†These authors contributed equally.
Academic Editor: Michael H. Dahan
Objective: Ovarian carcinoma is a malignant tumor with the highest mortality of any cancer occurring in female reproductive system. Cytoreductive surgery is the main treatment for ovarian cancer and has markedly improved. Mechanism: This article discusses the evolution and development of ovarian cancer cytoreductive surgery (CRS), including classical standard tumor cell reduction, visceral-peritoneal debulking (VPD) and ultra-radical cytoreduction (URC). Findings in Brief: we reviewed CRS in combination with radiotherapy, chemotherapy and immunotherapy for ovarian cancer (OC). Finally, we discussed the opportunity and challenges of ROC therapeutic. Conclusions: This study reveals that CRS and combination therapy can help clinicians to find the optimum treatment for ovarian cancer (OC).
Ovarian cancer is the malignant tumor that has the highest mortality rate of any malignancy in the female reproductive system. The incidence of ovarian cancer has increased yearly along with the trend of population aging [1, 2, 3, 4]. Frequently the early symptoms of the disease are not obvious, its onset is hidden, and the vast majority of patients are diagnosed in an advanced stage [3, 4]. Radical cytoreductive surgery (CRS) is a core treatment strategy that significantly improves patient survival [1]. Tumor cytoreduction has undergone the evolution of classical standard tumor cytoreduction (SC), visceral-peritoneal debulking (VPD) and ultra-radical cytoreduction (URC). The development process of tumor cytoreduction in advanced ovarian cancer is described in Fig. 1. This study reviews CRS and combination therapy which can assist clinicians in determining the optimum treatment for ovarian cancer (OC).
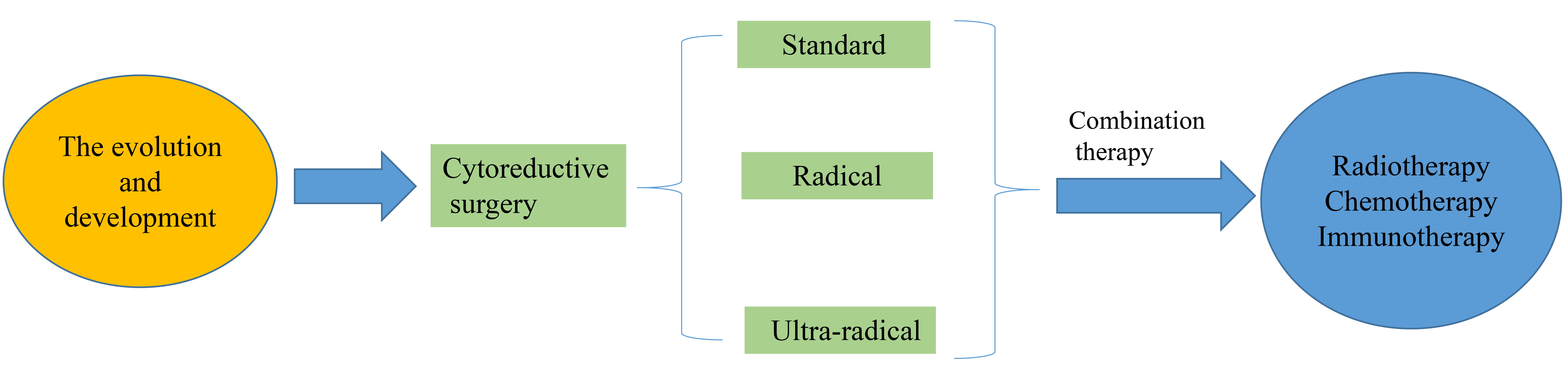 Fig. 1.
Fig. 1.schematic of review process in cytoreductive surgery (CRS).
