- Academic Editor
†These authors contributed equally.
Objective: Hemodynamic monitoring plays a crucial way in guiding the clinical decision-making process for the management of critically ill neonates. Noninvasive hemodynamic monitoring is characterized by continuous, convenient, and accurate assessment, presenting a viable option for implementation in neonatal intensive care units (NICU). This review article summarizes the research advancements made in noninvasive hemodynamic monitoring and electronic cardiometry (EC) applications in neonates, providing valuable reference resource for studies in the field of hemodynamic monitoring. Mechanism: The clinical significance of hemodynamic monitoring in neonates is first introduced and followed by a comprehensive description of both invasive and noninvasive techniques employed in hemodynamic monitoring. Furthermore, the research progress of EC in neonates is discussed, focusing particularly on its feasibility and accuracy. Finally, the application and influencing factors of EC in diverse diseases, encompassing neonatal conditions, are presented. Findings in Brief: Due to the risks associated with invasive cardiac output monitoring, noninvasive or minimally invasive alternative techniques are needed for hemodynamic monitoring. In recent years, noninvasive and minimally invasive techniques, such as ultrasound cardiac output monitor (USCOM) and impedance cardiography have been utilized. EC, as an impedance-based monitoring, facilitates noninvasive and real-time assessment of hemodynamic parameters. The integration of EC enables real-time and continuous monitoring of dynamic changes in cardiac and vascular functions in patients, thereby assisting in clinical evaluation and guiding the clinical decision-making. Conclusion: EC is a noninvasive, highly sensitive, and accurate monitoring technique that holds important guiding significance in clinical practice.
During the first several days of neonatal life, hemodynamic monitoring is a great challenge due to the existence of continuous shunt and complex physiological changes, as the transition from fetal circulation to adult circulation. Currently, circulatory monitoring indicators in most neonatal wards are limited to heart rate (HR) and blood pressure assessment. Arterial blood pressure is still the most commonly used indicator for evaluating the neonatal circulation status. Blood pressure is determined by a complex interaction of circulating blood volume, venous return (preload), myocardial contractility, systemic vascular resistance (postload), and pulmonary vascular resistance (in the case of patent ductus arteriosus (PDA)). However, as reported by Groves et al. [1], total perfusion of neonates generally shows only a weak positive correlation with blood pressure, but arterial blood pressure is largely affected by systemic vascular resistance. Newborns with reduced systemic perfusion tend to have normal or elevated blood pressure in the first few hours after birth, suggesting that high systemic vascular resistance may lead to reduced blood flow.
The commonly applied indexes for evaluating systemic hemodynamics generally include blood pressure, HR, capillary filling time, urine volume, and blood lactic acid. However, studies have shown that blood pressure, capillary refill time and urine volume are not well correlated with systemic blood flow [2, 3]. HR is innervated by sympathetic nerves and vagus nerves, and is also susceptible to drugs, pain, body temperature and other factors. As such, it can neither accurately reflect hemodynamic changes, nor evaluate the patient’s systemic blood flow and tissue perfusion. Yuan et al. [4] have pointed out that the severity and prognosis of shock can be assessed by dynamic monitoring of arterial blood lactic acid level. Blood lactic acid level increases with the severity of neonatal shock. Seri I [5] notes that the management of critically ill premature infants with hypotension remains challenging, requiring a better understanding of the pathophysiology of neonatal shock, and improving our ability to assess cardiac output, organ blood flow, and tissue perfusion at the bedside.
The neonatal intensive care unit (NICU) primarily treats premature infants. Preterm infants may present with conditions such as neonatal respiratory distress syndrome, absence of secondary pulmonary artery catheter, bronchopulmonary hypoplasia, congenital heart disease, neonatal meconium inhaled syndrome, neonatal infectious diseases, neonatal low blood volume shock, and septic shock [6, 7]. In addition, premature infants may also experience severe arrhythmia caused by neonatal heart failure, and multiple organ dysfunction syndrome caused by neonatal asphyxia [8]. Newborns are a special population characterized by fragile vitality. NICU real-time dynamic monitoring of neonatal hemodynamic changes is helpful for the early detection of unstable hemodynamics, and for timely adjusted strategy of treatments, thus reducing the viscera ischemia hypoxia and tissue damage [9]. It can also minimize the premature infant brain injury caused by cerebral blood supply insufficient and reduce premature neurological sequelae and disability. In order to improve the survival rate and quality of life, real-time comprehensive monitoring of children’s hemodynamic status is of vital clinical significance.
