Academic Editor: Valerio Gaetano Vellone
Objective: Superb Microvascular Imaging (SMI) Doppler is a novel technique. We aim to evaluate intra- and interobserver reliability of SMI Doppler for the assessment of normal placental microvasculature. Materials and Methods: A prospective observational study was conducted including 11 pregnant patients. Ultrasonography placental assessment was performed on 28-weeks pregnant patients, by two expert examiners using SMI Doppler in one single visit. To evaluate interobserver reliability, the first examiner took measurements using SMI Doppler, followed then by the second examiner. Afterwards, the first examiner performed a second evaluation, in order to assess intraobserver reliability. Intraclass correlation coefficients (ICC) and Cohen’s Kappa coefficient, and their 95% confidence intervals, were estimated for quantitative and qualitative parameters, respectively. Results: Intraobserver reliability was found to be excellent for all quantitative parameters, with all ICC values above 0.97. For qualitative variables, excellent reliability was obtained for the number of secondary villi, while the number of tertiary villi had an adequate reliability with a Cohen’s Kappa coefficient of 0.792 (95% CI 0.38–1.17; p = 0.007). Interobserver ICCs ranged from 0.92 to 1.00 for all quantitative parameters, thus finding excellent interobserver reliability for all of them. An excellent reliability was also obtained for the number of secondary villi, while the reliability for the number of tertiary villi was found to be adequate. Conclusions: Our findings show that placental microvasculature measurements obtained by a single or two different examiners are reliable and reproducible. The good intra- and interobserver reliability results of SMI Doppler showed in our study stress the value of this technique in the evaluation of placental microvasculature, and thus research in this field is the step forward for the assessment of placental insufficiency.
The placenta is one of the most important human organs, as its correct development is crucial for the sustenance of the pregnancy and a faulty placentation might entail the development of pregnancy complications like intrauterine growth restriction (IUGR), and yet, it remains one of the least understood organs [1]. Currently, evaluation of placental insufficiency is based on the traditional Doppler assessment of the Umbilical Artery (UA), although we know that resistances in the UA begin to increase when at least 70–80% of placental vessels are obliterated [2]. Given the great value of a correct assessment of placental insufficiency, finding techniques to adequately evaluate in vivo placental microvasculature has been a topic of research for many years.
Previous research focused on the use of three-dimensional color Doppler (3D-CD) and three-dimensional power Doppler angiography (3D-PDA) for the assessment of placental vasculature. The first results of this kind of research were quite promising, as authors like Konje et al. [3] were capable to create an in vivo 3-dimensional reconstruction map of the placenta vasculature. This was followed by various publications, with promising results in the depiction of the vascular branching of the villous tree and its intervillous space, as well as its reproducibility and correlation with biometrical fetal parameters [4, 5]. Rizzo et al. [6] tried to apply this technique to detect aneuploidies in the first trimester, which turned out unsuccessful, although they described normal vasculature patterns for normal placentas at this stage. The study published by Jones et al. [7] showed that 3D-PDA had good intra- and interobserver reliability, with intraclass correlation coefficients (ICC) over 0.90 and 0.60, respectively, although they admit the presence of bias in in the evaluation of pregnancies at 20 weeks. Nevertheless, its poor intra- and interobserver reproducibility in third-trimester pregnancies have called into question its clinical role, given that manifestations of placental insufficiencies take place at this stage of pregnancy [8].
Given the recent appearance of superb microvascular imaging (SMI) Doppler, a recently developed technique which uses an advanced algorithm to suppress motion artifacts and signals from overlaying tissue, allowing the capture of low-velocity blood flow vessels, research in this field has emerged. This novel technique has been applied to research on thyroid and other organs [9], and in recent years several authors have begun to use this technique in the obstetrics field. For instance, Hasegawa et al. [10, 11] were the first to use this technique to describe a case of placental infarction and later use it to describe placental microvasculature. Other authors like Hata et al. [12] have followed through, describing the vascular branching of normal placentas, and the application of SMI Doppler allowed them to clearly visualize up until the tertiary villous vessels, along with the jet flow of the spiral arteries. Other authors also applied in the evaluation of the placentas, documenting their experience with this technique with normal placentas, but also with placental anomalies such as adherent placenta adherent and the presence of chorioangiomas [13]. Still all these previous studies were carried out from a rather qualitative angle.
Sainz et al. [14] were the first to describe quantitative objective values of the villous functional unit. They performed a longitudinal study following 26 pregnant women until birth, describing the normal vascular branching process of the placenta throughout the pregnancy, stating that the secondary and tertiary villi were identifiable since the 20th week of pregnancy. They also performed a quantitative analysis of the vascular items of the villous unit, and established references curves for normal pregnancies, thus laying out a potential tool for the assessment of placental microvasculature.