Prior to the 18th century, ovarian cysts were considered incurable diseases [5, 6]. In 1775, William Hunter of Scotland extracted ovarian sac fluid. In 1807, Samuel Hartman first removed an ovarian cyst. In 1809, Dr. Ephraim McDowell of the University hospital of Kentucky reported oophorectomy in 2 cases of ovarian tumors. In 1934, Meigs et al. [7] proposed the idea that surgical removal of all visible tumor foci can help improve the prognosis of ovarian cancer patients. In 1969, Elclos and colleagues showed that maximal resection of lesions was beneficial in order to improve survival by comparing the prognosis of patients with or without residual tumor tissue after surgery [8]. In 1975, Griffiths et al. [9] further demonstrated this theory and popularized the procedure. Subsequent clinical studies have demonstrated that maximal tumor tissue debulking is directly related to patient prognosis [9, 10].
The concept of optimal cytoreduction has a long history with early studies
suggesting that patients with residual tumor tissue
The procedure for classic standard cytoreduction includes uterine and ovarian removal, omentectomy, appendectomy, lesions in the pelvic cavity and para-aortic lymphadenectomy [13]. According to the literature, postoperative survival time in patients who meet this criterion is significantly higher than in patients with unsatisfactory tumor reduction [14]. Lymphadenectomy is an important step in cytoreductive ovarian cancer, but it increases the duration of surgery, length of postoperative hospital stay cost of surgery and results in many postoperative complications due to the high incidence of pelvic and para-aortic lymph node metastasis [15]. There has resulted in a widespread debate about the need for lymph node dissection.
The first randomized clinical trial evaluating the role of lymphadenectomy in
advanced ovarian cancer was reported in 427 patients with advanced ovarian cancer
who underwent systemic lymphadenectomy or only resection of enlarged lymph nodes.
Results suggested that lymphadenectomy significantly improved progression-free
survival (29.4 months and 22.4 months) and reduced the rate of disease recurrence
but did not improve overall survival (respectively 58.7 months and 56.3 months).
European Society for Medical Oncology (ESMO) guidelines recommend that the
removal of enlarged lymph nodes is currently part of achieving satisfactory
debulking, but total lymphadenectomy should not be considered a standard
procedure until clinical trial results are available. The steps of pelvic
resection could be performed with different modalities including laparotomy,
laparoscopy or robotic [16]. National Comprehensive Cancer Network (NCCN)
guidelines recommend that attention be paid to the removal of suspicious or
enlarged lymph nodes, and that there is no need to remove clinically diagnosed
negative lymph nodes. Patients with extra pelvic tumor lesions
Radical neoplastic cytoreduction is a definitive procedure with resection of pelvic organs such as the rectum, sigmoid colon and pelvic peritoneum. In 1968, Hudson formally proposed a procedure in which the uterine appendages, affected intestinal tubes and all pelvic peritoneum are removed, resulting in a better prognosis for patients after surgery [17]. In recent years, the concept of holistic resection surgery has gradually developed and been perfected as ovarian cancer is a peritoneal disease with the peritoneum being both a transmission route and a barrier to limit the metastasis to retroperitoneal organs. En-bloc resection of the pelvis (EnBRP) is defined as the removal of all pelvic organs and peritoneum except the bladder. The specific surgical procedure is as follows: (1) entering the retroperitoneal space, free the ureter, ligation of pelvic funnel ligament; (2) truncation of sigmoid colon; (3) free the sigmoid colon from the sacrum by electrocoagulation and excision of the sigmoid mesentery; (4) enter the anterior sacral space; (5) free bladder peritoneum; (6) incision of the anterior vaginal wall; (7) retrograde excision of para-uterine tissues; (8) incise the posterior vaginal wall and enter the recto-vaginal space; (9) separation by electrocoagulation to remove pararectal tissue; 10 rectal or intestinal anastomosis. The goal of this procedure is to completely remove all pelvic lesions and reduce bleeding. Tozzi et al. [18] indicated that 98 patients with advanced ovarian cancer received EnBRP with all receiving satisfactory debulking with no intraoperative deaths and only two patients developing intestinal anastomosis fistula (2%). This procedure has been standardized and is safe and feasible for patients with advanced ovarian cancer. Diaphragmatic surgery and consequent pleural effusion have been reported following this procedure [19].
