Academic Editor: Paolo Ivo Cavoretto
Background: This study explored the guiding value of monitoring
pregnancy-induced hypertension syndrome (MP) for blood hypercoagulability in
combination with ultrasound monitoring of uterine artery blood flow in early
pregnancy and fetal growth and development in the second and third trimesters,
with the goal of preventing chronic hypertension with preeclampsia (PE) and its
clinical effects. Methods: The medical records of 189 pregnant patients
with chronic hypertension between June 2016 and June 2021 were retrospectively
analyzed; among them, 98 constituted the intervention group. The intervention
group received MP screening for blood hypercoagulability in combination with
ultrasound monitoring of uterine artery blood flow in early pregnancy and fetal
growth and development in the second and third trimesters of pregnancy. Those
with abnormalities were given timely symptomatic (low-molecular-weight heparin
with or without aspirin) and supportive treatment. The remaining 91 patients who
did not receive timely monitoring and intervention constituted the control group.
Fetal outcomes and PE rates were compared between groups. Results: The
PE incidence in the intervention group was significantly lower than that in the
control group (p
The incidence of pregnancy complicated by chronic hypertension is 1%–5% worldwide [1]. As maternal age at delivery continues to rise, the incidence of pregnancy complicated by chronic hypertension will continue to increase [2, 3]. In particular, the incidence of perinatal complications in pregnant women with chronic hypertension complicated by preeclampsia (PE) is high [2, 4, 5, 6, 7]. The harmful effects of chronic hypertension during pregnancy to both the mother and child have long been concerns of obstetric specialists. The adverse complications of chronic hypertension during pregnancy are attributable to uncontrolled severe hypertension with or without terminal organ damage or secondary PE and placental abruption, which are the main causes of adverse maternal and fetal outcomes [8]. Even if PE does not occur, the incidence of small-for-gestational-age (SGA) neonates among pregnant women with chronic hypertension is much higher than that among healthy pregnant women, and this condition is associated with abnormal placental etiology [9].
To date, numerous multicenter, prospective, randomized, case‒control studies have been conducted to prevent PE based on multiple parameters, such as clinical manifestations, mean arterial pressure (MAP), uterine artery pulsatility index (UtA-PI), pregnancy-associated plasma protein A (PAPP-A) and serum placental growth factor (PLGF) [10, 11, 12, 13, 14, 15, 16]. Despite their satisfactory outcomes, these studies were performed rather late in gestation (11–13 weeks +6d), and various indices were used; therefore, the methods of these studies are difficult to generalize more broadly, particularly in economically underdeveloped countries and remote areas. In addition, because scholars and researchers have not reached a consensus on the etiology and pathogenesis of PE, no breakthroughs have been achieved in the treatment of PE, and pregnancy termination remains the most effective treatment for PE patients.
From an epidemiological perspective, chronic hypertension is a high-risk factor for PE. Based on this consensus, in 2016, our institute implemented a screening approach involving the monitoring of pregnancy-induced hypertension syndrome (MP) for blood hypercoagulability combined with ultrasound monitoring of uterine artery blood flow in early pregnancy and of fetal growth and development in the second and third trimesters of pregnancy. The purpose of this combined monitoring was to identify PE in pregnant women with chronic hypertension as early as possible to provide timely intervention, thereby bringing the rates of good fetal outcomes among these patients to or close to the rates among healthy pregnant women, in addition to dropping the incidence of PE down to a minimum. Here, we retrospectively compared the pregnancy outcomes of women with chronic hypertension who received this intervention to those of women who did not receive this intervention. The results of this study might provide a basis for improving the prognosis of pregnant women with chronic hypertension and that of their infants.
In this retrospective study, 98 pregnant women with chronic hypertension who received treatment at the Department of Gynaecology and Obstetrics of the Second Xiangya Hospital beginning in the early stages of pregnancy between June 2016 and June 2021 were consecutively included. At the first prenatal examination, these women received routine MP screening for blood hypercoagulability combined with ultrasound monitoring of uterine artery blood flow. During the middle and late stages of pregnancy, fetal development was monitored. Patients who presented with blood hypercoagulability due to MP or abnormal uterine artery blood flow were subjected to positive case management and received early intervention. Those without abnormalities were excluded from consideration. The patients received reviews every 1–2 weeks until the fetus was born.