The primary index utilized in hemodynamic monitoring is cardiac output (CO), which is adapted to the metabolism of tissues and cells throughout the body. CO is an effective indicator for the evaluation of the efficiency of circulatory system. Besides, perfusion index (PI) and plethysmograph variability index (PVI) are also applied for monitoring early postnatal peripheral circulatory changes, with the advantages of convenience and noninvasiveness [10]. During circulatory disturbances, blood flow shifts from nonvital organs and tissues (skin, subcutaneous tissue, muscles, gastrointestinal tract) to vital organs (heart, brain, kidneys) [11]. Monitoring the peripheral perfusion of nonvital tissues helps in the early detection of inadequate perfusion in vital organs. The techniques of hemodynamic monitoring can be divided into two categories, depending on whether invasive or noninvasive procedures are performed. The first category is the invasive CO monitoring techniques, including traditional hot dilution method, pulmonary artery floating catheter and direct Fick law [12]. Regarded as the gold standard for CO measurement, the invasive hemodynamic monitoring approaches require a high-level technical know-how of operators due to intricate operation protocols, leading to elevated risks of complications, higher costs, and failure in an extensive application within NICU. The second category is minimally invasive and noninvasive CO measurement techniques, including the common pulse indicator continuous cardiac output (PiCCO) thermodilution technology, cardiovascular nuclear magnetic resonance imaging (MRI) technology, repeated partial respiration method, ultrasound cardiac output monitor (USCOM), and impedance cardiography [13].
The basic principle of thermal dilution, also known as temperature dilution, is related to temperature changes. A floating catheter is inserted into the subclavian vein, and CO and other hemodynamic indicators are calculated by tracing the area under the time-temperature curve [14].
Nachman et al. [15] have performed studies combined with the principle proposed by Fick, and demonstrated that Fick is accurate enough to serve as a basis for clinical decision-making. This technique is routinely used in cardiac catheterization and is considered as a reference technique for CO in children. Blood oxygen concentration and other precise measurements of respiration and inhalation are also helpful to calculate CO and oxygen consumption.
Invasive blood pressure monitoring requires the placement of an arterial indwelling needle, which is susceptible to factors such as the length of patency, hardness, and presence of gas of the pipeline. Central venous pressure monitoring needs operators with professional technical skills and provides a single monitoring index, and it fails to reflect other hemodynamic indexes. Moreover, there is noninvasive operation and its application is limited.
Pulse indicator continuous cardiac output (PiCCO) is actually a combination of pulmonary temperature dilution and invasive blood pressure waveform analysis technology (Fig. 1), incorporating the analysis of arterial pressure waveform and continuous output measurement, and the accuracy is likely to be compromised in the presence of severe anatomical, functional variations, and severe rapid hemodynamic changes in the cardiovascular system. The side effects mainly include the risks of bleeding, infection, and thrombosis after catheterization. Especially for critically ill patients with hemodynamic instability, its detection accuracy may not be satisfactory with relatively high cost of consumables, making it not suitable for neonatal diseases.
