Academic Editor: Paolo Ivo Cavoretto
Background: Our aim in this study was to evaluate whether endometrial
receptivity assay (ERA) test improves single, autologous euploid frozen-thawed
embryo transfer (FET) outcomes in patients with repeated implantation failure.
Methods: This was a retrospective cohort study which was conducted in a
University affiliated private hospital. The study included 135 patients with
repeated implantation failure who underwent single, autologous euploid ERA
adjusted and non-adjusted FET. Patients were stratified into three groups,
patients with receptive endometrium based on the ERA test, patients with
non-receptive endometrium based on the ERA test and patients who did not receive
the ERA test (control group). The three groups were compared in terms of FET
outcomes. Results: Of 135 patients, 73 had the ERA test results
available and 62 did not have the ERA test. Of 73 patients, 28 had non-receptive
endometrium and 45 had receptive endometrium. The three groups are all the same
in terms of age, body mass index, type of infertility, duration of infertility,
number of previously embryo transfers and infertility causes (p
For assisted reproductive technologies (ART), the implantation is still a rate-limiting step for the success of embryo transfer. A group of patients experience multiple implantation failures in the settings of ART. Although more than one definition are used, the term “repeated implantation failure (RIF)” implies the failure of two or more embryo transfer (ET) attempts using at least two or more good-quality embryos [1, 2]. Chromosomal aberrations are the most common cause of implantation failure. Fortunately, preimplantation genetic testing for aneuploidy (PGT-A) technologies render possible to eliminate the majority of genetically abnormal embryos. However, a proportion of euploid embryos do not achieve implantation even if no structural pathology is identified in the uterus. This raises the question of whether the timing of transfer based on the developmental stages of embryos is a good approach for successful implantation in all patients.
Implantation occurs with the invasion of the trophoblasts from epithelium into the stromal tissue of the endometrium. A complex sequenced cascade renders the process in which cytokines and mediators (fibronectine, adhesion molecules, integrins, etc.) have roles [3, 4, 5]. It has been proposed that the implantation can occur within a narrow period of time in a menstrual cycle, which is known as window of implantation (WOI). In a natural cycle, 5–7 day old embryos can implant into endometrial tissue in 18th–21st days of the cycle [6, 7]. With ART treatment, this frame spanned between 19th–23th cycle days [7, 8, 9]. During this time, the endometrium is considered to have the highest potential that allows an embryo to be able to implant. In routine clinical practice, the timing of embryo transfer is adjusted based on the developmental stage of embryo. However, the WOI has been found to be different in 25.9% of patients with RIF [10]. This finding suggests that the endometrial receptivity does not coincide with the time of embryo transfer in some patients, and implantation rate can be improved by modifying the day of embryo transfer in those whose period of WOI have shifted. The endometrial receptivity assay (ERA) has emerged as a diagnostic test to determine the receptivity status of the endometrium [11]. The ERA test results are reported as pre-receptive, receptive and post-receptive. The hypothesized idea is that an embryo should be transferred in the receptive period of the endometrium for a successful implantation, which may supposedly overcome repeated implantation failures. Thus, the day of embryo transfer is personalized according to the ERA results.
We hypothesized that if adjusting embryo transfer day based on the ERA test increases the chance of implantation. This can be more accurately shown in a homogenous patient population undergoing euploid embryo transfer which should overcome aneuploidy-dependent IVF failure. If the window of implantation difference is also the cause of failed IVF in this patient group, in patients who undergo euploid embryo transfer adjusted according to the ERA test, pregnancy rate will be higher than those who receive euploid transfer without ERA testing.
To be able to test this hypothesis, we compared single, autologous euploid embryo transfer outcomes between three patient groups: (1) those with receptive endometrium based on the ERA test, (2) those adjustment for transfer with non-receptive endometrium based on the ERA test and (3) controls that did not undergo the ERA test.
This retrospective study consisted of patients with repeated implantation failure who underwent single, autologous euploid embryo transfer at ART clinic of Acibadem Fulya Hospital for 3 years. The study was approved by Acibadem University Medical Research Ethics Committee (ATADEK 2019-10/3). Informed consent was obtained from all individual participants, allowing the use of their blinded clinical data for scientific purposes. Data from patients were obtained through our IVF unit’s electronic record and the process of data collection was consistent with data protection regulations.
