- Academic Editor
Background: To assess the variations in protein C (PC) activity
throughout pregnancy and investigate potential correlations between plasma PC
activities and adverse pregnancy outcomes. Methods: A retrospective
cohort study was conducted among 1511 women who underwent PC activity testing at
a hospital in China from June 2011 to August 2021. t-test, Kruskal
Wallis, Fisher’s exact test, logistic regression and receiver operator
characteristic (ROC) analysis were used for analysis of data. Results:
The PC activity demonstrated a significant increase during the second trimester
of pregnancy. The PC activity was found to be lower in pregnant women with a
history of thrombosis (median, 95.70% [interquartile range (IQR),
85.50–114.60%]) as compared to those without (median, 109.00% [IQR,
95.00–124.60%], p = 0.008) or with current thrombosis (median,
101.10% [IQR, 85.30–117.00%]) compared to those without such events (median,
109.00% [IQR, 95.00–124.78%], p = 0.013). History of thrombosis was
the independent risk factor of current thrombosis during pregnancy (odd ratio
(OR) 260.57; 95% confidence interval (95% CI), 76.751–884.689; p
Protein C (PC) is a vitamin K-dependent serine protease zymogen primarily synthesized in the liver and circulating in plasma. Its activation requires calcium ions and phospholipids, which occurs through thrombin or thrombin-thrombomodulin complex. The PC pathway plays a crucial role in regulating hemostasis by hydrolyzing and inactivating activated coagulation factor VIII and coagulation factor V, inhibiting the activation of coagulation factor X and prothrombin. Insufficient levels of PC can disrupt the balance between coagulation and anticoagulant systems, leading to a hypercoagulable state that increases the risk of thrombosis. Studies have shown that decreased PC activity is associated with venous thrombosis, arterial thrombosis, pulmonary embolism, as well as other thrombotic diseases [1, 2].
Due to changes in coagulation factors during pregnancy, a hypercoagulable state is present. In addition to activated protein C (APC) resistance in the second and third trimesters of pregnancy, there is an increased risk for thrombosis [3, 4]. Pregnancy requires an effective uteroplacental vascular system, and decidual vascular thrombosis may lead to fetal growth restriction (FGR), fetal death, and recurrent abortion. PC deficiency has been reported as being associated with early pregnancy losses in women [5], however researchers have also reported that there is no significant difference in the rate of PC deficiency between women with recurrent abortion and those who do not [6]. Due to the low incidence of PC deficiency, most existing literature consisted of small-scale studies. Furthermore, PC activity changes after pregnancy, resulting in a lack of unified criteria for diagnosis. We reviewed PC testing data from our hospital over the past 10 years and conducted a retrospective review to evaluate the alterations of plasma PC activity during pregnancy, as well as investigate potential associations between these activities and adverse pregnancy outcomes.
A retrospective cohort study was conducted at the Shandong Provincial Hospital affiliated with Shandong First Medical University, covering the period from June 2011 to August 2021. Women in the Obstetrics and Reproductive Department of our hospital who had a history of abortion or adverse pregnancy outcomes, as well as those with a history of thrombosis or other relevant conditions, underwent PC activity testing. A total of 1511 women with complete pregnancy data were included in the study. Exclusion criteria encompassed cases lost to follow-up or with incomplete data as well as those experiencing pregnancy loss attributed to other factors including cervical incompetence, genital malformation, severe endocrine disorders, cardiovascular diseases, chorioamnionitis, and fetal abnormalities. Diseases causing abnormal vaginal bleeding such as placenta previa, cervicitis and neoplastic lesions were also excluded.
Adverse pregnancy outcomes encompassed the following: threatened abortion, spontaneous abortion (termination of pregnancy prior to 28 weeks gestation), fetal death (FD), hypertensive disorders complicating pregnancy (HDCP), FGR, oligohydramnios, or placental abruption. The first trimester was defined as 5–13 weeks gestation, the second trimester as 14–27 weeks gestation, and the third trimester as after 28 weeks gestation. Criteria for diagnosing threatened abortion included: (1) occurrence in early or mid-pregnancy; (2) presence of slight vaginal bleeding accompanied by lower abdominal pain; and (3) closed uterine orifice, intact fetal membranes, and appropriate size of the uterus relative to gestational age [7]. Recurrent pregnancy loss (RPL) was defined as two or more failed pregnancies confirmed by ultrasonography or histopathologic examination [8]. The HDCP criteria encompassed gestational hypertension, chronic hypertension with superimposed preeclampsia, preeclampsia, and eclampsia. Pregnant women with chronic hypertension without preeclampsia were excluded from the study. The diagnostic criteria followed American College of Obstetricians and Gynecologists (ACOG) guidelines for both FGR and hypertensive disorders in pregnancy [9, 10]. Placental abruption was clinically assessed based on antepartum uterine tenderness and vaginal bleeding, which was later confirmed by placental inspection following delivery [11, 12]. Oligohydramnios was characterized by an amniotic fluid volume less than 5% for gestational age, an amniotic fluid index (AFI) of less than 5 cm, or a maximal deepest pocket of less than 2 cm [13]. The diagnostic criteria for fetal death included a gestational age of at least 28 weeks and the absence of fetal heart activity and movement following ultrasound diagnosis with subsequent skull collapse. Diagnostic criteria for antiphospholipid syndrome (APS) are based on the Sydney criteria [14].
