- Academic Editor
Background: Group B streptococcus (GBS) is commonly recognized as an opportunistic pathogen,
which can cause infections in pregnant women and their newborns. The aim of this
study was to explore the invasiveness of GBS by comparing various indices of
pregnant mothers and newborns.
Methods: This retrospective study involved 6892 consecutive GBS screened
pregnant women, and 48 GBS-positive newborns. The data of pregnant women and
newborns was compared by Chi-square test and Kruskal-Wallis test. A
p-value
Group B streptococcus (GBS or Streptococcus agalactiae) is both a normal commensal and an opportunistic pathogen that colonizes the gastrointestinal tracts of women, men, and children of all ages, and is the source of vaginal and urethral colonization [1, 2, 3]. GBS is a predominant microbe of perinatal infections that can cause puerperium complications, vertical transmission from mothers-to-newborns at the time of delivery, and can lead to severe neonatal sepsis, pneumonia, and meningitis [4, 5]. So, administering intravenous antibiotics during labor to GBS-positive women could prevent invasive disease of their newborns. However, intrapartum overtreatment with antibiotics increases the risk of maternal and neonatal untoward effects, such as antibiotic resistance, anaphylactic shock, and intestinal complaints [6]. Especially, the mucosal immune system of newborns is closely related to the initial bacterial colonization of the intestines, while overuse of antibiotics can alter intestinal flora, then lead to childhood asthma, diabetes, allergy, obesity, and autism [7]. To reduce the incidence of GBS disease in newborns [8] and the overuse of antibiotics, universal screening for GBS at 35‒38 weeks gestation and intrapartum antibiotic prophylaxis (IAP) is the standard of care [9].
It has been demonstrated by other studies that conventional bacterial culture methods have high requirements for transportation conditions and identification level. Time-consuming culture methods are another problem in labor which gets results within 24–72 hours after sampling and gets the antibiotic susceptibility testing results after 72 hours [10]. On the other hand, the GBS gene targeting real-time polymerase chain reaction (PCR) method is more accurate, more sensitive, and faster due to the results are available within 1–2 hours after sampling [11]. Thus, we collected the PCR results of pregnant women and newborns to identify the epidemiological characteristics of GBS from our hospital.
This retrospective study was conducted involving clinical data of 946 consecutive cases from 6892 pregnant women with late antenatal screening of GBS tests from January to December 2020, and the files of GBS-positive neonates from January 2019 to December 2020. Two swabs were collected from the anus and vagina of each pregnant woman, respectively, at 35–38 weeks gestation. The swabs were collected from the nasopharynx or ears of the newborns with clinical symptoms of infection after birth from GBS-positive mothers. All information was obtained from the laboratory information system of YuHuangding Hospital (Yantai, Shandong, China).
Inclusion and exclusion criteria, the 946 consecutive cases were during the period from all 6892 pregnant women with GBS tests and delivered in our hospital later. At the same time excluded the cases with other risk factors which can cause adverse pregnancy: myoma uteri, uterine malformations, multiple pregnancies, preeclampsia, hypertension, diabetes mellitus, fetal malformations, fetal growth restriction, intra-uterine fetal demise, and other genital tract pathogen infections. And then, to devided the 946 cases into three groups, in which 500 GBS-negative cases and 446 GBS-positive cases, shown in Tables 1,2,3. The first group was the premature delivery group at
| GBS test results | Swabs collection position | Number of positive pregnant women | GBS-positive rate | |
| Vagina | Anus | |||
| Single vaginal GBS-positive | Positive | Negative | 35 | 0.51% (35/6892) |
| Single anal GBS-positive | Negative | Positive | 233 | 3.38% (233/6892) |
| Vaginal and anal double GBS-positive | Positive | Positive | 197 | 2.86% (197/6892) |
| Total positive cases | 465 | 6.75% (465/6892) | ||
Each pregnant woman collected two swabs at the same time, so a total of 13,784 swabs were collected from 6892 pregnant women. The positive rate was calculated by the number of positive women.