In 2013, NICE proposed “ultra-radical” surgery for patients with advanced ovarian cancer. Ultra-radical surgery includes diaphragmatic resection, extensive peritoneal resection, splenectomy, cholecystectomy, endometrectomy, gastrectomy, and hepatic resection. With the expansion of the scope of surgery and the extension of the operative time, postoperative complications will increase. A retrospective analysis of study data from multiple centers in NICE in 2019 found that the postoperative complications of ultra-radical tumor cytoreductive reduction were not significantly different from the standard procedure [20].
Ultra-radical tumor cytoreduction is radical surgery along with any of the following surgical procedures: extensive peritoneal resection including partial diaphragmatic resection, diaphragmatic resection and repair; resection of hepatic surface lesions and exploration of the hepatic hilar area; splenectomy and pancreatic tail resection; other intestinal tube resection, partial gastric resection, etc.; and tumor resection at the thoracic cavity, such as excision of lymph nodes with angular enlargement of the heart. After the promotion of NICE recommendations, the operation has been performed in numerous locations for patients with advanced ovarian cancer. When the classic standard surgery has difficulty in achieving satisfactory tumor reduction, ultra-radical tumor cytoreduction is the recommended treatment method. Due to the complexity of the surgical procedure, which involves the removal of multiple organs of the epigastric, hepatobiliary, pancreas and spleen, multidisciplinary teamwork is required to minimize complications. Patsner [21] laser-cauterized diaphragmatic lesions in three patients with ovarian cancer, successfully removing isolated lesions of diaphragmatic metastases.
Subsequently, the international centers carried out and reported the analysis of diaphragm resection surgery and its postoperative complications in ovarian cancer. The results of multi-center studies consistently showed that diaphragmatic resection can improve the extent of tumor reduction. Thus, diaphragmatic excision is fully feasible [22], although there are pulmonary complications such as pleural effusion, pulmonary embolism, pneumothorax and lung infection.
Advanced ovarian cancer usually
metastasizes to the cardiophrenic lymph node (CPLN) and is usually seen on
preoperative imaging. The significance of diaphragmatic lymphadenopathy is
unclear, but in 2007 Lim et al. [23] defined CPLN diameter
Most patients with advanced ovarian cancer have metastasis to the peritoneum in the epigastric region, which is the main obstacle to achieving satisfactory debulking. In recent years, procedures such as pelvic peritoneal resection have been used in patients with advanced ovarian cancer with peritoneal metastasis and have achieved a satisfactory tumor reduction rate of 60 percent. Other investigators compared surgery vs chemotherapy for ovarian cancer recurrence to explore the best options in cases of ovarian recurrence [27].
There have been few reports of total peritoneal resection in patients with ovarian cancer. Total peritectomy is a viable resection method for patients with peritoneal metastases in advanced ovarian cancer, helping to achieve satisfactory attenuation and improve prognosis. At present, the resection of the whole peritoneum is widely promoted in Europe and the United States.
Tumor reduction surgery to remove the pancreas is relatively rare with numerous postoperative complications. Abdominal abscesses are usually caused by incomplete drainage and frequently require secondary open surgery [28, 29, 30, 31, 32]. Pancreatic resection surgery is complex and there are many postoperative complications, so multidisciplinary cooperation is required to timely discover and deal with postoperative complications.
Total colectomy has a risk of long-term postoperative diarrhea and anemia, and is only suitable for patients who are younger and have lesions involving multi-segment bowel and mesenteric root diffuse involvement. Postoperative diarrhea is frequently long-term and prolonged use of loperamide is required [33, 34].