During the medical treatment of the patients, physicians diagnosed connective tissue diseases such as systemic lupus erythematosus, rheumatoid arthritis, dermatomyositis, and Sjogren’s syndrome. The PC activity was determined using the chromogenic substrate method (HemosIL Protein C, Instrumentation laboratory Co., Bedford, MA, USA), with a normal value range of 70% to 140%.
Statistical analysis was conducted using IBM SPSS version 22.0 (IBM Corp., Armonk, NY,
USA). Categorical variables were analyzed by frequency distribution, while
quantitative continuous variables were expressed as median and interquartile
range (IQR) or mean
Among the 1511 patients, 1335 were tested during pregnancy and 176 before pregnancy. The Clinical characteristics of the pregnant participants are presented in Table 1. Women who were tested before pregnancy had higher rates of recurrent miscarriage, hypothyroidism, or APS. Because many of them were lost to follow-up during subsequent pregnancies, data during pregnancy were not collected. Complications observed among 1335 pregnant women included threatened abortion (9.36%), abortion (7.49%), FD (1.05%), HDCP (26.67%), FGR (7.87%), placental abruption (1.35%), oligohydramnios (9.66%), thrombogenesis (2.32%).
| Clinical characteristics | Before pregnancy (n =176) | During pregnancy (n =1335) | p |
| Age (years) | 34.95 |
34.29 |
0.094 |
| RPL (n) | 69 (39.2%) | 160 (11.9%) | 0.000 |
| Hypothyroidism (n) | 18 (10.2%) | 79 (5.9%) | 0.034 |
| History of thrombosis (n) | 1 (0.5%) | 19 (1.4%) | 0.721 |
| APS (n) | 25 (14.2%) | 91 (6.8%) | 0.010 |
| Connective tissue diseases (n) | 16 (9.0%) | 77 (5.7%) | 0.094 |
| Abortion (n) | - | 100 (7.4%) | - |
| Threated abortion (n) | - | 125 (9.3%) | - |
| FD (n) | - | 14 (1.0%) | - |
| FGR (n) | - | 105 (7.8%) | - |
| HDCP (n) | - | 356 (26.6%) | - |
| Placental abruption (n) | - | 18 (1.3%) | - |
| Oligohydramnios (n) | - | 129 (9.6%) | - |
| Thrombogenesis (n) | - | 31 (2.3%) | - |
PC, protein C; RPL, recurrent pregnancy loss; APS, antiphospholipid syndrome; FD, fetal death; FGR, fetal growth restriction; HDCP, hypertensive disorder complicating pregnancy.
Among the 1511 patients, 176 were tested before pregnancy, 283 in the first trimester of pregnancy, 480 in the second trimester and 572 in the third. The activity of PC was found to be at a level of 103.45% (IQR, 93.00–116.67%) among non-pregnant women, while it was observed to be at levels of 104.20% (IQR, 92.00–119.30%), 111.85% (IQR, 97.50–127.07%) and 108.80% (IQR, 94.10–124.80%) in pregnant women during their first trimester, second trimester and third trimester, respectively. The results indicated that PC activity is significantly higher during the second trimester compared to other stages of pregnancy (Fig. 1).