In addition, 91 pregnant women with chronic hypertension were randomly recruited from three municipal hospitals during the same period. These women received standard prenatal examination but did not receive MP screening. These patients constituted the control group. The general data of the two groups, such as their maternal age, number of pregnancies, body mass index (BMI), systolic blood pressure (SBP) and diastolic blood pressure (DBP) in early pregnancy, were collected, and their PE incidences and fetal outcomes were compared with those of the intervention group.
The procedures of this study were approved by the Ethics Committee of the Second Xiangya Hospital (2020-573). Written informed consent was obtained from each participant.
The inclusion criteria were as follows: (1) blood pressure
Patients who met any of the following criteria were excluded from consideration: (1) a history of a drug allergy; (2) serious dysfunction of the heart, liver, kidneys or other organs; (3) poor treatment compliance; or (4) contraindications for aspirin and low-molecular-weight heparin.
The observed indices included data obtained from records of clinical parameters including blood pressure, urinary protein, term birth, premature birth, neonatal asphyxia, birth weight of the newborn, fetal or newborn loss and transfer due to newborn complications.
Patients in this group received routine pregnancy examinations but did not receive routine screening for blood hypercoagulability.
In addition to routine pregnancy examinations, patients in this group also received the following examinations.
(1) MP screening. An MP monitoring system was utilized to noninvasively monitor
peripheral resistance (PR), vascular elasticity and blood viscosity (V), and
these indices can indirectly reflect the blood hypercoagulable state. The
procedures were as follows [17]. The patient laid on their left side at
45
(2) Color Doppler ultrasonography. Uterine artery blood flow during early pregnancy and fetal growth parameters during middle and late pregnancy, including fetal size, umbilical artery blood flow and amniotic fluid conditions, were monitored using ultrasound. A GE Voluson E10 color Doppler ultrasound diagnostic instrument (General Electric Medical System Co., Ltd., Brunn am Gebirge, Austria) was used. The frequency of the convex array probe was 2.5–5.0 MHz, and that of the abdominal probe was 3.5 MHz.
The procedures of uterine artery blood flow monitoring were as follows.
Bilateral uterine artery blood flow was monitored during early pregnancy (before
the end of the 12th week of gestation). The patient was in the supine position
with normal breathing. The gains and scanning speed of the instrument were
adjusted. The ultrasonic probe was placed above the groin. Because the
simultaneous display of the uterine artery and external iliac artery is required
for color Doppler flow imaging, the site within 1 cm of where the uterine artery
crosses the external iliac artery was chosen as the sampling site to obtain the
spectrum of uterine artery blood flow. Bilateral uterine artery information was
needed for each patient. The sampling volume was adjusted, and the sampling line
was maintained as parallel to the blood flow direction as possible (or the
included angle
Treatment for the intervention group is described below.
(1) Treatment method. For patients with a positive MP outcome, aspirin or low-molecular-weight heparin was administered; for those with a noticeable MP outcome, combined medication was provided [21, 22]. Treatment flow charts for this group are shown in Figs. 1,2.
 Fig. 1.