The study population was stratified into three groups: those with receptive endometrium and those with non-receptive endometrium (pre- and post-receptive) based on the ERA results and controls. The controls were those who did not undergo the ERA test. All comparisons were performed between these three groups.
All patients included in the study met the following inculusion criteria: (1) age between 25 and 44 years, (2) previous history of RIF, and (3) unexplained RIF was diagnosed according to infertility work-up, (4) euploid, single, autologous embryo transfer. Repeated implantation failure was defined as the failure of two or more good quality embryo transfers in previous fresh and/or frozen cycles. Good quality embryos were defined as Gardner et al. [12] described. All tranfers were performed on the 5th day with good quality embryos. This was the first cycle of participants undergoing the PGT-A procedure.
The exclusion criteria of the study were as follows: (1) age below 25 or over 44 years, (2) congenital or acquired uterine anomalies (i.e., myoma, polyps, cysts, etc.), (3) thrombophilia disorders, (4) chronic medical conditions (such as diabetes, liver, kidney disorders, thyroid dysfunctions, etc.), (5) abnormal karyotype analysis results, and (6) embryo transfers not screened by PGT-A. All patients had infertility work-up including hysterosalpingography, hysteroscopy and karyotype analysis. Couples with abnormal karyotype analysis results were not included in the study groups. Patients with hydrosalpinx underwent tubal ligation or salpingectomy by laparoscopy. Each transfer represents a different patient.
Collected demographic data included age, body mass index (BMI, kg/m
Endometrial Receptivity Array was performed in a hormone replacement cycle. Oral
estradiol valerate (2 mg/day) was started on the 2nd day of the menstrual cycle,
which was increased by 2 mg every four days to a maximum dose of 8–10 mg/day.
Endometrial thickness was assessed on the 14th day of menstrual cycle. When the
endometrial thickness was between 7–14 mm in conjunction with trilaminar pattern
and serum progesterone level was less than 1.5 ng/mL, daily intramuscular
progesterone (100 mg/day) was started to be administered for the secretory
transformation of the endometrium. The endometrial sampling was performed using
Pipelle on the sixth day of the progesterone administration. And the endometrial
tissue was laid in a cryotube which contains 1.5 mL RNA (Qiagen). The cryotube
was vigorously shaked for a few seconds, and then kept at 4 °C or in ice
box for
After the oocyte retrieval, intracytoplasmic sperm injection (ICSI) was performed. Embryos were cultured to the blastocyst stage and underwent assisted hatching, followed by trophectoderm biopsy on day 5. Biopsy specimens were analyzed by next generation sequencing (NGS). Next generation sequencing was performed by Veri-Seq protocol (Illumina Inc., San Diego, CA, USA) and was sequenced with MiSeq (Illumina) sequencer. BlueFuse Multi software (Illumina) was used for analyzing sequence files as defined before [13].
All patients had euploid embryo transfer in a hormone replacement cycle in one of subsequent menstrual cycles. In hormone replacement cycle, 2 mg oral estradiol was started on the second day of the cycle. And every four days, the dose was increased 2 mg, to a maximum dose of 10 mg/day. In those undergoing the ERA test, the timing of embryo transfer was determined based on the ERA results as follows: on the sixth day of progesterone administration in those with receptive endometrium or on the adjusted day in those with non-receptive endometrium. All embryo transfers were performed at the second or third menstrual cycle following the biopsy in the ERA group. In the control group, the timing of embryo transfer was on the sixth day of progesterone initiation. Endometrial preparation was performed using the same hormone replacement cycle protocol.
Embryo transfer outcomes were documented for all patients and given per transfer. The primary outcome was live birth rate, which was defined as a delivery after 24 completed weeks of gestation. The secondary outcomes included pregnancy, implantation, and clinical pregnancy. Pregnancy was defined as serum beta-human chorionic gonadotropin (beta-HCG) positivity while implantation was defined as the ultrasound evidence of intrauterine gestation sac at the 6 weeks of gestation and clinical pregnancy as the ultrasound evidence of fetal cardiac activity at the 7 weeks of gestation. Clinical pregnancy per ET was named as clinical pregnancy rate (CPR) while LB per ET was named as live birth rate (LBR) [14].
