage,
premature delivery, neonatal asphyxia, fetal distress, amniotic fluid fecal
staining, low birth weight) compared to the low TCA expression group (p
| Feature | Low TCA (n = 167) | High TCA (n = 63) | p-value |
| Postpartum hemorrhage | 4 | 9 | |
| Premature delivery | 9 | 13 | |
| Neonatal asphyxia | 2 | 6 | |
| Fetal distress | 4 | 8 | |
| Amniotic fluid fecal staining | 4 | 8 | |
| Low birth weight | 8 | 11 | |
| Total number of adverse outcomes | 31 | 55 |
Note: The number was expressed as n, and the comparisons were conducted using the Chi-square test.
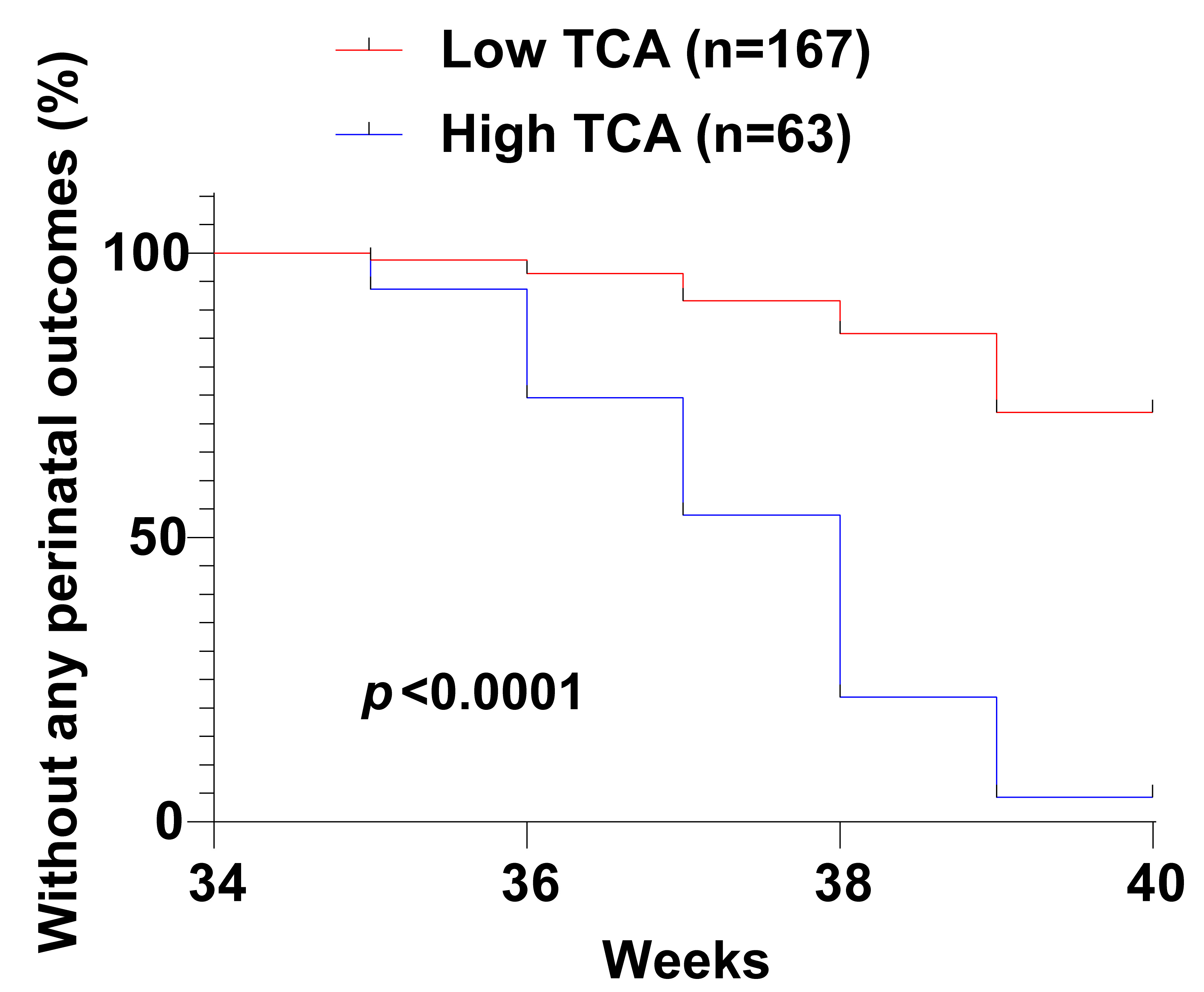 Fig. 4.
Fig. 4.Kaplan-Meier curve analyses of the impact of serum TCA levels on adverse maternal and fetal outcomes in ICP patients.
Multivariate logistic regression analyses were conducted to identify independent
risk factors for adverse maternal and fetal outcomes in ICP patients. Gestational
age at delivery, TBA, ICP severity, ALT, AST and TBIL, which showed significant
differences with p
| Variable | OR | 95% CI | p-value |
| Gestational age at delivery | 0.942 | 0.270–3.288 | 0.925 |
| TBA | 1.660 | 1.022–2.697 | 0.041 |
| ICP severity | 7.775 | 0.074–815.700 | 0.388 |
| ALT | 1.384 | 1.002–1.912 | 0.049 |
| AST | 1.233 | 0.990–1.535 | 0.061 |
| TBIL | 1.036 | 0.789–1.360 | 0.801 |
| TCA | 1.422 | 1.021–1.979 | 0.037 |
OR, odds ratio; 95% CI, 95% confidence interval.