In this study, we aim to take a step further and evaluate intra- and interobserver reliability of SMI Doppler for the assessment of normal placental microvasculature.
A prospective observational cross-sectional study was carried out including 11 pregnant patients who were recruited from the Protocol for Assistance in Pregnancy and Childbirth at Valme University Hospital.
All included patients were white women, with an uncomplicated singleton 28-weeks pregnancy and a low-risk combined first-trimester screening. All patients underwent spontaneous labor at term. Patients with co-morbidities or development of pregnancy complications were excluded from the study. Epidemiological variables collected were: maternal age; parity; MoM of ẞ-human chorionic gonadotropin (ẞ-hcg) and pregnancy-associated plasma protein A (PAPP-A); body mass index (BMI); newborn weight, length and head circumference; Apgar score at 5 minutes; and cord blood pH.
Ultrasonography evaluation was performed by two expert examiners in fetal ultrasound (J.A.S. and R.G.J.) from the Obstetrics Department at the Valme University Hospital. Placental ultrasound captures using SMI Doppler were obtained using a Canon Aplio 500 ultrasound machine (Tokyo, Japan) with a PUT-675 MV-3 probe. First, a conventional ultrasonography assessment was performed, using conventional Color and Spectral Doppler, collecting the following parameters: estimated fetal weight (EFW), EFW percentile, pulsatile index (PI) and peak systolic velocity (PV) of the umbilical artery middle cerebral artery, uterine arteries, and ductus venosus, as well as the cerebroplacental ratio.
Afterwards, we performed the ultrasonography evaluation of the placenta. To this end, the central part of the placenta was located, and then SMI Doppler was applied, following the technique described in our previous study [14]. Quantitative parameters collected were the PI and PV of the basal plate, chorionic plate, and primary, secondary and tertiary villi (Fig. 1). Qualitative parameters collected were the number of secondary and tertiary villi, classified as abundant or sparse depending on whether they occupied more than 50% of the Doppler gate.
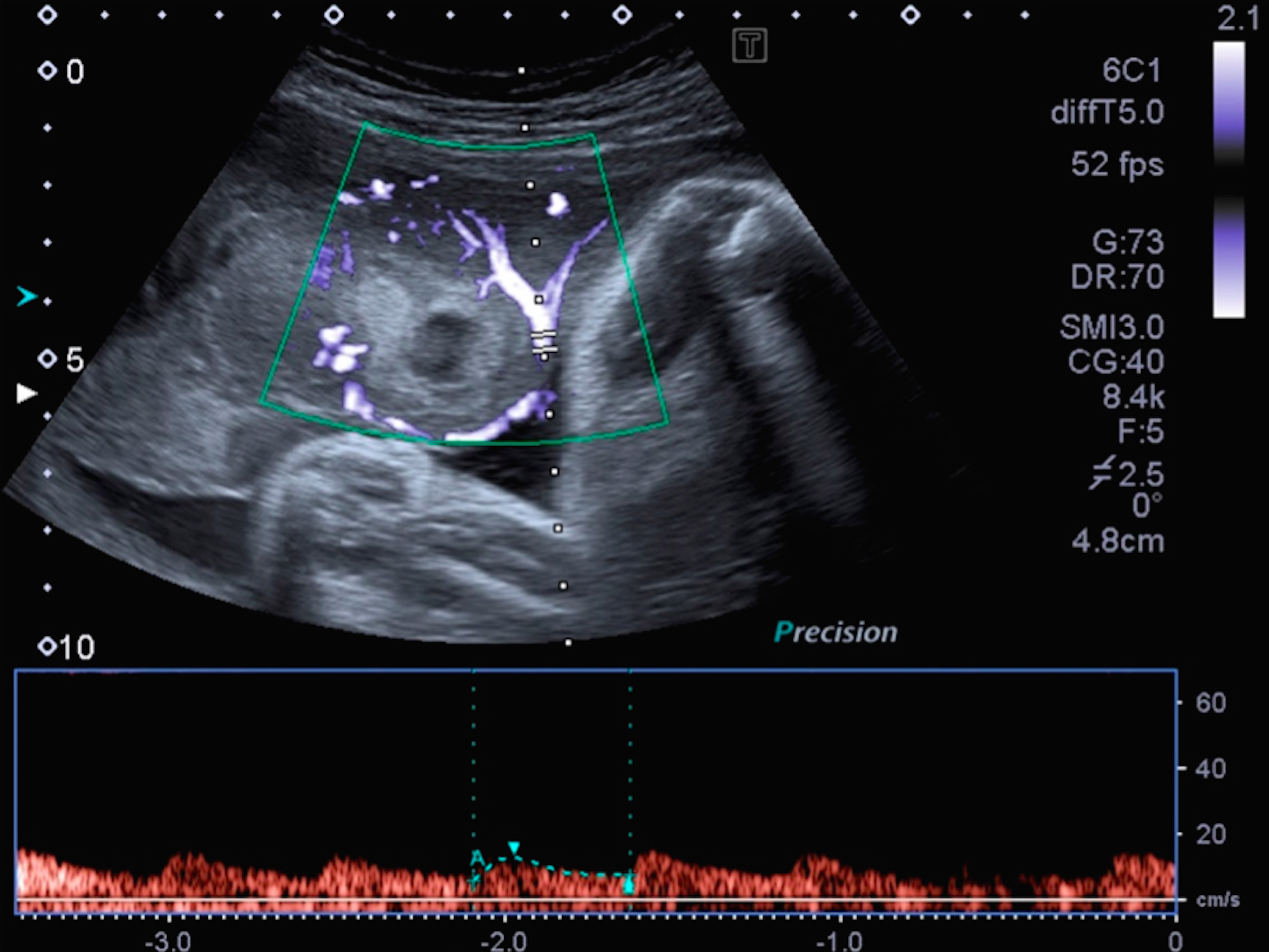 Fig. 1.
Fig. 1.Caption of SMI Doppler ultrasound assessment of the primary villi in a normal placenta.
All patients were evaluated by the two examiners in one single visit at 28 weeks of gestation. To evaluate interobserver reliability, the first examiner took measurements using SMI Doppler, followed then by the second examiner. Afterwards, the first examiner performed a second evaluation, in order to assess intraobserver reliability. During each evaluation, only one examiner was present in the examination room.