Patients with advanced ovarian cancer have a heavy tumor load and may require
extensive radical surgery which poses a challenge for centers or surgeons who are
not familiar with these procedures. Pleural effusion, malnutrition and lymphedema
may lead to a decline in physical function, further limiting the recovery ability
of ovarian cancer patients after surgery. In addition, many elderly patients
suffer from multiple comorbidities. Therefore, in order to improve the rate of
satisfactory tumor reduction and minimize surgical complications, neoadjuvant
chemotherapy (NACT) came into being
[35, 36, 37]. Compared with initial cytoreductive surgery, patients with NACT have low
blood loss, low transfusion rates, short surgical time, low incidence of serious
complications, short postoperative hospital stays, short ICU hospital stays, low
bowel resection rates, low splenectomy rates, a significantly lower frequency of
tumor invasion into the appendix and a low incidence of permanent colostomy [38].
A randomized controlled trial of 670 cases of primary ovarian cancer in
EORTC-NCIC demonstrated the need for NACT to reduce extensive surgery, in which
NACT reduced patients with metastases
A retrospective study conducted by the Korea National Cancer Center in 140 and 116 patients with advanced ovarian cancer who underwent primary tumor cytoreduction and NACT+ intermediate tumor cytoreduction, respectively, showed that NACT may improve patient outcomes, reduce blood loss and surgical complexity, and have a satisfactory tumor reduction rate similar to that of the primary tumor cytoreductive group, and that NACT can increase the success rate of tumor reduction surgery without affecting overall survival. However, even with the help of NACT, hyper radical resection is necessary to achieve satisfactory tumor reduction with 60 percent of patients receiving NACT still undergoing ultra-radical tumor cytoreduction [43].
In summary, a significant proportion of patients who receive NACT still require radical tumor cytoreductive or ultra-radical tumor cytoreductive surgery. While NACT may reduce surgical complexity and provide a greater chance for satisfactory tumor reduction, NACT has not completely eliminated the need for extensive surgery in ovarian cancer patients. If the goal is to achieve satisfactory tumor reduction, it is often necessary to require ultra-radical surgery [39, 41]. Target therapies as Poly (ADP-ribose) polymerase (PARP) [44] are also rising strategies for combination therapy.
With the development of three-dimensional conformal radiotherapy and intensity-modulated radiotherapy and the increase of concurrent chemoradiotherapy, radiotherapy for ovarian cancer has made new progress. Although chemotherapy has a good effect on advanced EOC, especially chemotherapy based on paclitaxel and cisplatin which has a significant short-term effect, it does not significantly improve survival [45]. The combination of radiotherapy and chemotherapy can play a synergistic anticancer role. Studies have shown [10] that secondary tumor cell reduction for platinum-sensitive patients can effectively prolong postoperative survival, but there is no clear effect on the effect of secondary surgery on drug-resistant patients. Because ovarian epithelial cancer cells are more sensitive to radiation, combinations with radiotherapy can effectively improve the therapeutic effect. Local radiotherapy can effectively relieve tumor pain, but the recurrent foci of ovarian cancer are mostly located in the abdomen and pelvis, so it is necessary to perform full abdominal radiotherapy for residual tumor to obtain the ideal therapeutic effect. The toxicity of radiotherapy is mild and well tolerated. Concurrent chemoradiotherapy for advanced ovarian cancer, especially for elderly patients, has been shown to be effective in the near term when surgery and chemotherapy are not suitable. At present, there are few studies on Biological Intensity Modulated Radiation Therapy (BIMRT) therapy [46], but it has been confirmed that BIMRT can improve the quality of life of cancer patients and is well tolerated. Dang et al. [47] reported that a 68-year-old female patient with ovarian cancer with abdominal metastasis received concurrent radiotherapy and chemotherapy with the tumor disappearing and the tumor markers of CA-125, CA19-9 and Carcinoma Embryonic Antigen (CEA) decreasing to normal levels. The patient’s symptoms and signs were significantly improved, and the survival time was prolonged. The use of Positron Emission Tomography-Computed Tomography (PET/CT) before and after treatment demonstrates its advantages combined with radiotherapy in tumor search, border demarcation and therapeutic effect evaluation [48]. Rochet et al. [49] verified IMRT total abdominal radiotherapy combined with surgery and chemotherapy in a phase I clinical trial.