GBS, Group B streptococcus.
| GBS-negative | GBS-positive | Total | p-value | ||
| The percentage of all cases | 6427 (93.25%) | 465 (6.75%) | 6892 | ||
| The percentage of different age groups | |||||
| 19–30 y | 2742 |
201 |
2943 | 0.031 | |
| 31–40 y | 3540 |
262 |
3802 | ||
| 41–52 y | 145 |
2 |
147 | ||
*Each subscript letter denotes a subset of GBS group categories (such as ‘a’ and ‘b’), the column proportions of which do not differ significantly from each other at the 0.05 level. The difference between different age groups was mainly in the positive rate of 45–50 group. y, years.
| Gestational weeks | GBS-negative | GBS-positive | Total | p-value | |
| Median | 39.21 w | 39.38 w | 0.262 | ||
| 25 percentiles | 38.54 w | 38.86 w | |||
| 75 percentiles | 40.29 w | 40.29 w | |||
| Premature delivery | |||||
| Present | 10 (1.06%) | 7 (0.74%) | 17 (1.80%) | 0.619 | |
| Absent | 490 (51.80%) | 439 (46.41%) | 929 (98.20%) | ||
| Total | 500 (52.85%) | 446 (47.15%) | 946 (100%) | ||
| PROM | |||||
| Present | 73 (7.72%) | 77 (8.14%) | 150 (15.86%) | 0.263 | |
| Absent | 427 (45.14%) | 369 (39.01%) | 796 (84.14%) | ||
| Total | 500 (52.85%) | 446 (47.15%) | 946 (100%) | ||
| Chorioamnionitis | |||||
| Present | 6 (0.63%) | 7 (0.74%) | 13 (1.37%) | 0.626 | |
| Absent | 494 (52.22%) | 439 (46.41%) | 933 (98.63%) | ||
| Total | 500 (52.85%) | 446 (47.15%) | 946 (100%) | ||
PROM, Premature rupture of membranes.
 Fig. 1.
Fig. 1.Flow chart of the inclusion criteria of the pregnant women.
There were 15,967 newborns whom were born in our hospital from January 2019 to December 2020, of which 38 cases were GBS-positive. The other 10 GBS-positive cases were transferred from other hospitals after the onset of GBS infection. The inclusion criteria for the newborns are shown in Fig. 2. All data from the 38 GBS-positive cases and the 10 GBS-positive cases transferred from other hospitals were compared in Table 4. According to the clinical symptoms of GBS-positive children, they were divided into three groups: GBS-colonization group; GBS-infection group; and death group. The deaths in the mode of delivery groups were merged into the corresponding infection groups in Table 4. With respect to the GBS-colonization site in the mother group, the death group was also merged into the corresponding infection groups. The result of pairwise comparison between groups by the Kruskal Wallis H-test is shown in Table 5.