Fig. 1.Intervention flow chart for the intervention group (
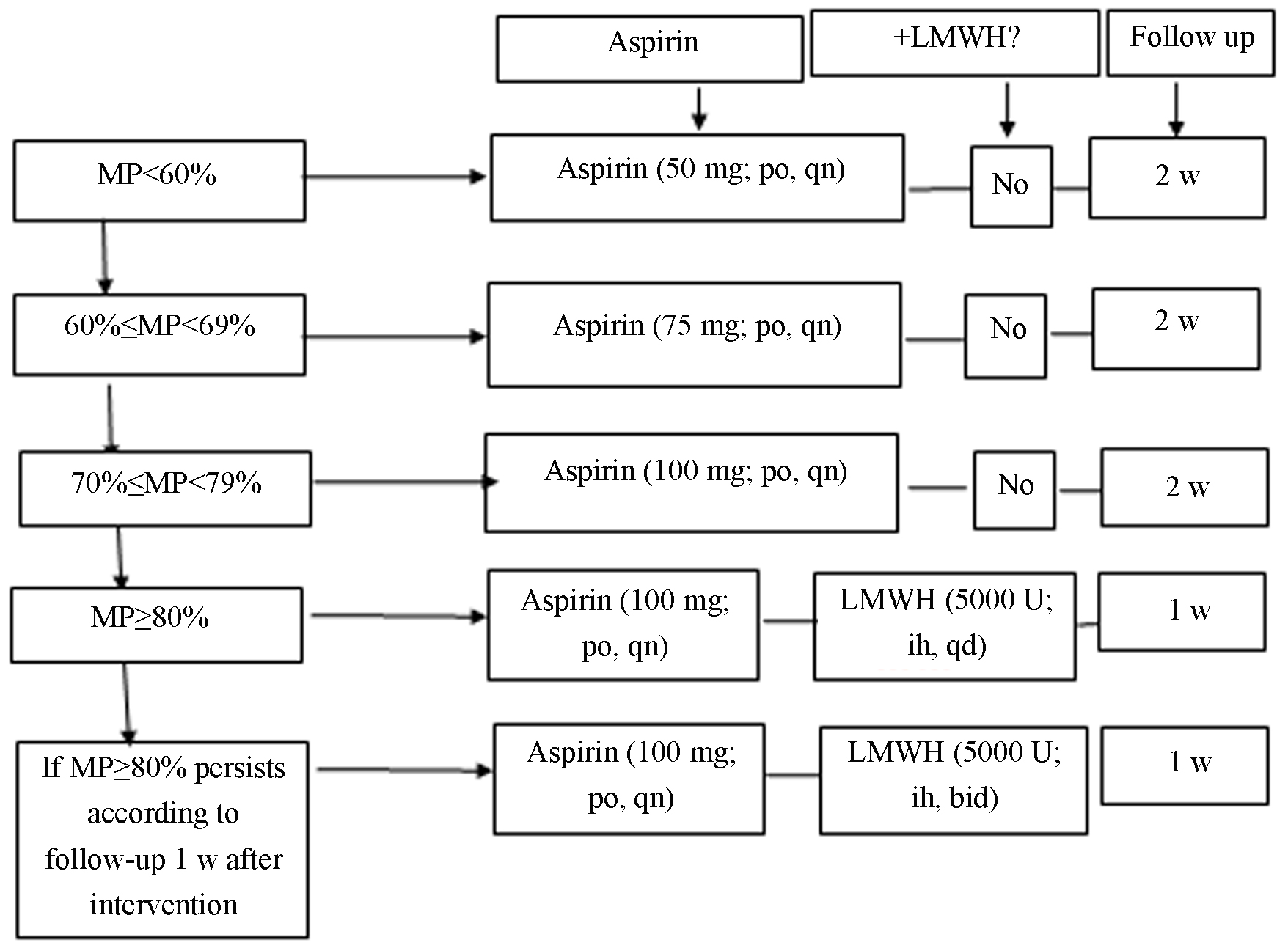 Fig. 2.
Fig. 2.Intervention flow chart for the intervention group (
For patients at
For patients at
At gestational week
(2) Antihypertensive treatment. The use and withdrawal of antihypertensive drugs
were based on several recommendations. According to the recommendations proposed
by the International Society for the Study of Hypertension in Pregnancy (ISSHP)
in 2018, the target blood pressure for the treatment of pregnancy complicated by
chronic hypertension is (110–140)/(80–85) mmHg, and the indication for drug
withdrawal is blood pressure
Apart from routine pregnancy examinations, blood pressure, urinary protein and platelets were monitored. The primary observed outcomes included term births, premature births, neonatal asphyxia, the birth weight of the neonate, neonatal transfer and fetal loss.
