Academic Editor: Michael H. Dahan
Background: Pelvic inflammatory disease (PID) complicating transcervical embryo transfer, not related to previous oocyte aspiration, is an extremely rare event. However, it can lead to severe complications. Case: We report two cases of pelvic infection related to transcervical frozen embryo transfer. The case of one patient was complicated by a pelvic abscess, which required percutaneous drainage, and the case of the other patient was complicated by ascites, which was positive for chlamydia. Conclusions: Despite there not being enough evidence to recommend cervical cultures or antibiotic prophylaxis in all cases of embryo transfer, it would be advisable in those cases with a personal history of PID.
Nowadays, embryo transfer in the context of in vitro fertilization (IVF) treatment is a procedure with a low incidence of complications [1].
Fresh embryo transfer has been the most common form of transfer in IVF cycles since the beginning of this technique. In recent years, improvements in vitrification protocols and an increase in preimplantation genetic testing of embryos have contributed to an increase in the number of frozen-thawed embryo transfer cycles. Consequently, the proportion of live births due to the success of this technique is increasing [2, 3, 4].
Initially, the main indication for this technique was to prevent ovarian hyperstimulation syndrome (OHSS). However, later studies have shown that controlled ovarian stimulation could lead to adverse effects in the endometrium, disrupting successful embryo-endometrium interaction. Therefore, it was suggested that performing frozen embryo transfer could improve the reproductive outcomes of IVF treatments [5, 6].
In our center, embryo transfer is a standardized procedure. Firstly, we clean the cervix with saline solution, and cervical mucus is aspirated. Before inserting the catheter, the cervical canal is flushed with Vitrolife’s G2 Plus medium. Then, a soft catheter (Labotect embryo transfer catheter, 190 mm) is loaded with embryos and handed to the clinician who inserts it through the cervical canal into the uterine cavity with abdominal ultrasound guidance. Finally, the embryos are placed 1–2 cm from the uterine fundus.
Reports regarding pelvic inflammatory disease (PID) after embryo transfer are extremely rare. Most of them are related to oocyte aspiration, where cervical and vaginal organisms could be inoculated during the procedure [7].
However, we report two cases of severe PID after frozen embryo transfer, which were not related to oocyte aspiration.
The first patient was a 34-year-old Caucasian woman with a medical history of
peritonitis after right ovarian cystectomy 17 years ago. The patient presented