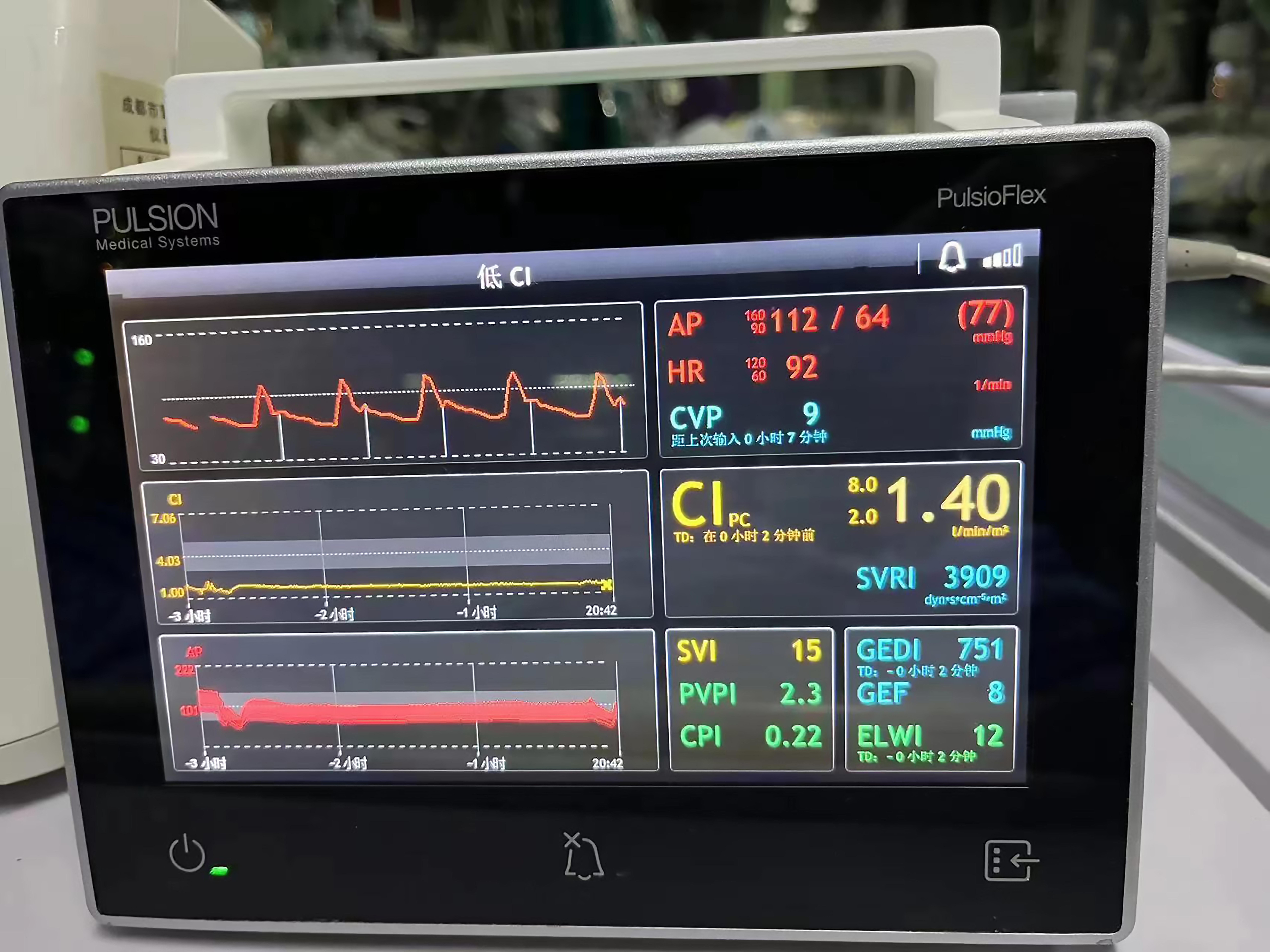 Fig. 1.
Fig. 1.Pulse indicator continuous cardiac output measuring instrument (Pulsion Medical Systems, Munich, Germany).
CO monitoring based on the MRI technique is reported with good repeatability, reflecting CO accurately and objectively, and it can be used to evaluate myocardial blood supply and cardiac function indicators (Fig. 2). However, it is difficult to be promoted in wards because of continuous detection and expensive equipment [16].