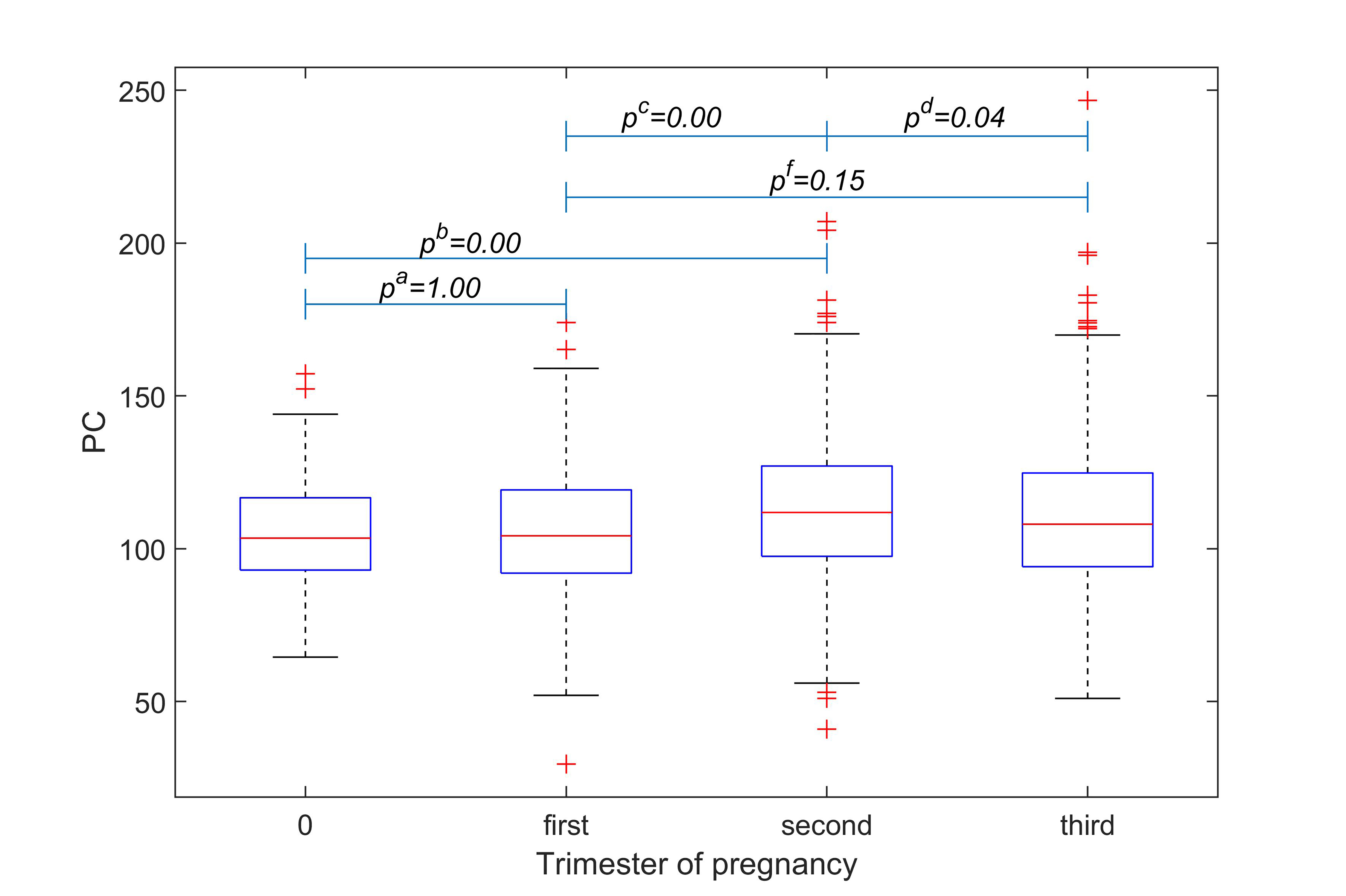 Fig. 1.
Fig. 1.Comparison of PC activity among women at
different stages of pregnancy. Statistical comparisons were
made between non-pregnancy and the first trimester (p
Among 1335 pregnant women, 19 had a history of thrombosis. The activity of PC
was significantly lower in pregnant women with a history of thrombosis compared
to those without (median, 95.70% [IQR, 85.50–114.60%] vs median,
109.00% [IQR, 95.00–124.60%], p = 0.008). Additionally, among the
cohort studied there were 31 cases of thrombosis observed and the activity of PC
was found to be significantly lower in these patients when compared to those
without thrombotic events (median, 101.10% [IQR, 85.30–117.00%]
vs median, 109.00% [IQR, 95.00–124.78%], p = 0.013)
(Fig. 2). Binary logistic regression analysis was conducted to identify factors
that may impact thrombosis, including age, gestational age, history of
thrombosis, APS, connective tissue disease, and use of anticoagulants. Only a
history of thrombosis was an independent risk factor for current thrombosis
during pregnancy (odd ratio (OR) 260.57; 95% confidence interval (95% CI),
76.751–884.689; p
 Fig. 2.
Fig. 2.Differences in PC activity were observed between pregnant women with a history of thrombosis (A) or current thrombosis (B) and those without.