Statistical analysis was performed using the SPSS version 22 (Statistical
Program for Social Sciences, IBM, Chicago, IL, USA). Demographic data were
characterized by means, standard deviations (SD) and percentages. Assumption of
normality was made using Kolmogorov-Smirnov Shapiro Wilk test. Means were
presented with SD and median values for continuous variables. Difference in mean
values and characteristics between groups were analyzed with independent samples
t test and chi-square test. Kruskal Wallis test was used to compare
continuous variables and chi-square test for categorical variables. To be able to
reveal the association of the ERA test with the chance of live birth, a logistic
regression analysis was conducted controlling for the following variables: age,
BMI, duration of infertility, number of previous embryo transfers and endometrial
thickness. Live birth was added to the model as the dependent variable and the
ERA test as an independent variable (binary). p value of
A total of 135 patients met the inclusion criteria of the study. Of those, 73
had the ERA test result available and 62 did not have the ERA test. Among those
with ERA test results, 45 (61.6%) had receptive endometrium and 28 (38.4%) had
non-receptive endometrium. Of the non-receptive results, 13 (46%) were
pre-receptive and 15 (54%) were post-receptive. The mean values of age, BMI and
duration of infertility were 36.6
| Receptive | Non-receptive | Control | p value* | ||
| (n = 45) | (n = 28) | (n = 62) | |||
| Age (years) | 36.6 |
36.4 |
36.6 |
0.972 | |
| Body mass index (kg/m |
25.3 |
25.3 |
26.2 |
0.360 | |
| Duration of infertility (years) | 6.2 |
5.9 |
5.9 |
0.737 | |
| Number of previous embryo transfer | 3 (2–6) | 4 (2–6) | 3 (2–6) | 0.792 | |
| Fresh ET | 1 (0–3) | 1 (1–2) | 1 (0–4) | 0.596 | |
| Frozen ET | 2 (1–5) | 2 (1–4) | 2 (1–5) | 0.533 | |
| Primary infertility | 40 (88.9) | 25 (89.3) | 56 (90.3) | 0.969 | |
| Causes of infertility (%) | 0.990 | ||||
| Tubal factor | 9 (20) | 6 (21.4) | 12 (19.4) | ||
| Ovulatory dysfunction | 8 (17.8) | 4 (14.3) | 10 (16.1) | ||
| Diminished ovarian reserve | 6 (13.3) | 4 (14.3) | 8 (12.9) | ||
| Endometrioma | 4 (8.9) | 2 (7.1) | 6 (9.7) | ||
| Male factor | 8 (17.8) | 5 (17.9) | 8 (12.9) | ||
| Combined male and female factor | 4 (8.9) | 3 (10.7) | 9 (14.5) | ||
| Unexplained | 6 (13.3) | 4 (14.3) | 9 (14.5) | ||
| Duration of estradiol use (days) | 12.8 |
13.8 |
12.7 |
0.659 | |
| On the day of starting progesterone | |||||
| Endometrial thickness (mm) | 10.3 |
9.8 |
10.3 |
0.455 | |
| Estrogen level (pg/mL) | 459.5 |
448.5 |
453.9 |
0.981 | |
| Progesterone level (ng/mL) | 0.25 |
0.19 |
0.20 |
0.471 | |
| Data was presented as mean *Independent sample t-test and Chi-square test were applied, p SD, standard deviation. | |||||
Table 2 presents the comparison of each group regarding embryo transfer outcomes. The implantation rate was 57.8%, 64.3% and 59.7%, respectively (p = 0.857). The clinical pregnancy rate was also similar among the groups (p = 0.929). The live birth rate was 46.7% in the receptive endometrium group, 50% in the non-receptive endometrium group and 51.6% in the control group, and these differences didn’t provide statistical significance (p = 0.879) (Fig. 1). Live birth rate and clinical pregnancy rate were similar between pre- and post-receptive groups in the ERA test group (p= 0.413). In addition, clinical pregnancy loss rates were similar between groups (p = 0.947).