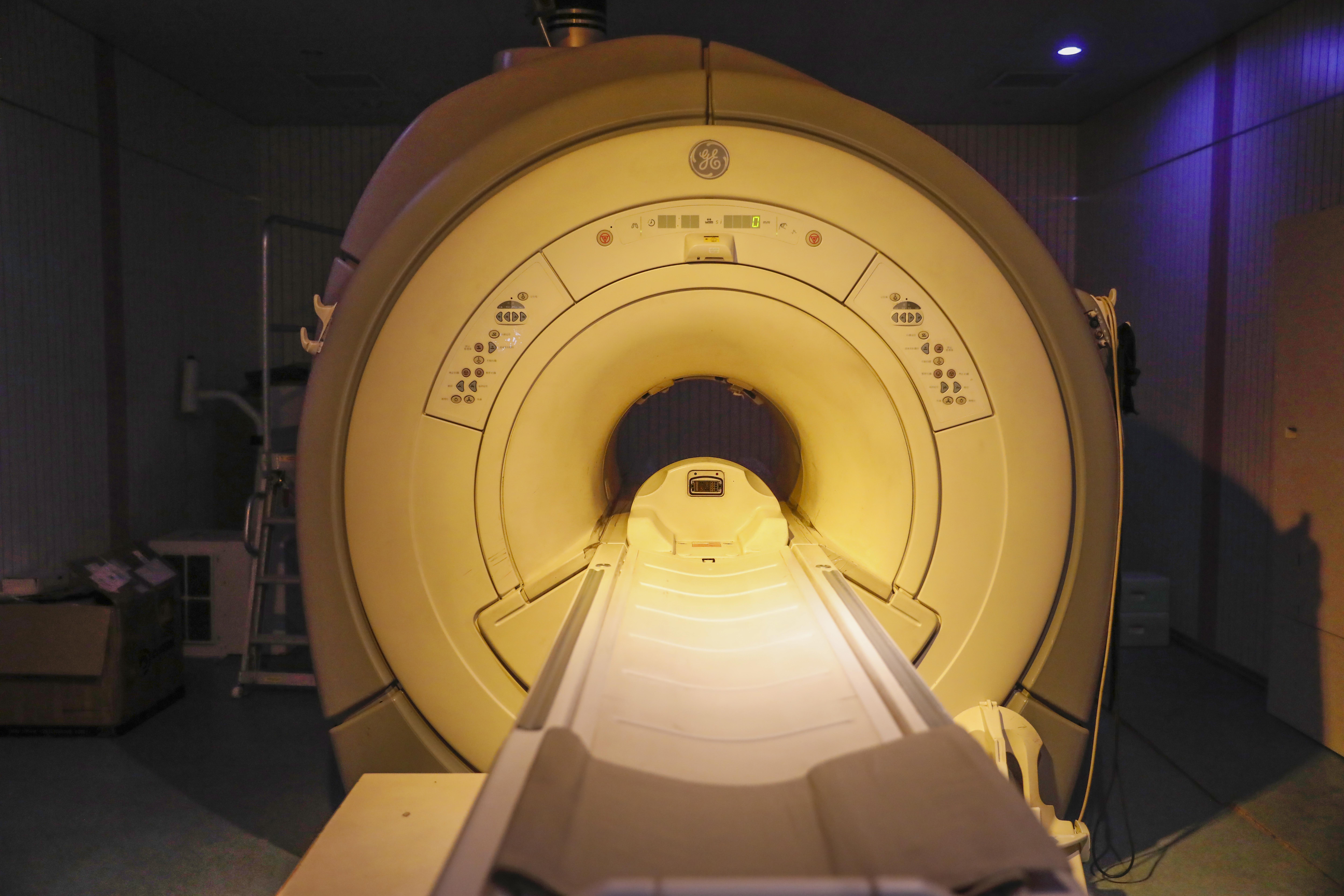 Fig. 2.
Fig. 2.Cardiovascular magnetic resonance imaging (SIGNA Voyager, General Electric Company, Pittsburgh, PA, USA).
USCOM has been applied in clinical for over 40 years (Fig. 3), which is now commonly used as hemodynamic noninvasive monitoring means. Despite the benefit of real-time dynamic monitoring, this approach requires stringent operation expertise and skills. Ultrasonic signals are susceptible to interference, overhaul time is longer than the other factors, such as in the NICU using a wide range of standardized training is needed.
