- Academic Editor
Background: This study aims to investigate the factors
affecting the recurrence in women of childbearing age after transcervical
resection of polyps (TCRP) and to construct a nomogram model predicting this
recurrence. Methods: We selected 190 patients with Endometrial
polyps (EP) who underwent surgical treatment in our hospital between December
2017 and December 2018. Multivariate logistic regression analysis was used to
analyze the factors affecting the recurrence of TCRP in women of childbearing
age, and the calibration curve. The receiver operating characteristic (ROC) was
used to assess the efficacy of the nomogram model for predicting recurrence in
women of childbearing age; Kaplan Meier curve analysis of recurrence rates among
patients with different factors. Results: Body mass index (odds
ratio (OR) = 5.417, 95% confidence interval (CI) = 1.344–21.834), polyp
diameter (OR = 3.595, 95% CI = 1.27–10.703), gravidity (OR = 3.647, 95% CI =
1.224–10.869), and polycystic ovary syndrome (OR = 3.625, 95% CI =
1.169–11.244) are independent risk factors for recurrence after TCRP in women of
childbearing age (p
Endometrial Polyps (EP) are gynecological diseases characterized by abnormal uterine bleeding, representing local overgrowth of stroma and endometrial glands. The prevalence of EP in different populations is 7.8%–34.9%, primarily diagnosed in women of childbearing age. While most are benign lesions, there’s a potential for malignancy. In the postmenopausal population, 4–6% of polyps are due to precancerous or malignant changes, and 1–2% of premenopausal patients are due to precancerous or malignant changes [1, 2, 3]. With the continuous improvement and development of hysteroscopy in the gynecological field, it has not only become the gold standard for diagnosing EP but is also widely recommended for the surgical treatment of symptomatic patients or those presenting with larger volumes, such as Transcervical Resection of Polyps (TCRP) under hysteroscopy [4, 5]. However, given the current uncertainty around the causes of EP onset and its potential for malignancy, there’s a notable risk of recurrence post-surgery. In recent years, the postoperative recurrence rate of endometrial polyps has been 2.5%–43.6%, depending on the follow-up time and the nature of the polyp. The risk of recurrence after surgery for non atypical proliferative polyps is higher than that for benign polyps (43.6% vs 8.3%, respectively), which affects the surgical outcome [6, 7]. Therefore, it is crucial to determine the risk factors post-TCRP to pinpoint and proactively intervene with high-risk individuals. To date, there is no existing research on personalized prediction of recurrence in women of reproductive age following TCRP. Nomograms, which are prediction model charts based on evidence-based medicine, can individually calculate the postoperative recurrence rate of a disease, offering high practicality [8], for example, application in endometriosis, cervical cancer, endometrial cancer, etc. [9, 10, 11]. In light of this, our study analyzes factors influencing the recurrence of TCRP in women of reproductive age, and constructs a nomogram to predict the recurrence after TCRP, aiming to assist in the early prevention and treatment of EP recurrence.
A total of 190 EP patients, aged between 20–40 years with an average age of
(30.29
Patient age, BMI, hypertension, diabetes, lipid metabolism disorder, polyp
location (anterior wall, posterior wall, or side wall), number of polyps (single
or multiple), polyp diameter (
All patients completed preoperative examinations, and TCRP was performed 5–7 days after the end of menstruation. An Olympus electrosurgical endoscope (model: A2031A, Guangzhou Chuangyi Medical Technology Co., Ltd., Guangzhou, Guangdong, China) was used. Intraoperative parameters: distension fluid was 0.9% saline (flow rate: 120 mL/min), distension pressure was 90–110 mmHg, coagulation and cutting power were 40–50 W and 70–90 W respectively. The lithotomy position was adopted, utilizing either epidural or general anesthesia. Hysteroscope was slowly inserted, the location, number, and diameter of the polyps were observed, and they were cut from the posterior wall of the uterine fundus gradually downward, checking visually for any residuals. After surgery, all patients took oral drospirenone and ethinyl estradiol tablets (trade name: Yasmin, National Medicine Permission No. J20171071, Jenapharm GmbH & Co. KG, Jena, Germany, specification: 21 tablets/box), 1 tablet/d, taken continuously for 21 days, then stopped for 7 days before starting the next course, in the same manner, for a total of 3 courses.
All patients underwent a 2-year follow-up via telephone or outpatient services every 3 months, ending on December 31, 2020, for the modeling group and October 31, 2021, for the validation group. If there were signs of recurrence such as menstrual irregularity and abnormal bleeding, the patient was advised to return to the clinic for a check-up. A diagnosis of recurrence was made if ultrasound and hysteroscopy indicated EP. Seven patients in the modeling group were lost to follow-up, a loss rate of 3.68% (7/190), leaving a total of 183 cases included; there were no lost patients in the validation group. Based on the results of the final follow-up, both groups categorized EP patients into either recurrence or non-recurrence groups.