Data were analyzed with SPSS 22.0 (IBM Corp., Chicago, IL, USA). Measurement
data are presented as the mean
The two groups did not show significant differences in the number of cases
requiring antihypertensive drugs before pregnancy, mode of conception, maternal
age, the number of pregnancies, BMI, SBP or DBP in early pregnancy (p
| Index | Group | p value | |
|---|---|---|---|
| Intervention (n = 98) | Control (n = 91) | ||
| Age (years) | 36.02 |
35.45 |
0.591 |
| Number of cases requiring antihypertensive drugs before pregnancy (n) | 17 | 14 | 0.703 |
| Natural/assisted conception (n/n) | 88/10 | 83/8 | 0.732 |
| Average duration of pregnancy | 3.18 |
3.24 |
0.899 |
| BMI | 22.38 |
0.749 | |
| SBP in early pregnancy | 87.61 |
87.43 |
|
| DBP in early pregnancy | 145.62 |
145.93 |
0.295 |
In the intervention group, pregnancy termination occurred in nine patients due
to fetal abnormalities and missed abortions, and in the control group, pregnancy
termination occurred in eight patients. The incidence of chronic hypertension
complicated by PE in the intervention group was significantly lower than that in
the control group (6 vs. 27; p
| Index | Group | p value | ||
|---|---|---|---|---|
| Intervention (n = 98) | Control (n = 91) | |||
| Actual number of pregnancies | 89 | 83 | 0.020 | 0.888 |
| PE | 6 | 27 | 18.421 | |
The intervention group had 81 term births and eight premature births. Among the newborns, 3 presented with mild asphyxia, but no cases of severe asphyxia occurred. The intervention group had six low-birth-weight infants, including two term-birth infants (one due to a unicornuate uterine pregnancy and one due to pregnancy after transcervical resection of adhesion (TCRA), a small placenta and a thin umbilical cord) and four prematurely born infants. Neonatal transfer occurred in seven cases. Labor was induced for four patients due to fetal abnormalities, and dilation and curettage were performed for five patients due to missed abortion during early pregnancy. No fetal losses occurred.
The control group had 53 term births and 23 premature births. Among the newborns, 12 suffered from mild asphyxia, and eight had severe asphyxia. The control group had 19 low-birth-weight infants, including eight term-birth infants and 11 (12.1%) prematurely born infants. Labor was induced for three patients due to fetal abnormalities, and dilation and curettage were performed for six patients due to missed abortion during early pregnancy. In addition, six fetal losses occurred in the control group. Among the fetal losses, four were due to small for gestational age, a loss of umbilical artery blood flow and severe FGR; therefore, abortion was performed (two cases of intrauterine fetal death and two cases of rescue withdrawal because of an extremely low birth weight), and cesarean section was performed in two pregnancies due to intrauterine death (one complicated by placental abruption and one complicated by hemolysis, elevated liver enzymes and low platelet (HELLP) syndrome). Neonatal transfer occurred in 29 cases.
Except for their rates of fetal abnormalities and missed abortion (p
| Index | Intervention (n = 98) | Control (n = 91) | p | |
|---|---|---|---|---|
| ctual number of pregnancies | 89 | 83 | ||
| Term births (n) | 81 | 53 | 18.401 | |
| Premature births (n) | 8 | 23 | 12.160 | 0.001 |
| Mild neonatal asphyxia (n) | 3 | 12 | 7.650 | 0.006 |
| Severe neonatal asphyxia (n) | 0 | 8 | 9.846 | 0.002 |
| Low-birth-weight infants | 6 | 19 | 10.630 | 0.001 |
| Neonatal transfers | 7 | 29 | 22.053 | |
| Fetal losses | 0 | 6 | 7.291 | 0.007 |
| Abortions due to abnormalities | 4 | 3 | 0.108 | 0.742 |
| Missed abortions | 5 | 6 | 0.191 | 0.662 |
Among the patients in the intervention group, blood hypercoagulability positivity was high according to the findings of MP screening. After timely intervention, a significant change in the positive rate of uterine artery blood flow was observed (Table 4).
| Detection method | + | – |
|---|---|---|
| MP | 87 | 11 |
| Uterine artery blood flow | 34 | 64 |
| 60.668 | ||
| p | ||
In the intervention group, fetal growth was closely monitored by ultrasound every two weeks. When an increase in umbilical cord blood flow resistance was observed or the growth curve was not satisfactory, the patient was hospitalized and received symptomatic and supportive treatment (7–10 d as a treatment course). Satisfactory pregnancy outcomes were achieved.