 Fig. 3.
Fig. 3.Doppler ultrasound diagnostic device (GE-E10, General Electric Company, Pittsburgh, PA, USA).
In 1966, Kubicek et al. [17] proposed the bioimpedance method to
measure CO. Through a large number of studies, the correlation between
bioimpedance method and thermal dilution method is as high as 0.87–0.91. In the
1990s, the US food and drug administration (FDA) approved the noninvasive cardiac
output monitor (NICO
At present, this noninvasive CO chest impedance method is mainly used to detect blood flow in newborns by ECG measurement and biological resistance (BR). Its basic principle is that the artery blood flow volume change as the main measurement, with each heart beat caused artery blood flow changes caused by the change of blood volume and blood flow velocity makes the impedance of the conductive information transmission along the aorta or the corresponding change in resistance, calculated as per stroke volume (SV), CO, and hemodynamic indicators.
Bernstein and Osypka [18] developed and described the technical background of
EC, a new model for interpreting chest bioimpedance. The NICO
 Fig. 4.
Fig. 4.Noninvasive cardiac output monitor (ICON, Osypka Medical Inc., Berlin, Germany).
Electronic cardiometry (EC), using internal calibration, is a method for measuring SV and CO that considers the weight and length of newborns. In recent years, research efforts have been focused on examining the correlation, accuracy and precision of ultrasonic monitoring methods, bioimpedance methods, and nuclear magnetic resonance methods. Katheria et al. [19] have studied the feasibility of using EC in the first 5 minutes after delivery in 20 full-term babies with complete umbilical cord and have recorded the measurement results used for trend monitoring. Freidl et al. [20] have also used EC in the delivery room to monitor the first 15 minutes of the life of full-term infants. Studies show that EC can provide fast and accurate bedside monitoring.
Torigoe et al. [21] have applied EC in 28 infants with very low birth weight (VLBW) and low birth weight (LBW), implying that PDA and ventilator’s influence on this method. Grollmuss et al. [22] compared SV and HR of 228 premature infants with transthoracic echocardiography (TTE) (134 LBW, 94 VLBW) and EC (134 LBW, 94 VLBW) by bland-Altman diagram. The accuracy of the method was expressed by calculating coefficient of variation (CV). This study evaluated whether EC and TTE were interchangeable, even in LBW and VLBW infants, suggesting that EC was also suitable for LWB/VLBW infants, and it was a safe and easy method to perform. Song et al. [23] evaluate the practicability of EC monitoring by comparing the results of CO and system blood flow, measured by EC and echocardiography (ECHO). Through prospective observation, 40 premature infants received 108 pairs of EC and ECHO measurements, and analyzed the correlation between EC, left ventricular opacification (LVO), and right ventricular opacification (RVO), indicating that EC is expected to become the trend of CO monitoring for premature infants. EC is considered as a noninvasive method for continuous measurement of CO. Blohm et al. [24] showed that EC is comparable to the aortic flow-based TTE method for pediatric patients. Boet et al. [25, 26] showed that EC method is feasible, reproducible, fast, and it is a useful tool for continuous monitoring, and hemodynamic evaluation of neonates. EC is particularly meaningful for the clinical treatment of premature infants.
EC was once applied to monitor stroke and CO in 79 premature infants with
hemodynamic stability (mean gestational age (GA): 31
CO, a product of SV and HR, is particularly important for ensuring organ
perfusion, especially in the NICU. Due to the risk of complications associated
with invasive CO monitoring, noninvasive alternatives are required. Ren Xinrui
et al. [29] selected 17 children with congenital heart disease using EC
and TTE to examine SV, stroke volume variation (SVV) and
distensibility index of the inferior vena cava (dIVC), and found that EC had good
consistency with TTE monitoring results. Critchley et al. [30] conclude
that when bias and accurate statistics are used, CO bias, consistent limit, and
percentage error standards are proposed, acceptance of new technology should rely
on a consistent limit of
Huang et al. [34] reported that the use of EC to guide the intraoperative treatment of target fluid in patients undergoing surgery for congenital heart disease significantly reduce the dose of artificial colloid during surgery, and the tissue perfusion and oxygen supply are sufficient, thus reducing the postoperative tracheal catheter removal time.