Among 160 pregnant women with a history of two or more spontaneous abortions, there were 8 cases of abortion (5.0%) and 5 cases of fetal death (3.13%). The PC activity of these 13 women with fetal loss was not significantly different from that of the other 147 women with successful pregnancies (median, 120.20% [IQR, 102.50–140.50%] vs median, 111.62% [IQR, 95.70–124.90%], p = 0.287).
As shown in Table 2, among the 1335 pregnant women included in this study, 125
presented with symptoms of threatened abortion. The PC activity was found to be
significantly lower in pregnant women with threatened abortion compared to
asymptomatic women (median: 100.80% [IQR, 91.30–113.15%] vs median:
110.00% [IQR, 95.65–125.00%], p
| Adverse pregnancy outcomes | n | PC activity | p | |
| Median (IQR) | ||||
| Threated abortion | absent | 1210 | 110.00 (29.35) | 0.000 |
| present | 125 | 100.80 (21.85) | ||
| Fetal loss | absent | 1221 | 109.00 (29.00) | 0.715 |
| present | 114 | 107.90 (23.32) | ||
| FGR | absent | 1230 | 109.00 (29.53) | 0.819 |
| present | 105 | 108.00 (26.50) | ||
| HDCP | absent | 979 | 108.00 (28.00) | 0.272 |
| present | 356 | 110.00 (34.68) | ||
| Oligohydramnios | absent | 1206 | 108.50 (29.90) | 0.079 |
| present | 129 | 112.00 (29.90) | ||
| Placental abruption | absent | 1317 | 109.00 (29.15) | 0.565 |
| present | 18 | 113.85 (37.88) |
IQR, interquartile range.
| B | S.E. | Wald | p | Exp (B) | 95% CI | ||
| Low | Up | ||||||
| Gestational age | –0.040 | 0.008 | 27.219 | 0.000 | 0.960 | 0.946 | 0.975 |
| PC | –0.15 | 0.005 | 10.854 | 0.001 | 0.985 | 0.976 | 0.994 |
| Hypothyroidism | 0.635 | 0.297 | 4.568 | 0.033 | 1.888 | 1.054 | 3.381 |
B, regression coefficient; S.E., standard error; 95% CI, 95% confidence interval.
The PC activity did not show any significant differences among pregnant women with fetal loss, FGR, oligohydramnios, HDCP, placental abruption and those without in a total of 1335 pregnancies (p
In this review, it was observed that the PC activity of women in their second trimester of pregnancy was higher compared to other stages of pregnancy, which is consistent with Fu M et al.’s findings [15]. Fu M et al. [15] conducted a study involving 465 non-pregnant women and 1972 pregnant women and discovered that PC activity slightly increased from 13 to 20 weeks of gestation and remained at this level until delivery. The median PC activity levels were found to be significantly higher during the periods of 13–20, 21–27, 28–33, and 34–42 weeks of gestation when compared to the non-pregnant group. In this review, a notable decline in PC activity was observed during the third trimester of pregnancy, which distinguished it from Fu M’s findings [15]. Said observed a potentially biologically significant increase in PC activity throughout the first 22 weeks of pregnancy in 440 otherwise asymptomatic women [16]. However, Cui et al. [17] reported a declining trend in plasma levels during pregnancy among Han women from North China, including Shandong province.
Previous literature consistently suggested that PC deficiency is associated with an increased risk of thrombosis in non-pregnant women, as well as an elevated risk of pregnancy-related venous thromboembolism [18]. Venous thromboembolism occurred in 12–19% of pregnancies in women with PC deficiency [19]. A study of 74 women with pregnancy-related thrombosis found that those with a history of venous thromboembolism had a higher incidence of PC deficiency, and those with PC deficiency were at greater risk for venous thromboembolism prior to delivery [20]. In light of the low incidence rate of PC deficiency and the limited sample size in previous reports, this study compared plasma PC activity levels between pregnant women with a history of thrombosis or thrombotic pregnancy and those without, revealing a significant decrease in PC activity that is consistent with these research findings. Because PC activity varies with gestational age, we included factors such as gestational age, chronologic age, and anticoagulant use in the multivariate analysis and found that PC activity was not an independent risk factor, although the p value reached 0.07. However, women with a history of thrombosis have a significantly increased risk of thrombosis during pregnancy, and the odds ratio can be as high as 260.57. Therefore, we recommend enhanced screening for thrombosis during pregnancy in women with a past medical history of thrombosis.