with sterility of 2 years evolution. The basic sterility study based on hormone
analysis, seminogram, serology and ultrasound revealed the presence of a right
hydrosalpinx (65
Two days after frozen embryo transfer, the patient developed a high fever (39
She was discharged 7 days after hospitalization due to clinical improvement.
However, 11 days later, she came back to the emergency department due to
recurrent abdominal pain and high fever. Her white blood cell count was
20,000/mm
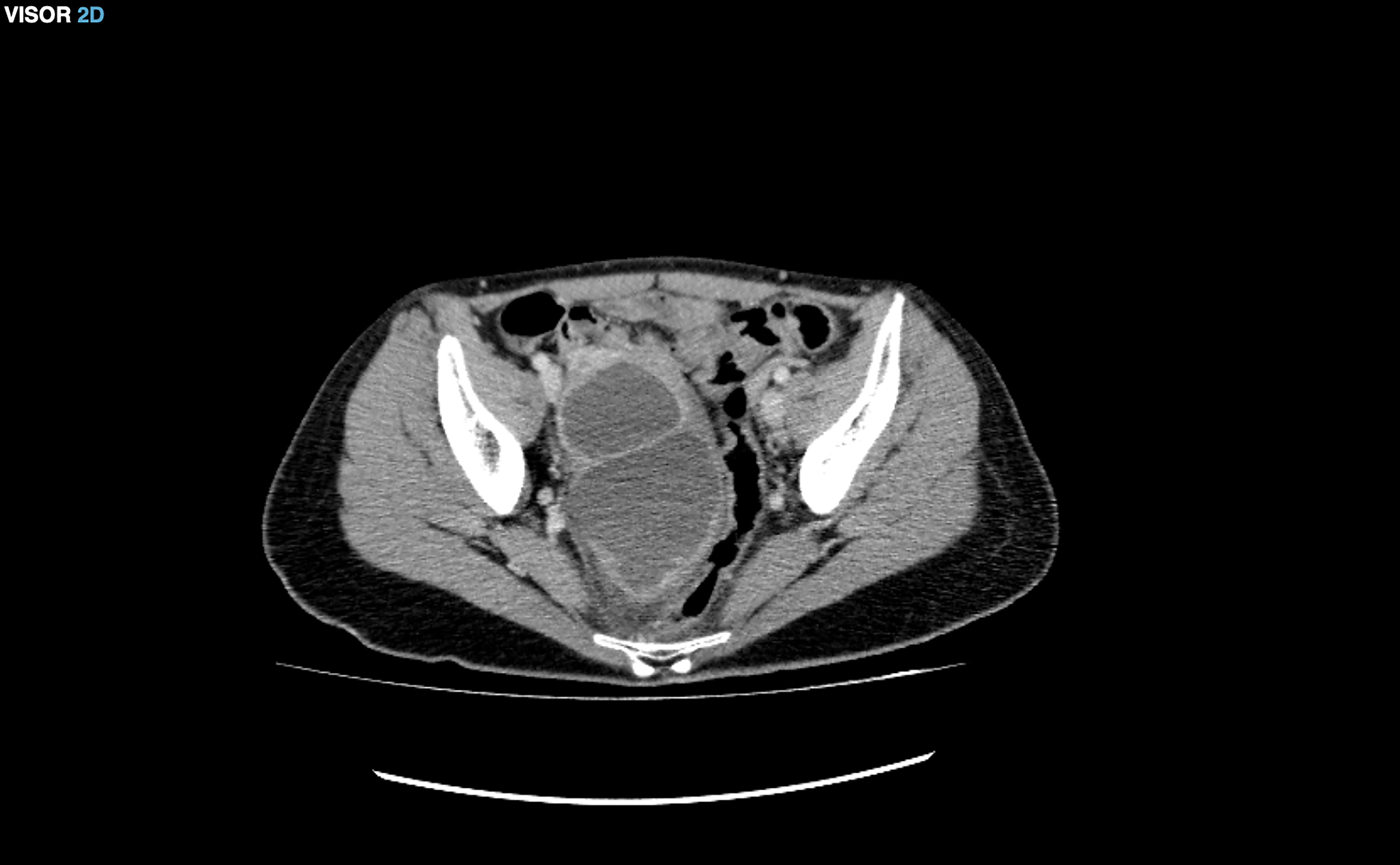 Fig. 1.
Fig. 1.Right tubo-ovarian abscess.
Endocervical and vaginal cultures were positive for Enterococcus faecalis. The patient received intravenous antibiotic treatment with ceftriaxone, doxycycline and metronidazole without clinical improvement, so a percutaneous drainage of the abscess was performed by interventional radiology, obtaining a positive culture for E. coli infection. The antibiotic treatment was changed to piperacillin-tazobactam. The patient remained afebrile after abscess drainage. Several ultrasounds were performed during her admission to check the drainage and evolution of the abscess. She was discharged 8 days after drainage with a complete resolution of the abscess. Finally, she had a negative beta-hCG test after the resolution of the PID.
The second patient was a 28-year-old, secundigravid and Caucasian woman, with a history of hyperthyroidism treated with radioactive iodine and levothyroxine. Twelve years ago, she required laparoscopic drainage of a pelvic abscess due to PID. Since then, she had no history of pelvic infection, surgery or abdominal pain. She had undergone a voluntary interruption of pregnancy. The basic study of secondary sterility demonstrated no anomalies in both the patient and her husband. Neither vaginal nor endocervical cultures were performed during the sterility study.
The patient came to the emergency department reporting intense abdominal pain and abdominal swelling for a month after having suffered a miscarriage at 6 weeks after a frozen embryo transfer performed at an external center. An emergency abdominal CT was performed, showing moderate ascites predominantly in the pelvic region (Fig. 2), which was initially treated with diuretics, albumin and anticoagulants.
 Fig. 2.
Fig. 2.Moderate ascites in the pelvic region.
However, due to the poor clinical evolution of the patient, a culdocentesis guided by ultrasound was performed 4 days after her admission and 500 mL of peritoneal fluid was aspired and analyzed, being positive for chlamydia. The endocervical culture was also positive for chlamydia. Antibiotic treatment with doxycycline was initiated, and the patient was discharged 2 days after culdocentesis with relevant clinical improvement.