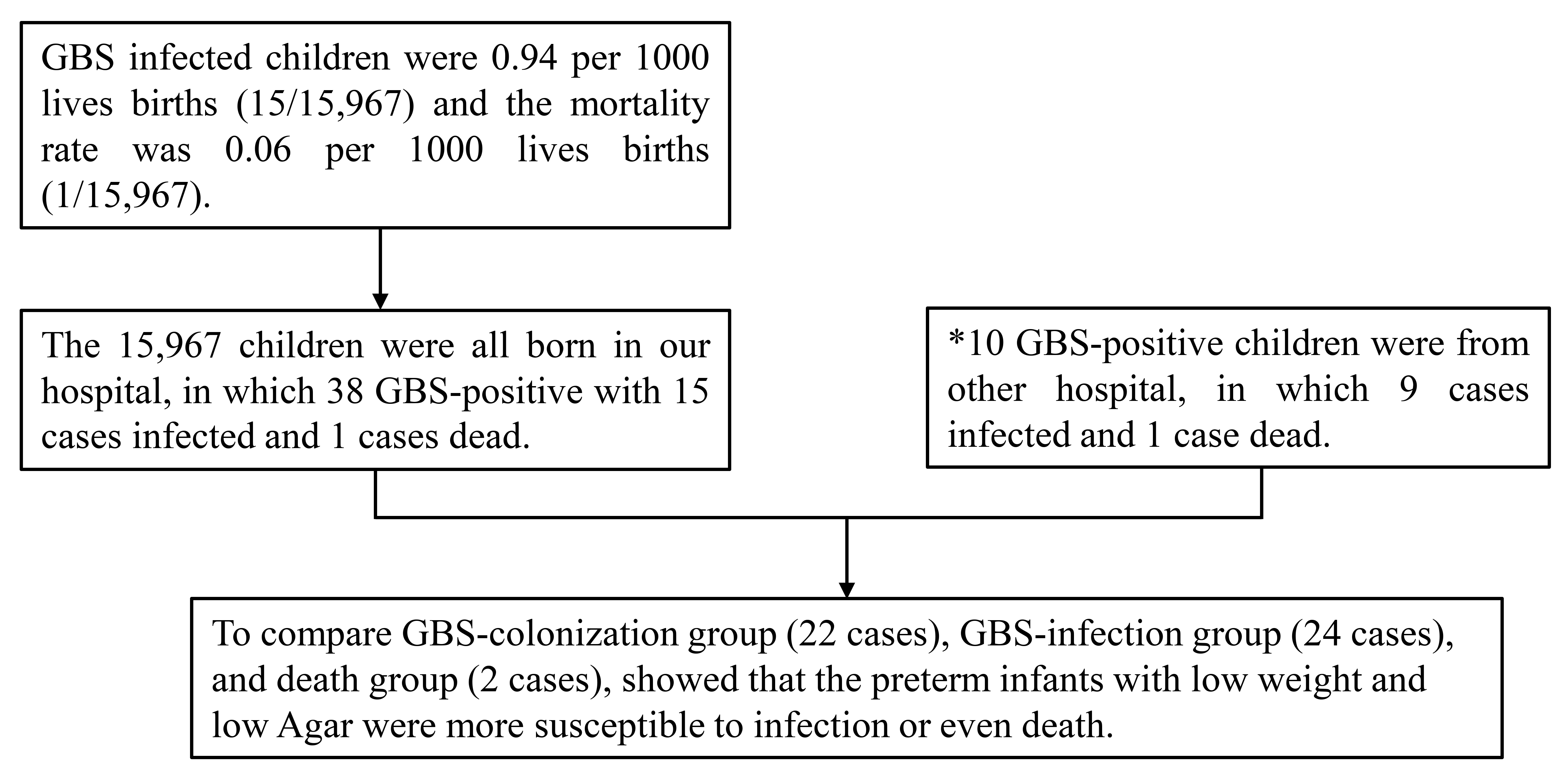 Fig. 2.
Fig. 2.Analysis of infants. *When calculating the infection rate and mortality rate of newborns, the 10 cases born in the other hospitals were not included in the 15,967 cases born in our hospital.