The literature has presented inconsistent findings regarding the association between PC deficiency and pregnancy loss. While some studies have identified PC deficiency as a risk factor for reduced pregnancy rate in women with RPL [21, 22], others have found that the detection rate of PC deficiency is higher among women who experience multiple miscarriages within the first three months of pregnancy compared to those do not [6]. A meta-analysis did not find any significant association between PC deficiency and RPL [23]. This study found no significant difference in PC activity between women with RPL or a history of abortion and those without, and there was no evidence to support the necessity of screening for PC activity during pregnancy for women with RPL.
Although PC activity did not decrease in pregnant women with abortion, it decreased significantly in those with bleeding in threatened abortion. In addition, there was a correlation between gestational age and threatened abortion, the earlier the abnormal PC appeared, the greater the risk of threatened abortion. The available data about relationship between PC and bleeding events are scarce. PC was usually accepted to be associated only with thrombosis but not bleeding [24], while Hsu et al. [25] reported a case of recurrent gastrointestinal bleeding secondary to thrombophilia due to PC deficiency. Whether the bleeding in our study was related to thrombophilia remains unknown.
In this study, the PC activity of pregnancies with FGR, preeclampsia, oligohydramnios, and placental abruption did not differ significantly from those without. However, Okoye et al. [26] found that blood PC activity was higher in pregnant women with preeclampsia than in the control group. Dehkordi et al. [27] failed to observe a significant difference in plasma PC activity between the pre-eclampsia group and the control group. Similarly, Berks et al. [28] reported no correlation between PC deficiency and preeclampsia in a cohort of 844 pregnant women. Ebina et al. [29] detected PC activity in 1220 women with gestational ages ranging from 8–14 weeks but found no association between PC activity and adverse pregnancy outcomes such as preeclampsia, or fetal growth restriction. In some studies, investigating the correlation between thrombophilia and adverse pregnancy outcomes, most of the literature does not support a link between PC activity or lack thereof and adverse pregnancy outcomes such as pre-eclampsia and FGR [30, 31, 32].
Untreated hypothyroidism during pregnancy can lead to fetal deaths and abortions [33]. While Kaduskar et al. [34] reported abortion rate of pregnant women diagnosed with hypothyroidism during pregnancy were not different from those without. In this study, women with hypothyroidism who received thyroxine did not have an increased miscarriage rate, but they had an increased rate of threatened abortion and bleeding events (OR, 1.88). As early as 1965, Simone et al. [35] demonstrated significantly reduced levels of factor (F) VIII, FIX and FXI in hypothyroid patients. An observational cohort study showed that hypothyroidism resulted in hyperfibrinolysis and a reduced activated thrombin-activatable fibrinolysis inhibitor (TAFIa)-dependent prolongation of clot lysis [36]. It was reported that hypothyroidism leads to an increased risk of abnormal vaginal bleeding, and recently it has been suggested that low plasma levels of FT4 within the reference range also influence the risk of bleeding [37, 38]. We also found in pregnant women that hypothyroidism, although treated with thyroxine, increased the risk of vaginal bleeding.
This study had several limitations, including its retrospective nature and being a single-center cohort study conducted in China. The majority of the population consisted of Han ethnicity, with other ethnic groups such as Hui, Korean, Manchu and Mongolian accounting for only 0.73%. Additionally, the subjects included had a higher incidence of abnormal pregnancies or thrombophilia in their previous pregnancies. While the PC activity detection equipment was replaced once in 2018, the method and reference range remained consistent.
A decrease in PC activity was observed in pregnant women with a history of thrombosis, current thrombosis, and threatened abortion. History of thrombosis was the independent risk factor for current thrombosis during pregnancy. Decreased PC activity is an independent risk factor for threatened abortion. However, no significant correlation was observed between PC activity and fetal loss, HDCP, FGR, oligohydramnios or placental abruption.
The datasets used during the current study are available from the corresponding author on reasonable request.
All the authors have contributed to the document retrieval, conception and design of this study. XW, JX collected and analysis data. YW designed the work and drafted the manuscript. XZ interpretated data and edited the manuscript. SW analyzed statistics and revised the manuscript. All authors participated in the discussion of analysis and interpretation of data in this article. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol received approval from the Ethics Committee of Biomedical Research Ethic Committee of Shandong Provincial Hospital prior to initiation (NO. SWYX 2022-98). Our study was retrospective with data from the medical record system, and it was applicated for waivers of informed consent.
We wish to extend our sincere appreciation to all those who provided assistance during the composition of this manuscript.
This study was supported by the Central Government Guides Local Scientific and Technological Development Fund Projects (YDZX2022096), the National Key Research and Development Program of China (2022YFC2704600), the National Natural Science Foundation of China (81741038), as well as the Jinan Science and Technology Program (202134014).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