SPSS 22.0 (IBM Corp., Chicago, IL, USA) and R3.6.3 software (R Development Core
Team, Auckland, New Zealand) were used to process data and generate graphs. Count
data were expressed as (n (%)). The recurrence and non-recurrence groups were
compared using chi-square tests
In the modeling group of 183 EP patients, the 2-year postoperative recurrence
rate was 10.93% (20/183). The measured data of age, BMI, polyp diameter,
gravidity, and parity were converted into binary variables using the median as
the boundary. The results showed that the recurrence of childbearing women after
TCRP was not related to hypertension, diabetes, lipid metabolism disorder, polyp
location, parity, uterine fibroids, and endometriosis (p
| Clinical data | Recurrent group (n = 20) | nonrecurrent group (n = 163) | p | ||
| Age (year) | |||||
| 4 (20.00) | 75 (46.01) | 4.913 | 0.027 | ||
| 16 (80.00) | 88 (53.99) | ||||
| BMI (kg/m |
|||||
| 3 (15.00) | 82 (50.31) | 8.928 | 0.003 | ||
| 17 (85.00) | 81 (49.69) | ||||
| Hypertension | 5 (25.00) | 24 (14.72) | 0.745 | 0.388 | |
| Diabetes | 3 (15.00) | 17 (10.43) | 0.057 | 0.811 | |
| Abnormal lipids metabolism | 6 (30.00) | 30 (18.40) | 0.871 | 0.351 | |
| Polyp site | |||||
| Anterior wall | 5 (25.00) | 44 (26.99) | 0.195 | 0.907 | |
| Posterior wall | 9 (45.00) | 65 (39.88) | |||
| Side wall | 6 (30.00) | 54 (33.13) | |||
| Number of polyps | |||||
| Single | 8 (40.00) | 104 (63.80) | 4.251 | 0.039 | |
| Multiple | 12 (60.00) | 59 (36.20) | |||
| Polyp diameter (cm) | |||||
| 7 (35.00) | 122 (74.85) | 13.598 | 0.000 | ||
| 13 (65.00) | 41 (25.15) | ||||
| Gravidity (order) | |||||
| 8 (40.00) | 121 (74.23) | 10.036 | 0.002 | ||
| 12 (60.00) | 42 (25.77) | ||||
| Parity (order) | |||||
| 8 (40.00) | 101 (61.96) | 3.568 | 0.059 | ||
| 12 (60.00) | 62 (38.04) | ||||
| Fibroid | 4 (20.00) | 19 (11.66) | 0.497 | 0.481 | |
| Endometriosis | 6 (30.00) | 29 (17.79) | 1.018 | 0.313 | |
| Polycystic ovary syndrome | 5 (25.00) | 9 (5.52) | 7.008 | 0.008 | |
BMI, body mass index.
Whether or not there was a recurrence after TCRP in childbearing women was taken
as the dependent variable (no = 0, yes = 1), and the statistically significant
indicators in Table 1: age (
| Variable | SE | wald | p | OR | 95% CI | |
| Age | 0.487 | 0.573 | 0.722 | 0.395 | 1.628 | 0.529~5.005 |
| BMI | 1.690 | 0.711 | 5.643 | 0.018 | 5.417 | 1.344~21.834 |
| Number of polyps | –0.242 | 0.574 | 0.177 | 0.674 | 0.785 | 0.255~2.418 |
| Polyp diameter | 1.279 | 0.557 | 5.282 | 0.022 | 3.595 | 1.207~10.703 |
| Gravidity | 1.294 | 0.557 | 5.394 | 0.020 | 3.647 | 1.224~10.869 |
| Polycystic ovary syndrome | 1.288 | 0.578 | 4.974 | 0.026 | 3.625 | 1.169~11.244 |
TCRP, transcervical resection of polyps; OR, odds ratio; CI, confidence interval; SE, Standard Error.