| GBS-colonization | GBS-infection | Death group | p-value | ||
| GBS-positive neonates | 22 | 24 | 2 | ||
| Diseases | |||||
| Pneumonia | / | 14 | |||
| Pyemia | / | 6 | 1 | ||
| Pneumonia with Pyemia | / | 2 | 1 | ||
| Pneumonia with bacteriaemia | / | 1 | |||
| Pneumonia with bacteriaemia with Meningitis | / | 1 | |||
| Onset time (cases) | |||||
| / | 19 | 2 | |||
| / | 2 | ||||
| / | 3 | ||||
| Mode of delivery | |||||
| Caesarean section | 2 (4.17%) | 10 (20.83%) | 1 (2.08%) | 0.010 | |
| Spontaneous delivery | 20 (41.67%) | 14 (29.17%) | 1 (2.08%) | ||
| Gestational weeks | 0.004 * | ||||
| Median | 39.68 w | 38.43 w | 29.50 w | ||
| 25 percentiles | 38.86 w | 34.00 w | 25.29 w | ||
| 75 percentiles | 40.86 w | 41.14 w | – | ||
| Neonatal birth weight | 0.022 * | ||||
| Median | 3.40 kg | 3.23 kg | 1.25 kg | ||
| 25 percentiles | 3.11 kg | 2.47 kg | 0.80 kg | ||
| 75 percentiles | 3.70 kg | 3.68 kg | – | ||
| Apgar scores at 1 min | 0.000 * | ||||
| Median | 10.0 | 10.0 | 5.50 | ||
| 25 percentiles | 10.00 | 9.00 | 3.00 | ||
| 50 percentiles | 10.00 | 10.00 | – | ||
| GBS-colonization site of mother | |||||
| Negative | 1 (2.08%) | 2 (4.17%) | 0.002 | ||
| Rectovaginal positive | 18 (37.50%) | 9 (18.75%) | 1 (2.08%) | ||
| Rectal positive | 1 (2.08%) | 1 (2.08%) | |||
| Without GBS tested mother | 2 (4.17%) | 12 (25.00%) | 1 (2.08%) | ||
*The results were compared by the Kruskal Wallis H-test and shown in Table 5.
| Medians (p-value) | |||
| GBS-colonization–GBS-infection | GBS-infection-Death group | GBS-colonization-Death group | |
| Gestational weeks | 39.68–38.43 w (0.036*) | 38.43–29.50 w (0.312) | 39.68–29.50 w (0.035*) |
| Neonatal birth weight | 3.40–3.23 kg (0.526) | 3.23–1.25 kg (0.100) | 3.40–1.25 kg (0.029*) |
| Apgar scores at 1 min | 10.0–10.0 (0.002*) | 10.0–5.50 (0.033*) | 10.0–5.50 (0.000*) |
*p
The GBS-specific CAMP gene was detected by polymerase chain amplification and
fluorescent labeling of the GBS reagent (Taipu Biological Co. LTD, Fuzhou,
Fujian, China), and the results were analyzed
using an ABI 7500 Real-time PCR System
(Thermo Fisher Scientific Inc., Waltham, MA, USA). After sampling, 1 mL of cleaning
solution was added to elute the cotton swabs and shaken for 2 min to make the
suspension. All suspensions were put into 1.5 mL centrifuge tubes and the
supernatants were discarded after centrifuging at 13,000 rpm for 5 min. The above
process was repeated twice, then 50 µL of cleaning solution was added to
make a suspension. To draw 50 µL of the suspension washed from the sample
swabs, a 50 µL positive control and 50 µL negative control was
dispensed into 1.5 mL centrifuge tubes. Ten microliters of internal reference
were added to each tube, then dry-bathed at 95 °C for 2 min and ice-bathed at –20
°C for 2–5 min. After centrifugation at 13,000 rpm for 1 min, 5 µL of the
supernatant was used for PCR amplification with 44.3 µL of PCR reaction
solution, 0.5 µL of Taq DNA polymerase, and 0.2 µL of uracil
N-glycosylase (UNG). The PCR reaction solution contained buffer, MgCl
Data are presented as a percentage, median and interquartile range for different
variables. A Chi-square test was utilized to assess the categorical data and the
Kruskal-Wallis test was used for measurement data with SPSS 24.0 (IBM Corp.,
Armonk, NY, USA). p-value
Two swabs were collected from the anus and vagina of each pregnant woman. So, the results with three situations were recorded (vaginal GBS-positive, anal GBS-positive, the vaginal and anal double GBS-positive; Table 1). The total GBS-positive rate in pregnant women was 6.75% (465/6892; Table 1), the vaginal GBS-positive rate was 0.51% (35/6892), the anal GBS-positive rate was 3.38% (233/6892), and the vaginal and anal double GBS-positive rate was 2.86% (197/6892). The GBS-positive rate was different among the age group and the GBS-positive rate was 6.83% (201/2943) in the 19–30 years (y) group, 6.89% in the (262/3802) the 31–40 y group, 1.36% (2/147) in the 41–52 y group, (p = 0.031), in Table 2.