ICP is a common liver disorder that occurs during pregnancy and can have adverse effects on both the mother and the fetus [6]. While the prognosis for mothers with ICP is generally favorable, they often experience intense pruritus, which significantly impacts their quality of life [6]. On the other hand, the fetus is at increased risk of adverse outcomes, including stillbirth, spontaneous preterm birth, and meconium staining of the amniotic fluid [4, 5, 11]. Therefore, it is crucial to identify biomarkers that can predict adverse perinatal outcomes in ICP patients, as this can aid in fetal surveillance and treatment to prevent and reduce the occurrence of such outcomes. ICP is a liver disorder that only happens during pregnancy [12]. The changes in enzyme activity in liver cells during liver disease are reflected in the changes in enzyme activity in the serum [13]. For instance, alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase in serum are indicators of hepatocyte injury [14]. ADH has been proposed as a marker for the diagnosis of ICP in pregnant women [12]. Currently, there are a few highly specific biomarkers for ICP that are undetectable in healthy individuals, but can be detected in small amounts in the early stages of the disease. Previous studies have suggested the potential role of postprandial bile acids in predicting perinatal outcomes in ICP, with primary bile acid species, especially glycocholic acid (GA) and TCA, being prominently elevated in severe ICP patients [15]. Therefore, we hypothesized that TCA, as a crucial bile acid, might serve as a predictive factor for adverse maternal and infant outcomes. In this study, we aimed to investigate the relationship between serum TCA levels and adverse outcomes in pregnant women with ICP. Our results demonstrated that serum TCA levels had predictive value for adverse outcomes in pregnant women with ICP. However, further research is necessary to validate these findings and establish the clinical utility of TCA as a predictive biomarker.
Pruritus and a decrease in LFT are common clinical manifestations of ICP, along with elevated levels of TBA [16]. Elevated concentrations of serum bile acid have been associated with an increased risk of preterm birth and stillbirth, particularly in singleton pregnancies and ICP patients with serum bile concentrations of 100 µmol/L or higher [17]. Monitoring prenatal indexes such as AST, ALT, TBA, and TBIL levels in pregnant women with ICP is crucial for predicting perinatal prognosis [18]. Consistent with previous findings, our study revealed significant differences in gestational age at delivery, TBIL, AST, ALT, and TBA levels, and ICP severity between the normal and adverse groups. These results highlight the importance of these parameters in assessing the prognosis of ICP and predicting adverse outcomes.
TCA has been shown to have significant effects on fetoplacental arterial
pressures in a dual perfusion placental cotyledon model, suggesting its potential
role in ICP [19]. Consistent with previous studies, our results revealed elevated
serum levels of TCA in ICP patients with adverse perinatal outcomes. Severe ICP
patients have been reported to exhibit higher serum levels of TCA, TCDCA, TUDCA,
GA, and GDA normal pregnancy or mild ICP [9]. To further evaluate the predictive
value of serum TCA levels for adverse perinatal outcomes in ICP pregnant women,
we conducted ROC curves analyses, which yielded an AUC of 0.8430, with 66.22%
sensitivity and 91.03% specificity. The results indicated that
the sensitivity of serum TCA levels to predict maternal and infant adverse
outcomes in ICP pregnant women was low (
Furthermore, we classified ICP patients into low and high TCA level groups, and
observed that those with high TCA levels had significantly decreased gestational
age at delivery, elevated ALT, TBA, TBIL and AST levels, a higher proportion of
severe ICP, a greater number of adverse perinatal outcomes (including premature
delivery, postpartum hemorrhage, fetal distress, neonatal asphyxia, low birth
weight, and amniotic fluid fecal staining), and an increased
risk of developing perinatal outcomes. Consistent with our
findings, previous studies have reported higher TCA levels in pregnant women with
more severe ICP, which were associated with increased incidence of preterm birth
and reduced gestational age at delivery [21, 22].
Additionally, we identified TBA, TCA, and ALT
as independent risk factors for adverse maternal and infant outcomes in ICP
patients. Similar studies have shown that elevated ALT levels and the presence of
meconium-stained amniotic fluid, delivery before 34 weeks of gestation, and
composite adverse perinatal outcomes are more common in severe
ICP cases compared to mild cases [23]. In twin pregnancies complicated by ICP,
TBA
In summary, our study provides evidence supporting the high predictive value of serum TCA levels for adverse perinatal outcomes in pregnant women with ICP. TCA also serves as an independent risk factor for adverse outcomes in ICP patients. However, there are limitations to our research. Lipid profiling is a targeted metabolomics platform that can comprehensively analyze lipid types. Mass spectrometry can provide molecular weight information by measuring the mass-to-charge ratio (m/z) of the ionized substance. Many modern mass spectrometers can achieve mass accuracy of 0.001–0.002 m/z, which makes the lipid profile of compounds similar to m/z searches through databases or identification of ionized molecules via various commercial or open-sourced software. We did not conduct lipid profiling experiments due to limited resources and funding. In our study, serum TC, TG, and GLU were assessed using an automatic biochemical analyzer. Besides, the sample size of this study is relatively small, and we will further expand the sample size for in-depth research in the future.
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Guarantor of integrity of the entire study, study concepts, study design: YC, HL, HG; definition of intellectual content, literature research, clinical studies, experimental studies: YC, HL; data acquisition, data analysis: HG; statistical analysis: YC, JZ; manuscript preparation, manuscript editing, manuscript review: YC, JZ. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The present study was reviewed and approved by the Academic Ethics Committee of the Third Affiliated Hospital of Zhengzhou University, and conducted in accordance with the principles outlined in the Declaration of Helsinki (approval number: 2023-168-01). Informed consent was obtained from all participants after providing a detailed explanation of the study objectives and procedures.
Not applicable.
This work was partially supported by grants from the Joint construction project of Henan Province Medical Science and Technology Public Relations Plan (Grant Number: LHGJ20190359).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