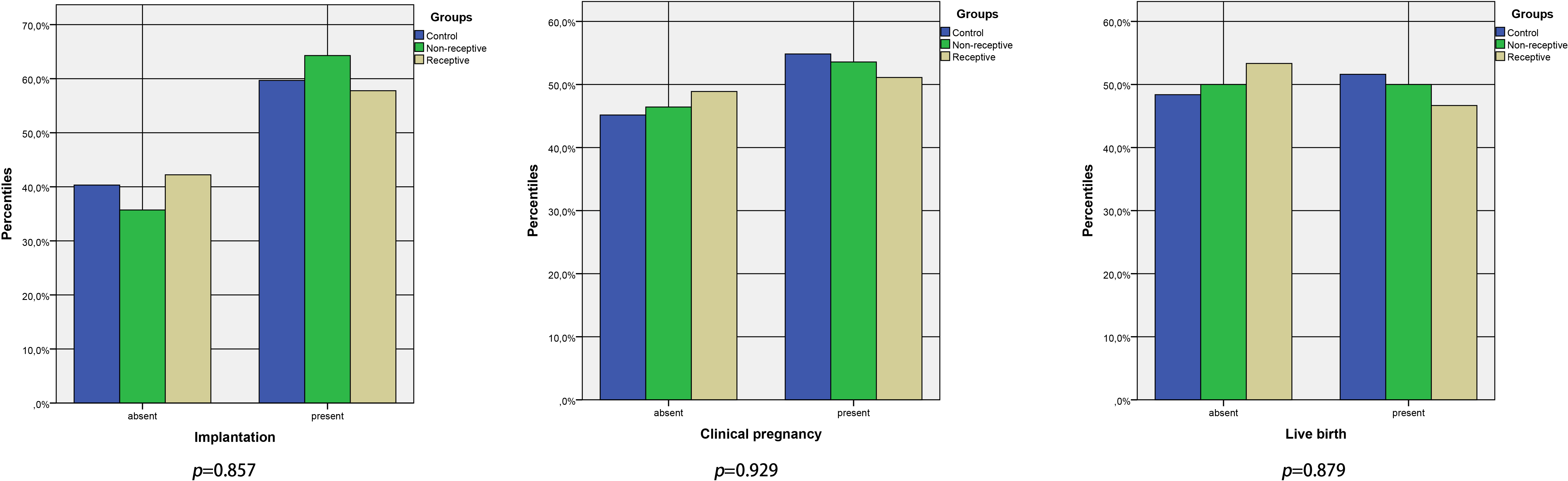 Fig. 1.
Fig. 1.Reproductive outcomes of the patients who were grouped according to their ERA test status.
| Receptive | Non-receptive | Control | p value* | |
| (n = 45) | (n = 28) | (n = 62) | ||
| Positive pregnancy test | 29 (64.4) | 20 (71.4) | 40 (64.5) | 0.788 |
| Implantation | 26 (57.8) | 18 (64.3) | 37 (59.7) | 0.857 |
| Clinical pregnancy | 23 (51.1) | 15 (53.6) | 34 (54.8) | 0.929 |
| Live birth | 21 (46.7) | 14 (50) | 32 (51.6) | 0.879 |
| Data was presented as number (percentiles).
*Chi-square test were applied, p | ||||
The multivariate regression analysis proved that the utility of the ERA test is not a discriminative factor of live birth in those with repeated implantation failure who underwent single, autologous euploid embryo transfer when controlled for the following confounding factors including age, BMI, duration of infertility, number of previous embryo transfers and endometrial thickness (adjusted OR: 0.917, 95% CI: 0.458–1.836, p = 0.806) (Table 3).