In Table 3, there were not any differences in the three common complications among the two groups. In the GBS- negative and -positive groups, the incidence of prematurity, PROM, and chorioamnionitis were 1.06% and 0.74%, 7.72% and 8.14%, 0.63% and 0.74%, respectively. The corresponding p-values were 0.619, 0.263, and 0.626. Accordingly, there were no differences in the gestational weeks group (p = 0.262).
In the 15,967 newborns group, the neonatal general GBS-positive rate was 0.24%
(38/15,967). A total of 48 GBS-positive newborns were identified, among which 38
were born in our hospital. There was a 0.24% (38/15,967) GBS-positive rate among
15,967 births, the infection rate was 0.94 per 1000 live births (15/15,967), and
the mortality rate was 0.06 per 1000 live births (1/15,967) (in Fig. 2).
Analysis of the data revealed significant differences in delivery mode,
gestational age, neonatal birth weight, and Apgar scores among the
GBS-colonization, GBS-infection, and death groups (p = 0.010, 0.004,
0.022, and 0.000
In the neonatal GBS-infection group, 19 neonates had symptoms of infection
within 24 hours after birth. In the
The mothers of 58.33% (28/48) GBS-positive neonates were mostly GBS rectovaginal double-positive. The mothers of 31.25% (15/48) GBS-positive neonates did not undergo GBS testing, in which 25% (12/48) had infections and 2.08% (1/48) died. Three (6.25%, 3/48) GBS-positive neonates had GBS-negative mothers. There were statistical difference between groups (p = 0.002); however, the neonatal infection rate of mothers who did not undergo GBS screening was higher than the other groups (Table 4).
The GBS-colonization rate of pregnant women in our study was 6.75%, which was close to the average level of the GBS-positive rate in mainland China (3.7–14.52%) [8]. The GBS-positive rates in the vagina, anus, and rectovaginal were 0.51% (35/6892), 3.38% (233/6892), and 2.86% (197/6892), respectively; thus, the GBS-positive rate in the anus was higher than the vagina because the GBS primary reservoir is the gastrointestinal tract [3]. The positive rate of GBS was different among the different age groups. It showed that the prevalence of GBS in younger women was higher than in older women. These results are similar to previous studies [12, 13, 14].
Between GBS-positive group and GBS-negative group, there was no difference in gestational weeks, PROM, preterm delivery, and chorioamnionitis groups. Contrary to previous studies [11, 15, 16], in our current research there was no significant difference between the two groups regarding adverse pregnancy outcomes, which is consistent with the findings by Goel et al. [13] and Ngonzi et al. [17]. Accordingly, in the Tano et al. [18] study, there was no pathologic evidence to support a connection between GBS-infection and chorioamnionitis, which is consistent with our study.
We excluded all cases with diabetes, hypertension, and preeclampsia, which may cause premature labor or PROM [19, 20, 21]. The cases with Ureaplasma urealyticum, Chlamydia trachomatis, Candida, Neisseria gonorrhoeae, and Gardnerella vaginalis, which have a pathological role in PROM and chorioamnionitis [12, 22] were also eliminated. Rocchetti et al. [12] confirmed that candidiasis and cytolytic vaginosis also increase GBS-colonization. Therefore, the seco-infection cases were deleted because it was difficult to determine the actual pathogenic factor leading to the maternal infection.