After delivery, all placentas were submitted for histopathologic assessment. For this evaluation, at least five full-thickness disk samples were taken for each one, along with samples from the umbilical cord and the membrane roll. To verify the absence of signs of maternal or fetal vascular malperfusion, the analysis was made based on the Amsterdam Placental Workshop Group Consensus Statement [15].
An IBM SPSS Statistics software version 26 (IBM, Armonk, NY, USA) was used to conduct the statistical analysis. Quantitative variables were described with means and standard deviations, while percentages were used for qualitative. The intra- and interobserver concordance was analyzed using intraclass correlation coefficients (ICC) and their 95% confidence intervals (CI), for quantitative variables. For qualitative variables, Cohen’s Kappa concordance coefficients and their 95% CI were used [16]. Reliability values under 0.2 were considered as poor reliability; 0.21–0.40 as fair; 0.41–0.60 as moderate; 0.61–0.80 as adequate; and 0.81–1.00 as excellent reliability. The mean difference between examiners, or biases, was estimated with the Bland-Altman and 95% limits of agreement (LOA) methods [17].
A total of 11 patients were included in the study. The mean age of patients were 30.82 years with a standard deviation of 4.8 years, with a 72.7% of nulliparous patients. Mean newborn weight at birth was 3120.9 grams, and mean Apgar score at 5 minutes was 9.55, with a 77.8% rate of cord blood pH above 7.2. The rest of the epidemiological variables assessed are displayed in Table 1.
| Variables | Median |
| Maternal age | 31.5 |
| Nulliparous | 8 (72.7%) |
| ẞ-hcg (MoM) | 1.2 |
| PAPP-A (MoM) | 1.1 |
| BMI | 25.6 |
| Newborn weight | 3110 |
| Newborn length | 49.3 |
| Newborn head circumference | 33.5 |
| Apgar score at 5 minutes | 10 |
| Cord blood pH |
7 (77.8%) |
| BMI, Body mass index; ẞ-hcg, ẞ human chorionic gonadotropin; PAPP-A, pregnancy-associated plasma protein A; MoM, multiple of the median. | |
In Table 2 the results of the conventional ultrasonography assessment are displayed. Mean EFW of patients was 1198.45 grams, with the mean percentile being 33.55. Mean values of the PI of the umbilical artery and middle cerebral artery were 1.02 and 1.93, respectively. The rest of the Doppler parameters values were normal for the gestational age, as displayed in Table 2.