The feasibility and effectiveness of IMRT in treating patients with advanced ovarian cancer suggest that IMRT is a novel therapeutic option for treating advanced EOC [46, 47, 50]. Advanced EOC has a high rate of recurrence, and consolidation therapies including total abdominal irradiation (WAR) have had limited success. Shetty et al. [51] reported a feasibility study, in which the whole abdomen (PTV) irradiation dose was 25 Gy/25 times and a single dose was 1Gy for 8 patients by using spiral tomography radiotherapy, and the PTV irradiation dose in the pelvic cavity was 45 Gy/25 times and a single dose was 1.8 Gy. The median follow-up time was 15 months. Five patients recovered and three had abdominal recurrence. These results demonstrate that total abdominal irradiation with IMRT can be sustained and accepted as consolidation therapy in patients with epithelial recurrent ovarian cancer at FIGO-III. It is feasible to use PET-CT, spiral tomography and CT techniques for radiotherapy of ovarian cancer with the clinical effect of PET-CT-guided radiotherapy being better.
The direction of ovarian cancer treatment in the future requires comprehensive treatment and immunotherapy. Immunotherapy, as an important component of comprehensive therapy, has recently gained medical attention. The prepared mouse monoclonal anti-idiotypic antibody 6B11 (Ab2) mimics antigen. Oc166-9 can bind to COC166-9 (Ab1) Mab and inhibit it in animal models. Anti-idiotypic antibodies can be induced by anti idiotypic antibody 6B11 (Ab3) and cellular immunity, so the antibody has the potential to act as an anti-idiotype. Antibody vaccine has been used in the treatment of ovarian cancer [52]. Immune cells (CIK, DC, etc.) were injected into the body for biotherapy. Currently, high immune system and combined immunotherapy with radiotherapy and chemotherapy, in addition to CRS has been utilized to treat ovaries cancer.
Hyperthermic intraperitoneal chemotherapy (HIPEC) is an innovative surgical technique for the treatment of primary and metastatic abdominal tumors, including certain gynecological cancers [53]. It uses highly concentrated, heated chemotherapy directly into the abdomen during surgery. After the tumor is surgically removed, heated chemotherapy fluid is circulated through the abdomen. HIPEC delivers chemotherapy directly to cancer cells in the abdomen, allowing higher doses of chemotherapy to be used and improving the effectiveness of treatment, as it is better absorbed by tumors and microscopic cancer cells remaining in the abdomen [54].
Successful implementation of satisfactory cytoreductive surgery relies heavily on surgical skills, multidisciplinary team collaboration and perioperative management of critically ill patients. The diagnosis and treatment level of ultra-radical surgery for advanced ovarian cancer in China has lagged behind other countries and the level of diagnosis and treatment is varied among centers. As a country with a relatively high incidence of ovarian cancer, China urgently needs to strengthen regional and international exchanges, standardize the surgical quality control for advanced ovarian cancer [55, 56, 57], form a domestic multidisciplinary team to jointly learn and discuss cooperation, and improve the specialized surgical ability and multidisciplinary team collaboration ability for ovarian cancer in order to improve the diagnosis and treatment level of advanced ovarian cancer patients.
We reviewed CRS in combination with radiotherapy, chemotherapy and immunotherapy for ovarian cancer (OC). And, we discussed the opportunity and challenges of ROC therapeutic. Therefore, this review reveals that CRS and combination therapy can help clinicians to find the optimum treatment for OC.
XLL, WYC, TQ, and YLD designed the review, wrote and edited the manuscript. All authors read and approved the final version.
Not applicable.
Not applicable.
This research is supported by the Liuzhou Municipal Science and Technology Bureau (No. 2018BJ10502) and Self-financed Scientific Research Program of Guangxi Public Health Department (No. Z20190250) (No. Z20190359).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