The independent risk factors (BMI, polyp diameter, gravidity, polycystic ovary
syndrome) determined by multivariate logistic analysis in the modeling group were
introduced into R software to construct a nomogram model for predicting the
recurrence of childbearing women after TCRP. The results showed that BMI
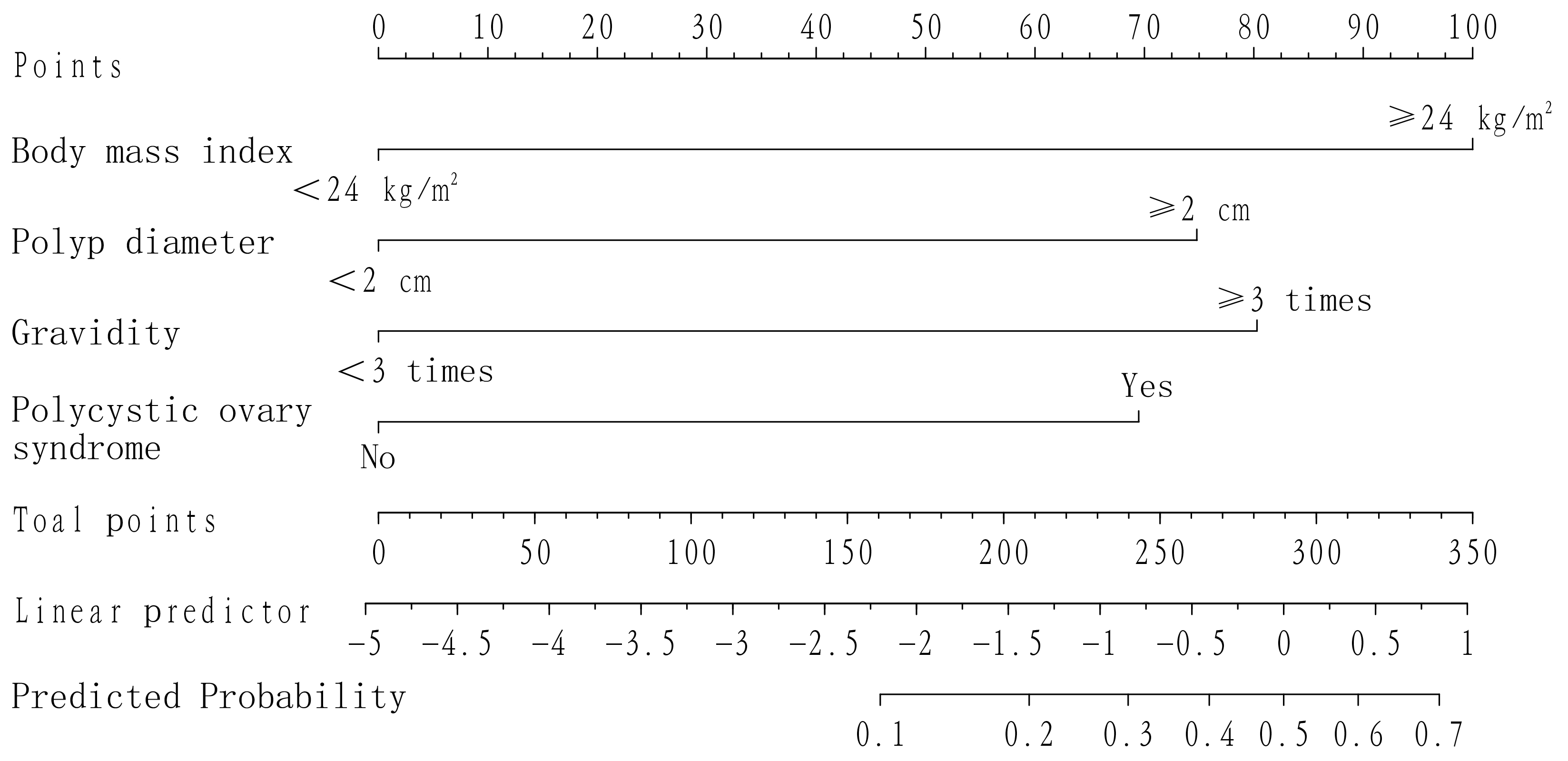 Fig. 1.
Fig. 1.Line graph model for predicting recurrence after TCRP in women of childbearing age.
Fig. 2A shows that the slope of the calibration curve of the nomogram model
predicting the recurrence of childbearing women after TCRP is close to 1; Fig. 2B
shows that the area under the ROC curve of the nomogram model predicting the
recurrence of childbearing women after TCRP is 0.781 (95% CI =
0.669–0.894); and the Hosmer-Lemeshow goodness of fit test
 Fig. 2.
Fig. 2.Internal validation of the nomogram model for predicting the recurrence of childbearing women after TCRP. (A) Calibration curve for predicting recurrence using the column chart model. (B) ROC curve: receiver operating characteristic of the nomogram model for predicting recurrence.