Additionally, either diabetes or hypertension are risk factors for premature labor and PROM [23], and also increase the likelihood of GBS-colonization [10, 15]. These confounding factors not only increase the rate of GBS-colonization, but also induce adverse pregnancy outcomes. Therefore, it is necessary to exclude the confounding factors and to compare a single factor for GBS to confirm the role of GBS in adverse pregnancy outcomes. Moreover, these confounding factors may be the reason for the differences in results between studies.
Most of the GBS-infected neonates had pneumonia, followed by pyemia, bacteriaemia, meningitis, and even death. The emergence of symptoms of infection and death were mostly concentrated within the first 24 hours. There were significant differences in the three indices (gestational age, neonatal birth weight, and Apgar scores), reflecting the health status of newborns among the three groups. Infants with poor basic conditions are concentrated in the infection and death groups.
In the weight group, there was only a difference between the colonization and death groups. The two dead cases in our study were premature babies with low weight, and similar cases were also reported by Todorova-Christova et al. [24], that low weight at birth or prematurity was confirmed as a substantial risk factor of GBS-infection. In the Apgar score group, there were differences among all three groups. Therefore, we believe that the occurrence and progression of GBS-infections were related to the basic physical health status of newborns, which is consistent with Mousavi et al. [10], which reported that GBS can give rise to life-threatening infections in some vulnerable hosts, especially infants with chronic diseases.
A difference also existed in the mode of delivery so that the colonization and GBS-infection rates in newborns that were delivered spontaneously were higher than newborns delivered by cesarean section, which is consistent with the results of Verani et al. [3] and Joachim et al. [25]
In the GBS-colonization site group, most of the rectovaginal-positive mothers caused vertical transmission (37.5%, 18.75%, and 2.08%). Three GBS-positive newborns were born from GBS-negative mothers, which confirmed the intermittent and transient nature of GBS-colonization [11, 26]. Of the 48 GBS-positive newborns, the mothers of 15 did not have GBS screening or had false-negative results, thus accounting for 57.7% (15/26) of infected children and the infection rate was higher than the newborns from the maternal screening group, which confirmed that prenatal screening of GBS is beneficial to reduce GBS-infections in neonates.
Among the 30 mothers who had GBS screening, there were 10 newborn infections, 1 newborn dead, and 19 newborns colonized. 1 dead newborn was a premature infant at 33 weeks + 5 days gestation with a birth weight of 1700 g, and an Apgar score of 8 at 1 min. Although the mother received IAP, the prophylactic antibiotics failed to effectively prevent fetal infection and death. The mother of another dead newborn was not screened for GBS; the newborn was born at 25 weeks + 2 days gestational age with a birth weight of 800 g and Apgar score of 3 at 1 min. The two deceased children had poor basic conditions and were difficult to survive in the case of combined GBS-infection. All of the above data are consistent with most studies that concluded that premature infants in poor health with GBS-infections usually have a poor prognosis [10, 24].
In our study we found that GBS may not be a single-factor pathogenic microorganism. After excluding other related factors, the pathogenicity of GBS in pregnant women was not as significant as described in some other studies [10, 18]. The colonization rate and invasiveness of GBS were increased when one or more pathogenic factors or clinical complications exist at the same time [12, 15]. In fact, GBS is more likely to attack premature newborns with poor health status, and poise life-threat to vulnerable individuals.
To reduce the pathogenic effects caused by GBS-infections, IAP has significantly altered the adverse outcomes of neonatal infection. Moreover, to reduce maternal adverse pregnancy related factors and improving the basic conditions of newborns can also prevent GBS-infection of newborns.
All data generated or analyzed during this study are included in this published article.
HY–perception and design, drafting of the article. SMZ–data collection and analysis. Both authors contributed to editorial changes in the manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. Both authors read and approved the final manuscript.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Yantai Yuhuangding Hospital (approval number: 2023-268). The manuscript was a retrospective case review, and consent to participate not applicable.
We thank Ms Huo Ran of Ludong University and International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