| Risk factors | OR (95% CI) | p value* |
| Age (years) | 0.999 (0.916–1.089) | 0.979 |
| BMI (kg/m |
1.044 (0.944–1.155) | 0.405 |
| Period of infertility (years) | 1.019 (0.876–1.186) | 0.806 |
| Number of previous embryo transfers | 0.835 (0.631–1.106) | 0.209 |
| Endometrial thickness (mm) | 1.096 (0.904–1.328) | 0.352 |
| Use of ERA test | 0.917 (0.458–1.836) | 0.806 |
| Used binary logistic regression analysis, p R, odds ratio; CI, confidence interval; BMI, body mass index; ERA, endometrial receptivity array. | ||
To the best of our knowledge, this is one of the largest study that evaluated the utility of the ERA test in patients with repeated implantation failure who underwent single, autologous euploid embryo transfer. Based on our findings, the ERA test was “non-receptive” in 38.4% of patients with the ERA test results. The day of embryo transfer was adjusted in these patients according to the ERA results, which were translated into increased implantation, clinical pregnancy and live birth rates compared with those with receptive endometrium. Moreover, these rates were similar in the control group, as well. Our study showed the use of the ERA test to provide a benefit to the patients with repeated implantation failure who underwent single, autologous euploid embryo transfer, as confirmed in both the univariate and the multivariate analyses.
Depending on our knowledge about the implantation failures, which not always related with embryonic abnormalities; transferring euploid embryos never provides us 100% success rate in IVF. As the important role of the good quality embryo, RE is also very important in successful implantation. Performing the embryo transfer when the endometrium is receptive would improve implantation chances in RIF cases. Tan et al. [15] conducted a study to determine the role of the ERA in patients who were unseccessfull after euploid embryo transfers. They found that implantation and ongoing pregnancy rate after personalized ET were higher compared to patients without personalized ET despite no significance (73.7% vs. 54.2% and 63.2% vs. 41.7%, respectively). Thus, by transferring euploid embryos in a personal WOI, much better PRs are expected.
The first studies on the ERA test have shown that the endometrial receptivity is displaced in 26–39% of infertile women [16]. Thus, it is sensible to expect an improvement in the success of embryo transfer conducted in the receptive stage of endometrium. Histological evaluation of endometrial receptivity dates back to 1980s, limited studies on this subject make the evidence of its effectiveness on implantation limited [17, 18, 19, 20]. In 2011, Diaz et al. [11] described a new method of evaluating endometrial receptivity status, which is based on the analysis of 238 different mRNAs expressed on the endometrium. The results of the ERA test are expressed as pre-receptive, receptive and post-receptive. The timing of ET is personalized in patients with a pre- or post-receptive result. It is well-known that the success of implantation depends on the harmony between the embryo and the endometrium [21]. Even though aneuploid embryos can be eliminated with high accuracy using PGT-A technologies, the receptivity status of the endometrium remains enigmatic [22]. Thus, the premise behind the determination of receptive time of the endometrium seems reasonable. However, it remains to be determined whether building a harmony between the embryo and the endometrium by the ERA test is translated into an increased chance of implantation. Moreover, some authors are concerned about the ERA test as they noticed that a patient can have different ERA results within a narrow period of time [23], which is contrary to the claim that the ERA test is reproducible for 29–40 months [24].
In this study, we observed that all IVF outcomes were comparable between the patients having a receptive endometrium who had a routine ET versus those who had a non-receptive endometrium and underwent a personalized ET (implantation rate: 57.8% vs. 64.3%, p = 0.857, clinical pregnancy rate: 51.1% vs. 53.6%, p = 0.929).
Similar results were obtained by Ruiz‑Alonso who observed that IVF outcomes after personalized ET in non-receptive endometrium cases (implantation rate: 38.5% and pregnancy rate: 50%) and the receptive ERA cases (implantation rate: 33.9% and pregnancy rate: 51.7%) [10]. Thus, clinical output of personalized ET in these RIF patients with non-receptive endometrium was supported by our results where implantation rate and pregnancy rate increased to the level of RIF patients with RE (64.3% vs. 57.8% and 53.6% vs. 51.1%). Even in patients with receptive ERA results, which embryos were transferred in a subsequent HRT cycle, clinical results of pregnancy rates in those RIF were similar to general infertile couples.
Previous studies found that the ERA test could alter the timing of embryo transfer in 25–44% of patients with previous implantation failure [10, 15], which was 38% in our study. Bassil et al. [25] reported a high rate of non-receptive ERA results (64%) in good prognosis patients. In fact, current literature does not provide strong evidence regarding the rate of non-receptive ERA results in different groups of infertile population.