Chronic hypertension is an important risk factor for PE, and a blood hypercoagulable state serves as the pathological basis of PE [25]. Scholars have utilized MP screening to monitor the blood hypercoagulable state in pregnant women, and satisfactory prediction rates have been reported [26, 27, 28, 29]. However, all these reported studies were performed after the 20th week of gestation. In our previous study, we found that blood hypercoagulability was detected noticeably earlier in MP screening than the presence of clinical manifestations. By monitoring changes in blood hypercoagulability in older women in early pregnancy and providing timely anticoagulant and antithrombotic intervention accordingly, namely, by providing reasonable intervention according to the blood hypercoagulable state rather than a blood pressure increase after 20 w of gestation, we significantly decreased the incidence of PE in older pregnant women as well as the incidence of the associated complications and greatly improved the prognoses of the mothers and infants, thereby achieving satisfactory results. Therefore, the effect of MP screening for blood hypercoagulability is satisfactory in early intervention for pregnant women with chronic hypertension.
By the end of the 20th week of gestation, the pathophysiological process of PE is basically completed, and some patients may present with typical clinical manifestations of PE even at this time. Therefore, normally, once abnormalities in uterine arterial blood flow and umbilical cord blood flow are observed, PE is beyond the initiation stage and has already entered the developmental stage. Vascular remodeling normally starts from the 8th gestational week and is completed by the end of the 20th gestational week [30]. That is, if attention is given to only the developmental stage of PE, namely, from the 20th gestational week onward, the optimal time for early intervention and the correction of placental and maternal vascular dysfunction, as well as for improving fetal prognosis, may be missed. Therefore, in clinical practice, early prediction or early screening should be performed during the initiation stage, when trophoblasts infiltrate the spiral arterioles of the myometrium, and intervention is provided before the completion of vascular remodeling. This strategy might be of greater clinical significance and have wider application prospects.
The blood hypercoagulable state in PE pregnant women causes insufficient placental blood flow perfusion and blocks material transportation between villous vessels, thereby affecting the intrauterine growth of the fetus [31]. FGR is associated with a low PLGF circulating level; low-molecular-weight heparin promotes the generation and release of the PLGF from placental villi and vascular endothelium in vitro, and monitoring of the uterine artery blood flow in early pregnancy [32] and of the growth of the fetus during middle and late pregnancy for women who are prone to a blood hypercoagulable state [33], as well as the use of aspirin at a low dose combined with low-molecular-weight heparin when necessary, can reduce the development of PE and its complications such as FGR [34]. In this study, the intervention group received MP for blood hypercoagulability combined with ultrasound monitoring of uterine artery blood flow in the early stage of pregnancy, and when necessary, these women were given treatment with low-molecular-weight heparin and aspirin. In addition, during the second and third trimesters of pregnancy, fetal growth was closely monitored using B-ultrasound [33], and when necessary, supportive treatment promoting fetal growth and development was provided. For these reasons, satisfactory clinical effects were achieved. Our results were consistent with those reported in the literature [34]. Therefore, MP screening is well suited as an “early warning” platform for the early prediction of blood hypercoagulability. The monitoring system is easy to operate, with high accuracy and satisfactory repeatability. The monitoring system can provide early hypercoagulability parameters for the prevention of and effective intervention for PE. In addition, the combination of the MP monitoring system with ultrasound monitoring of fetal growth and development in the second and third trimesters of pregnancy significantly improved the prognoses of the fetuses.
In this study, the intervention group received early MP screening, and if abnormalities were observed, anticoagulant drugs were administered. Our results showed that the maternal and fetal outcomes of the intervention group were significantly better than those of the control group. This finding is consistent with that reported by Rolnik et al. [16]. In their randomized, case‒control study, a total of 1620 patients from multiple centers were involved; the results showed that the use of aspirin (150 mg/d) for high-risk pregnant women at gestational ages of 11–14 weeks decreased the incidence of PE by 82% [16]. Aspirin is an antiplatelet drug that has antipyretic, analgesic and anti-inflammatory functions. The main mechanism of action of aspirin is that it reduces endothelial cell injury and inhibits platelet aggregation and thrombosis, thereby having antihypertensive and anticoagulant effects [35, 36]. Aspirin at a low dose does not increase the incidence of maternal thrombocytopenia, maternal postpartum hemorrhage, or prenatal and postpartum hemorrhage, nor does it increase the bleeding tendency and neonatal disease rate of the newborns; therefore, oral administration of low-dose aspirin during pregnancy is relatively safe for mothers and infants [37, 38, 39].