Chen Bailang et al. [35] report that EC is used to monitor fluid management and treatment for early resuscitation of patients with septic shock, so as to guide fluid resuscitation and accurate fluid replenishing, which is conducive to disease recovery and reduced complications caused by blind fluid replenishing.
Yuan et al. [36] used EC to monitor newborn children with congenital heart disease, and they found that the differences in cardiac index (CI), left ventricular ejection fraction (LVEF), N-terminal pro-brain natriuretic peptide (NT-probNP) and pulmonary arterial pressure (PAP) among groups with heart failure were statistically significant. CI to some extent reflects the degree of heart failure and holds significant clinical applicability in assessing heart failure in newborns with congenital heart disease.
Zhang et al. [37] have indicated that patients with sepsis used EC to dynamically and continuously monitor the changes of CI, integrated cardiopulmonary operating network (ICON) and other indicators of cardiac function, so as to evaluate the changes of patients’ early cardiac function and guide clinical decision-making. Liu et al. [38] report 62 cases of neonatal meconium aspiration syndrome with pulmonary hypertension who are treated with mechanical ventilation using electronic cardiac hemodynamics measurement, implying that electronic cardiac measurement is a noninvasive, a highly sensitive, and a highly accurate monitoring tool.
Wu et al. [39] used EC and ECHO to detect the change of CO in 20
children with moderate and severe HIE during rewarming. CO measured by EC and
ECHO during rewarming increased from 153
Neonatal Acute Respiratory Distress Syndrome (NARDS) is a common respiratory
system disorder in neonatal care units, often leading to critical conditions. It
typically occurs shortly after birth (
Bohanon et al. [41] have found that HR variability analysis is more sensitive than routine vital signs in the diagnosis of neonatal sepsis, being the largest preventable disease of neonatal death in the world. Heart rate (HR) variability (HRV) analysis and noninvasive CO shown in many patients with sepsis are a useful auxiliary recognition, unconventional vital signs than traditional vital signs (HRV and CO) and mean arterial pressure are more sensitive to the confirmation of VLBW neonates with sepsis, which helps early recognition of sepsis.
The survey of Katheria et al. [42] show that an increase in LV output and a decrease in average blood pressure, indicating the necessary management of clinically significant PDA. Rodríguez et al. [43] used EC to detect hemodynamic changes in neonates undergoing surgical ligation or drug closure of PDA, and found that CI, stroke volume index (SVI), and ICON decreased in children with closed ductus arteriosus.
Eriksen et al. [44] have found that normal neonatal CO is stable within 0.5~6 hours after birth, and infants with perinatal asphyxia have lower CO before and during treatment. The lower rate of lactic acid loss is found to be less likely to be related to CO but more likely to be an indicator of organ damage due to asphyxia.
Literature reported that three small cohort studies compared the effect of infant position on CO and the presence of differences between prone, left decubitus, and supine positions [45, 46]. Ma et al. [45] found that neonatal EC detection in different positions resulted in reduced CO and stroke output in prone position compared with supine position. Wu et al. [46] used EC (Electrocardiogram) and ECHO (Echocardiography) techniques to confirm that in healthy full-term infants, the decrease in cardiac output (CO) in the prone position is due to a reduction in stroke volume (SV) rather than heart rate (HR) decrease. The cardiac output recovers when in the supine position. These results investigate the changes in systemic and cerebral haemodynamics between supine and prone sleep in healthy term infants, which may have important implications for clinical use, especially as many premature babies tend to be prone to feeding. Paviotti et al. [47] used the EC technique to compare left and supine positions, indicating that SV and CO were reduced.
Invasive mechanical ventilation is reported to affect hemodynamics by changing intrathoracic pressure and venous return volume. Studies are reported that positive end-expiratory pressure (PEEP) have little influence on hemodynamics in the range of 1–10 cm, and high-frequency ventilation affects hemodynamics changes.