There are few retrospective studies that assessed whether the use of ERA increases the likelihood of implantation and/or live birth in some groups of patients. In these retrospective studies investigating embryos not screened by PGT-A did not show that the adjustment of embryo transfer day based on the ERA test changed pregnancy, implantation and live birth rates [26, 27, 28]. Bassil et al. [25] reported ongoing pregnancy rate as 33% in the non-receptive group, 50% in the receptive group and 35% in the control group. These differences were not statistically significant. Their control group did not undergo the ERA test, as similar to that of our study. However, different from our study, the embryos used in their study were not tested by PGT-A. Only two studies have investigated the utility of the ERA test in euploid embryo transfers. Tan et al. [15] found comparable ongoing and live birth rates between those with non-receptive ERA result and those with receptive ERA result in the study including subsequent euploid embryo transfers of patients with a previously failed euploid embryo transfer. A recent study by Neves et al. [29] remarked the ERA test was significantly associated with decreased pregnancy rates in euploid donor embryo transfers. The data gathered from the above-mentioned studies shows that adjusting embryo transfer day according to the ERA results does not seem to change outcomes. Our study is one of the largest one that evaluated the use of the ERA test in three groups, showing that euploid embryo transfer outcomes were seemed to be affected by whether the ERA test resulted in receptive or non-receptive. Out of phase patients according to ERA test results had change in ET day.
In addition, some studies have found that the ERA test improves IVF outcomes.
Among these studies, the one with the highest level of evidence is the randomized
controlled study of Simon et al. [30] which was recently published. In
this multicenter study, 458 patients were randomized to 3 study arm; personalized
embryo transfers guided by the ERA test result, frozen embryo transfer or fresh
embryo transfer. According to the results of this study, the cumulative live
birth rates were 71%, 55% and 49% for personalized ET, FET and fresh transfer,
respectively (p
In another study, Diaz et al. [31] divided the pre-receptive group into two subgroups as early-receptive and late-receptive groups and the receptive group into two subgroups as optimal receptive and late-receptive groups. The retrospective analysis of data from 771 patients showed that the late-receptive and optimal receptive groups had significantly better IVF results than the late-receptive group. However, we did not analyse subgroups of non-receptive results due to the small sample size.
Our study has certain strengths and limitations. One of the strengths was that we included homogeneous patient population into the study. All patients had a history of at least two failed embryo transfers and subsequently underwent single, autologous euploid embryo transfer. This allowed us to evaluate the effect of the ERA test on embryo transfer outcomes by minimizing selection bias. Most studies in the literature used embryos with unknown ploidy status and thus introduced significant bias into their results. The fact that we used a control group that did not undergo the ERA test was another strength of our study. This group enhanced the accuracy of our comparisons. The retrospective design of our study was main limitation of it. Another limitation was that our sample size was small especially when divided into groups. In addition, we could not evaluate perinatal outcomes of the patients. This would also be a stronger work if there was a separate “uncorrected” non-receptive group for comparison.
Our study suggests that the ERA seems to improve embryo transfer outcomes in patients with repeated implantation failure with corrected non-receptive endometrium. Randomized-controlled studies which compare uncorrected non-receptive groups for comparison are needed to better understand whether the ERA test plays a role in improving outcomes in the context of implantation failure.
SO—protocol/project development; OT—protocol/project development, data collection or management, manuscript writing/editing; HGC—data analysis, manuscript writing/editing; EY—data collection or management; EO—data collection or management; MG—protocol/project development, data collection or management, manuscript writing/editing; JY—manuscript writing/editing; EB—protocol/project development, data collection or management, data analysis, manuscript writing/editing.
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was taken from all couples in the study. The study was approved by Acibadem Mehmet Ali Aydinlar University Medical Research Ethics Committee (ATADEK 2019-10/3). Approval was obtained from ClinicalTrials.gov with NCT03355937 approval number.
The authors would like to thank the participants of this study and to staff of Acibadem Fulya Hospital Embryology Laboratory for their help to access the data.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