Low-molecular-weight heparin is the heparin fragment of unfractionated heparin (UFH) obtained after chemical decomposition or enzymatic cleavage, with a molecular mass ranging from 4000 U to 6500 U. It is an antithrombin-dependent thrombin inhibitor and has a stable dose effect in vivo. As low-molecular-weight heparin has a rather large molecular mass, it does not penetrate through the placenta and therefore has no teratogenic effect on the fetus. Apart from the anticoagulant and platelet aggregation-inhibiting effect, low-molecular-weight heparin also has anti-inflammatory, anti-apoptotic, antithrombotic and vascular endothelial cell-protecting effects; it can reduce blood V, improve placental function, increase the blood supply to the fetus, improve fetal intrauterine reserve capacity and promote fetal growth [40]. Low-molecular-weight heparin treatment was reported to have a positive effect on PE as early as the 1960s [41]. Low-molecular-weight heparin plays an important role in the infiltration and proliferation of early trophoblasts [42] and improves the pathogenesis of “superficial placental implantation” in PE. Low-molecular-weight heparin has satisfactory safety during pregnancy, and side effects are seldom reported [43].
The occurrence of PE is associated with the immune system [44]. Fetal loss is associated with a decrease in Treg cells, and Treg cell deficiency can lead to embryo implantation failure [45]. In PE, the number of peripheral and decidual Treg cells decreases [46]. Both low-molecular-weight heparin and aspirin influence the function of T lymphocytes [47]. Low-molecular-weight heparin and aspirin also improve the blood hypercoagulable state and regulate the functions of T lymphocytes. Therefore, the incidence of PE is reduced, and satisfactory pregnancy outcomes are achieved. The underlying mechanism may be that low-molecular-weight heparin and aspirin reduce the development of PE by regulating peripheral and decidual Treg cells. However, to validate this presumption, further studies are needed.
This study has some limitations. The sample size was small, and most of the patients enrolled in the control group were from different hospitals (some came to visit our department once the pathological condition became severe). These factors might lead to bias in the observed results of this study. To overcome these limitations, multicenter, randomized, case‒control trials with a larger sample size need to be conducted in the future.
Our previous study used the MP monitoring system to screen the blood hypercoagulability state of older pregnant women beginning in early pregnancy and guided early clinical intervention according to the screening results. In that study, we achieved satisfactory clinical results in the prevention of PE. In this study, to analyze another PE high-risk factor, chronic hypertension, the simple MP monitoring system was also used to screen the blood hypercoagulability state and initiate interventions in a timely manner. At the same time, ultrasound was used to monitor the growth and development of the fetus and provide symptomatic and supportive treatment in a timely manner, resulting in the achievement of ideal maternal and infant outcomes. It is reasonable to believe that for all pregnant women with PE high-risk factors, blood hypercoagulability can be monitored before pregnancy or in early pregnancy, and according to the screening results, timely and effective intervention can be administered and expected to achieve the same satisfactory effects. The use of this method, especially for pregnant women in economically underdeveloped and remote areas, may have notable societal benefits.
All data analyzed in this study are available from the corresponding author on reasonable request.
MP and SYX devised the study plan, MP and SYX led the writing of the article, MP, JYL, BL, LingY, YLDeng, WZ, YTN and WSL collected the data. MP, HYL, YZ, LiY, YW and BZ sorted the data and conducted the analysis, MP, YHZ and LYX validated the data, MP and YLDing were responsible for visualization, and MP attained the funding and supervised the whole process. All authors read and approved the final manuscript.
The procedures of this study were approved by the Ethics Committee of the Second Xiangya Hospital (2020-573). Written informed consent was obtained from each participant.
Not applicable.
This study was financially supported by the Research Project on Degree and Graduate Education and Teaching Reform of Nanjing University (grant no., 2021JGB149).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