| Variables | Median |
| EFW | 1206 |
| EFW percentile | 35 |
| Mean PI Uterine arteries | 0.8 |
| Mean PV Uterine arteries | 63.3 |
| PI Umbilical artery | 0.95 |
| PV Umbilical artery | 39.9 |
| PI Middle cerebral artery | 1.9 |
| PV Middle cerebral artery | 34.3 |
| Cerebroplacental ratio | 1.9 |
| PI Ductus venous | 0.4 |
| PV Ductus venous | 26 |
| PI, Pulsatile index; PV, Peak systolic velocity. | |
To evaluate the intraobserver reliability, an expert examiner took measurements of the same patients in two separate occasions. Intraobserver reliability was found to be excellent for all quantitative parameters, with ICCs values above 0.97 in all cases (Table 3). For qualitative variables, excellent reliability was obtained for the number of secondary villi, while the number of tertiary villi had an adequate reliability with a Cohen’s Kappa coefficient of 0.792 (95% CI 0.38–1.17; p = 0.007), as shown in Table 4.
| Variables | Examiner 1 | Examiner 1 | ICC | 95% CI | p |
| 1st measures | 2nd measures | ||||
| PI Basal plate | 0.37 |
0.30 |
0.97 | 0.90–0.99 | |
| PV Basal plate | 17.50 |
18.00 |
1.00 | 0.99–1.00 | |
| PI Chorionic plate | 0.55 |
0.55 |
0.99 | 0.96–0.99 | |
| PV Chorionic plate | 15.60 |
16.00 |
0.99 | 0.99–1.00 | |
| PI Primary villi | 0.75 |
0.75 |
0.98 | 0.94–1.00 | |
| PV Primary villi | 12.10 |
12.10 |
0.99 | 0.99–1.00 | |
| PI Secondary villi | 0.62 |
0.90 |
0.99 | 0.97–1.00 | |
| PV Secondary villi | 9.30 |
9.30 |
0.99 | 0.99–1.00 | |
| PI Tertiary villi | 0.87 |
0.87 |
0.99 | 0.98–0.99 | |
| PV Tertiary villi | 5.80 |
5.80 |
0.99 | 0.99–1.00 | |
| PI, Pulsatile index; PV, Peak systolic velocity; ICC, Intraclass correlation; | |||||
| Variables | Examiner 1 | Examiner 1 | κ | 95% CI | p |
| 1st measures | 2nd measures | ||||
| Abundant secondary villi | 9/11 (81.8%) | 9/11 (81.8%) | 1.00 | 1.00–1.00 | 0.001 |
| Abundant tertiary villi | 3/11 (27.3%) | 4/11 (36.4%) | 0.792 | 0.38–1.17 | 0.007 |
To assess interobserver reliability, measurements of the same patients were taken by two examiners. Interobserver ICCs ranged from 0.92 to 1.00 for all quantitative parameters, thus finding excellent interobserver reliability for all of them, as shown in Table 5. An excellent reliability was also obtained for the number of secondary villi, while the reliability for the number of tertiary villi was found to be adequate (Table 6).
| Variables | Examiner 1 | Examiner 2 | ICC | 95% CI | p |
| PI Basal plate | 0.37 |
0.30 |
0.92 | 0.74–0.98 | |
| PV Basal plate | 17.50 |
18.00 |
1.00 | 0.99–1.00 | |
| PI Chorionic plate | 0.55 |
0.55 |
0.96 | 0.87–0.99 | |
| PV Chorionic plate | 15.60 |
15.90 |
0.97 | 0.92–0.99 | |
| PI Primary villi | 0.75 |
0.57 |
0.93 | 0.75–0.98 | |
| PV Primary villi | 12.10 |
12.10 |
0.99 | 0.97–0.99 | |
| PI Secondary villi | 0.62 |
0.90 |
0.98 | 0.90–0.99 | |
| PV Secondary villi | 9.30 |
9.30 |
0.99 | 0.96–1.00 | |
| PI Tertiary villi | 0.87 |
0.87 |
0.99 | 0.98–1.00 | |
| PV Tertiary villi | 5.80 |
6.00 |
0.99 | 0.98–1.00 | |
| PI, Pulsatile index; PV, Peak systolic velocity; ICC, Intraclass correlation; | |||||
| Variables | Examiner 1 | Examiner 2 | κ | 95% CI | p |
| Abundant secondary villi | 9/11 (81.8%) | 9/11 (81.8%) | 1.00 | 1.00–1.00 | 0.001 |
| Abundant tertiary villi | 3/11 (27.3%) | 4/11 (36.4%) | 0.79 | 0.38–1.17 | 0.007 |
At last, the post-delivery histopathologic examination of the placentas was normal in all cases, without pathologic findings, according to the Amsterdam Placental Workshop Group Consensus Statement [15].