Hsu et al. [48] report that PDA may affect EC measurement of CO in 79 premature infants with pulmonary PDA by comparing EC measurement with transthoracic echocardiography. Artery catheter refers to the fetal period significantly connected to the pulmonary artery; the aorta artery catheter function closes shortly after childbirth. However, for various reasons, it leads to the increase of pulmonary vascular resistance, ductus delay shut down and continue to exist. A large number of aorta shunt blood flow to the pulmonary artery, resulting in a large amount of blood flow from left to right shunt, and circulatory blood volume reduction. Increased blood flow in the pulmonary circulatory system leads to abnormal hemodynamic indicators.
The principle of ECG electromechanical measurement is based on the electrical conductivity of blood, and the content of red blood cells in the catheter affects the conduction of electrical signals. In the early stage, our research group conducted a study on two groups of premature infants with gestational age of 32~33 weeks, measured CO at 0.28 L/min in the non-transfusion group and 0.17 L/min in the transfusion group. This indicates that measurements of CO in children with anemia requiring transfusion are lower than those in the non-transfusion group.
A number of studies reported that CO decreases in all infants during mild hypothermia treatment, and the reason after analysis mainly lies in the reduction of HR and the constant output per wave [49, 50]. Study has found that lactic acid clearance rate has nothing to do with CO in HIE children [51].
Walther et al. [52] found that left ventricular output and stroke output of preterm infants significantly increased from day 1 to day 7 after caffeine treatment. Caffeine increased left ventricular output of preterm infants through the combination of positive inotropic and chronotropic effects, and such inotropic effects were accompanied by pressure-boosting effects.
In summary, EC is an impedance-based monitoring method that provides a noninvasive, real-time assessment of hemodynamics. Despite the newborn’s reference has not yet been determined, the birth weight of different gestational age segment, the volatile CO range, how the children’s diseases and other complicated factors affect hemodynamic indexes, the noninvasive hemodynamic monitoring is widely used in the common severe neonatal diseases, and the clinical guidance is of great significance. The use of noninvasive cardiac displacement meter electronic heart monitoring method can be real-time, continuous understanding of patients’ heart and vascular function changes, allowing to perform the clinical evaluation of diseases, guiding clinical decision-making, improving the success rate of rescue, and reducing the death rate, which is worthy of clinical application.
NICO, Noninvasive Cardiac Output Monitor; NICU, Neonatal Intensive Care Units; EC, Electronic Cardiometry; USCOM, Ultrasound Cardiac Output Monitor; HR, Heart Rate; PDA, Patent Ductus Arteriosus; CO, Cardiac Output; PI, Perfusion Index; PVI, Plethysmograph Variability Index; PiCCO, Pulse indicator Continuous Cardiac Output; MRI, Magnetic Resonance Imaging; FDA, Food and Drug Administration; ECG, Electrical Cardiography; BR, Biological Resistance; SV, Stroke Volume; LV, Left Ventricular; ET, Ejection Time; VLBW, Very Low Birth Weight; LBW, Low Birth Weight; TTE, Transthoracic Echocardiography; CV, Coefficient of Variation; ECHO, Echocardiography; LVO, Left Ventricular Opacification; RVO, Right Ventricular Opacification; GA, Gestational Age; SVV, Stroke Volume Variation; dIVC, distensibility Index of The Inferior Vena Cava; CI, Cardiac Index; LVEF, Left Ventricular Ejection Fraction; NT-probNP, N-Terminal pro-brain Natriuretic Peptide; PAP, Pulmonary Arterial Pressure; ICON, Integrated Cardiopulmonary Operating Network; NARDS, Neonatal Acute Respiratory Distress Syndrome; DR, Chest Digital Radiography; TFC, Thoracic Fluid Content; HRV, HR Variability; PEEP, Positive End-Expiratory Pressure.
FL and WD designed the research study. FL and WD performed the research. FL and WD wrote the manuscript. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