Among the 150 EP patients in the validation group, the 2-year postoperative
recurrence rate was 11.33% (17/150). The recurrence of childbearing women after
TCRP was related to BMI, polyp diameter, gravidity, and polycystic ovary syndrome
(p
| Clinical data | Recurrent patients (n = 17) | Nonrecurrent patients (n = 133) | p | ||
| BMI (kg/m |
|||||
| 2 (11.76) | 65 (48.87) | 8.398 | 0.004 | ||
| 15 (88.24) | 68 (51.13) | ||||
| Polyp diameter (cm) | |||||
| 5 (29.41) | 87 (65.41) | 8.238 | 0.004 | ||
| 12 (70.59) | 46 (34.59) | ||||
| Gravidity | |||||
| 7 (41.18) | 98 (73.68) | 7.585 | 0.006 | ||
| 10 (58.82) | 35 (26.32) | ||||
| Polycystic ovary syndrome | 4 (23.53) | 7 (5.26) | 4.957 | 0.026 | |
 Fig. 3.
Fig. 3.External validation of the nomogram model for predicting the recurrence in childbearing women after TCRP. (A) Calibration curve for predicting recurrence using column chart model. (B) ROC curve: receiver operating characteristic of nomogram model for predicting recurrence.
The Kaplan-Meier curve analysis found that the recurrence rate of patients with
a BMI
 Fig. 4.
Fig. 4.Kaplan Meier curves for recurrence rates in different body mass indices, polyp diameters, gravidities, and polycystic ovary syndrome subgroups.
TCRP is the preferred treatment method for EP patients, with the advantages of accurate positioning and minimal trauma [13]. At the same time, it was found that the location and morphology of the polyps under direct hysteroscopy can be clearly displayed, the polyp base in the endometrial basal layer can be accurately removed, and the surrounding endometrial tissue is not damaged [14]. Although TCRP has obvious advantages, due to the strong regenerative ability of the endometrium, surgical treatment cannot fundamentally change the intrauterine environment. Therefore, there is a risk of polyp recurrence after surgery [15]. This study followed up women of childbearing age for two years after TCRP and found a recurrence rate of 10.93%, indicating a high risk of polyp recurrence in women of childbearing age after TCRP. Therefore, exploring the risk factors for recurrence in women of childbearing age after TCRP has important significance for improving the effect of TCRP and avoiding or reducing polyp recurrence.
In this study, factors such as age, BMI, polyp location, number of polyps, polyp diameter, gravidity, parity, uterine fibroids, endometriosis, and polycystic ovary syndrome were selected to analyze the relationship with the recurrence of women of childbearing age after TCRP. The results showed that BMI, polyp diameter, gravidity, and polycystic ovary syndrome are independent risk factors for recurrence in women of childbearing age after TCRP. However, this result cannot calculate the polyp recurrence rate of women of childbearing age after TCRP in a personalized way, and its practicality is relatively poor. The nomogram has the ability to predict individual clinical outcomes and has been applied to the recurrence of cervical dysplasia [8], recurrence after ischemic stroke [16], and recurrence after radiofrequency ablation in patients with non-valvular atrial fibrillation [17], with good results. Therefore, this study further introduced BMI, polyp diameter, gravidity, and polycystic ovary syndrome into R software to construct a nomogram for predicting the recurrence of polyps in women of childbearing age after TCRP, providing a relatively clear basis for personalized prevention and treatment of polyp recurrence.
The model developed in this study to predict the recurrence of polyps in women
of childbearing age after TCRP shows that the nomogram score for those with a BMI
In summary, the nomogram model constructed in this study based on BMI, polyp diameter, number of pregnancies, and polycystic ovary syndrome has good predictive power. It is beneficial for the early identification of high-risk groups for recurrence after TCRP in women of childbearing age. Targeted follow-up can be conducted on patients to be able to timely administer medication for treatment, thus preventing and reducing the risk of polyp recurrence. However, all the analysis variables in this study have only two values, and the contribution of each variable to polyp recurrence cannot be effectively evaluated. Moreover, patients who start taking oral contraceptives after hysteroscopic polypectomy may have a lower recurrence rate than expected, and the results may be limited because only symptomatic patients who underwent ultrasound examinations were included, potentially missing asymptomatic recurrences. In the next phase of research, it’s suggested to design experiments using larger sample sizes to obtain more accurate results.
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
XC and JL designed the research study. JL, HL, and NL collected the data. XC and NL analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This retrospective study involving human participants adhered to the ethical standards of the institutional research committee and the 1964 Helsinki Declaration, including its later amendments or comparable ethical standards. The study received approval from Meizhou People’s Hospital ethics review board (approval number: 2022-C-126). All subjects provided their informed consent for inclusion before participating in the study.
We thank all of our colleagues working in the Department of Gynecology, Meizhou people’s Hospital.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