The placenta possesses the ability to reflect several aspects of fetal development and as such has been defined as the “diary of intrauterine life” [18]. Its distinct vasculature is based in a root-like branching with low velocity flow, with the main functional unit being the villous tree surrounded by the intervillous space. The normal development of this particular vascular pattern is crucial for a proper fetal growth and alterations of this process may alter the consequent results, manifesting in the form of IUGR [19]. Given the importance of this entity, it is fundamental to find an adequate technique to assess the placental microcirculation in pursuit of a proper evaluation and management of cases of placental insufficiency.
In recent years, some authors have begun to apply SMI Doppler in obstetrics to evaluate placental microvasculature proving the potential of this technique in the obstetrics field [10, 11, 12, 13]. Although investigation in this field is still scarce, recent studies have been published applying this technique to assess fetal organs [20, 21] or placental anomalies such as the placenta accreta spectrum [22, 23]. With regard to placental vasculature, hitherto most studies have focused on describing the observations made by explores, but it was not until the study made by our group, that objective quantitative values were described for normal placentas. This provided a basis for research in this field, and thus SMI Doppler proved to be a valid technique to evaluate placental microvasculature throughout pregnancy, with normal references curves having been described for quantitative parameters [14].
Furthermore, SMI Doppler has been found to be more effective than conventional
Doppler in the depiction of the villous tree, detecting with more precision the
vessels of the functional unit [24]. In the study made by Sun et al.
[24] SMI Doppler detected significatively a greater number of vessels per unit
than color Doppler flow imaging (CDFI) (0.26/cm
In this study, we intended to take a step further and evaluate the reliability of SMI Doppler for the assessment of placental microvasculature. Our findings have shown that SMI Doppler has excellent intra- and interobserver reliability values in the assessment of placental microvasculature, except for the number of tertiary villi which had adequate reliability in both intra- and interobserver analysis. All quantitative variables, along with the number of secondary villi, showed excellent reliability values, with ICCs and Cohen’s Kappa values above 0.92. The reliability values for the number of tertiary villi, albeit adequate, was lower than for the rest of the parameters (0.792). This might be due to their small size which might cause some difficulty in their visualization and counting. Nevertheless, these results contrast with the reliability results of 3D-PDA and 3D-CD, which showed limited results in the third trimester of pregnancy [7, 8]. All patients included in our study were at their 28th week of gestation, which is especially relevant given that it is at this stage of pregnancy that manifestations of IUGR begin to appear.
Given potential of this technique to evaluate placental function, some authors have begun to research its application to evaluate placental insufficiency, like Hata et al. [25] which first described their experience in a case of a thick placenta with IUGR. In our previous study, we described a series of cases of normal pregnancies, as well as cases of placental insufficiency such as small for gestational age, late IUGR and early IUGR. The results showed lower values for most quantitative parameters in relation to reference curves developed by our group, especially in cases of early on-set IUGR cases. There was also a marked decrease of vascular branching of the villous tree, with scarce secondary and tertiary villi. Although the conclusions made are to be taken cautiously, the results of this study were promising [26]. A recent study made by Furuya et al. [27] evaluated the accuracy of SMI Doppler to properly visualize placental pathologic findings. They found that the highest accuracy was for the detection of placental infarction in cases of IUGR, with a positive predictive value of 100% and sensitivity of 89% (area under the curve of 0.945). This reinforces the potential of this tool for the assessment of placental insufficiency.
To date there are not studies regarding the reproducibility of SMI Doppler in this field, and we consider this to be one of the strengths of our study. Nonetheless, there are limitations of our study, with the small sample size being one of them, along with the fact that the examination was made in a single visit, which might have affected the intraobserver reliability. Future research should investigate if this reproducibility and accuracy values remains the same in pathologic pregnancies like fetal growth restriction or placental infarction, as well as with more than two examiners. Despite all, ours remains the only study to assess the reproducibility of SMI-Doppler to assess placental microvasculature.
Although the usefulness of SMI Doppler for the assessment of normal placental microvasculature has been broadly endorsed, specific studies are needed for the application of this technique to placental insufficiency which might allow to improve management of these complex cases.
Our findings show that placental microvasculature measurements obtained by a single or two different examiners are reliable and reproducible in the third trimester of pregnancy. The good intra- and interobserver reliability results of SMI Doppler showed in our study stress the value of this technique in the evaluation of placental microvasculature, and thus research in this field is the step forward for the assessment of placental insufficiency.
JASB designed the research study. CB, IV and RGJ performed the research. AFP and RGJ analyzed the data. RGJ and JAGM wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Ethical approval was given by the Biomedical Ethics Committee of Valme University Hospital (1001-N-18). Informed consent was obtained from all patients to participate in the study.
Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest. JAGM is serving as one of the Guest editors of this journal. We declare that JAGM had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to VGV.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