Pelvic infection after embryo transfer presenting as a pelvic abscess or ascites is an extremely rare event. Most cases are related to oocyte aspiration, where cervical and vaginal microorganisms could be inoculated into pelvic cavity [7, 8].
Some authors propose the theory that oocyte aspiration could reactivate a latent infection in the fallopian tubes, as it has been described in other techniques, such as hysterosonography or hysterosalpingography [9, 10].
However, pelvic infections not related to recent oocyte aspiration are not a frequent event. One of the cases in the literature reported an agonadal woman undergoing embryo donation, demonstrating that infection may result from transcervical embryo transfer, despite the precautions taken during the performance of this procedure [11]. Some reports have described cases with pelvic abscesses after transcervical frozen embryo transfer, which required laparoscopy for drainage [8, 12].
Our cases illustrate the potential of embryo transfer to result in a severe pelvic infection. Both patients had a medical history of PID and peritonitis, so the reactivation of a latent infection could be the mechanism of the infection in these cases. The patient reported by Friedler et al. [12] also had history of PID.
Likewise, the placement of embryos into the uterine cavity using a catheter that passes through the cervix also entails the possibility of bacterial contamination during the embryo transfer procedure. In fact, there is growing evidence that bacterial contamination of the uterine cavity following transcervical embryo transfer can negatively affect the implantation rates and pregnancy outcomes [13, 14, 15]. Hamdoun et al. [16] observed that all patients with catheter contamination did not achieve pregnancy during embryo transfer. However, the pregnancy rate after IVF in patients with no catheter contamination was 61.2% in their study.
On the other hand, pelvic infections, such as chlamydia, mycoplasma, and ureaplasma, seem to be related to a longer period to achieve pregnancy and increased rates of pregnancy loss [17, 18]. Bacterial vaginosis has also been considered an obstetrical and gynecological issue, and the link between bacterial vaginosis and preterm birth has been intensely studied. Likewise, some studies among infertile women have also suggested that bacterial vaginosis could negatively affect spontaneous female fecundity and IVF results [19].
This evidence makes us wonder if it would be necessary to recommend antibiotic prophylaxis in all patients before embryo transfer to prevent complications and to improve pregnancy rates.
Most of these studies conclude that standard aseptic measures, including sterile conditions during the procedure, help to minimize the risk of infection, but there is no evidence to recommend antibiotic prescription at the time of the embryo transfer, as it does not seem to improve pregnancy rates or to reduce complications [20, 21].
In 2012, a Cochrane review evaluated the effectiveness and safety of antibiotic administration prior to embryo transfer during assisted reproductive technology cycles and concluded that administration of amoxicillin and clavulanic acid prior to embryo transfer reduced upper genital tract microbial contamination but did not alter clinical pregnancy rates, so this review does not recommend its general use [22]. Otherwise, antibiotic prophylaxis is recommended by some authors in patients with a recent history of pelvic infection [8].
Therefore, considering the rarity of this complication, nowadays, there is not enough evidence to recommend the routine performance of cervical cultures or antibiotic prophylaxis before embryo transfer. However, it could be considered in patients with a personal history of PID or risk factors for pelvic infections.
Frozen embryo transfer is a standardized procedure with a low rate of complications. PID after embryo transfer is an extremely rare event, but this complication could lead to pelvic abscesses or ascites, which can compromise the patient’s health status. Despite its potential severity, nowadays, there is not enough evidence to recommend cervical cultures or antibiotic prophylaxis in all cases of embryo transfer. However, cervical cultures could be performed in those patients with risk factors or a personal history of PID in order to prevent complications and improve pregnancy rates.
MBV—detailed data collection and careful writing of the manuscript. JNS—literature review and manuscript writing. CGM—literature review and manuscript writing. MNS—literature review and manuscript writing. RNM—clinical management and review of the scientific research. MPCM—clinical management and review of the scientific research.
An authorization from the Medical Records Department of our hospital was obtained to access and review the medical records of the women included in this study. Similarly, the authors obtained the approval of our local ethics committee. The number is C.P. - C.I. PI20/208. Informed consent was obtained from both patients included in this manuscript.
Thanks to all the peer reviewers for their opinions and suggestions which improved the quality of our manuscript.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
