1 Department of Clinical Laboratory, Women’s Hospital, School of Medicine, Zhejiang University, 310006 Hangzhou, Zhejiang, China
2 School of Obstetrics and Gynecology, School of Medicine, Zhejiang University, 310006 Hangzhou, Zhejiang, China
3 Traditional Chinese Medicine Department, Women’s Hospital, School of Medicine, Zhejiang University, 310006 Hangzhou, Zhejiang, China
†These authors contributed equally.
Academic Editor: Johannes Ott
Abstract
Background: To investigate the correlations between serum levels of tumor markers [including carbohydrate antigen 125 (CA125), carcino-embryonic antigen (CEA) and alpha fetoprotein (AFP)] and the total testosterone of the patients with polycystic ovary syndrome (PCOS). Methods: The data was collected from 890 healthy Chinese women and 480 women with PCOS, including serum total testosterone (TTE), follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), progesterone (PGN), prolactin (PRL), CA125, AFP and CEA. The serum levels of reproductive hormone and tumor markers in the women with PCOS were compared among the subgroups in accordance with the classification of TTE quartiles. To further explore the association between CA125, AFP, CEA and the TTE levels, Spearman correlation analysis was performed. Results: PCOS had significantly lower CA125, and higher AFP and CEA levels in the serum than the healthy controls (p = 0.000, p = 0.015 and p = 0.001, respectively). Four subgroups divided by TTE showed significant differences in CA125 levels (p = 0.017). The Spearman correlation analysis also showed that CA125 was significantly negatively associated with serum TTE levels (p = 0.022). Conclusions: The serum level of CA125 is significantly lower and significantly correlated to the hormonal status of PCOS. AFP and CEA are significantly higher in PCOS.
Keywords
- polycystic ovary syndrome
- tumor marker
- total testosterone
- reproductive hormone
Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder, with a prevalence of about 6% to 21% in women of reproductive age [1]. The most commonly used criteria for diagnosing PCOS is the Rotterdam criterion proposed by The American Society for Reproductive Medicine in 2003 [2], which includes: (1) oligomenorrhoea or chronic anovulation; (2) the features of clinical and/or biochemical signs of hyperandrogenism; (3) polycystic ovaries on ultrasonography. In addition, congenital adrenal hyperplasia, Cushing’s syndrome, androgen secreting tumor and other diseases should be excluded. Hyperandrogenemia is the most prominent clinical manifestation of PCOS which might result in cutaneous manifestations, like hirsutism, acne and alopecia. Androgens of female are mainly derived from adrenal cortex and ovary, and some from peripheral tissues. A total of five types of androgens in women are dehydroepiandrosterone sulfate (DHEAS), dehydroepiandrosterone (DHEA), androstenedione (A4), testosterone (TTE), and dihydrotestosterone (DHT) [3]. Luteinizing hormone (LH) is a glycoprotein gonadotropin secreted by adenohypophysial cells that promotes the conversion of cholesterol into steroid hormone such as TTE, estradiol (E2) and progesterone (PGN) in gonadal cells. Excessive androgen promotes the development of PCOS by inducing apoptosis, autophagy, mitochondrial dysfunction and endoplasmic reticulum stress of granulosa cells (GCs) and oocytes [4]. Another notable feature of PCOS is ovulatory dysfunction and chronic oligomenorrhoea and consequent infertility. The possible causes of chronic anovulation in PCOS are as follows: (1) endocrine and metabolic abnormalities like hyperinsulinaemia (and/or insulin resistance); (2) endometrial dysfunction or impaired oocyte function; (3) a global up-regulation of steroidogenic enzymes in theca cells and inappropriate (premature) responsiveness to LH in GCs of part small antral follicles; (4) abnormal preantral folliculogenesis resulting from an imbalance between GCs number and oocyte size and decreased expression of anti-mullerian hormone (AMH) in small pre-antral follicles [5]. Besides, PCOS is associated with an increased risk of complications such as type 2 diabetes mellitus, cardiovascular and cerebrovascular disease, mood disorders and risks of cancers [6, 7].
Carbohydrate antigen 125 (CA125) is a glycoprotein antigen recognized by murine monoclonal antibody OCl25 prepared with ovarian epithelial cancer cell line OVCA433 as an antigen reported by American scientists Bast et al. [8] in 1981. The exact molecular characteristics of CA125 was defined until 2001 when Yin et al. [9] sequenced the first CA125 complementary DNA (cDNA) clone encoding a mucin-like glycoprotein designated Mucin 16 (MUC16) [9, 10]. Despite extensive and fundamental research, our knowledge of its biological role has remained elusive. Radioimmunoassay test was used to detect elevated CA125 levels in 1% of normal women, 6% of patients with benign disease, 28% of patients with non-gynecological cancers and 82% of patients with surgically demonstrated ovarian carcinoma based on a cut-off value of 35 U/mL [11]. Many other physiological or pathological conditions such as menstruation, pregnancy, endometriosis and pleural effusion led to elevated serum CA125 levels [12]. However, CA125 still functions as the most reliable tumor marker for ovarian carcinoma, being used to early detection of ovarian carcinoma as well as differential diagnosis of benign tumors [13]. The relationship between PCOS and ovarian carcinoma was unclear. Two previous studies had reported an increased risk of ovarian cancer among PCOS compared with healthy women [14, 15], while Olsen et al. [16] demonstrated that PCOS was not associated with ovarian carcinoma.
Alpha fetoprotein (AFP) is a glycoprotein that belongs to the albumin family and is first synthesized by fetal liver cells and yolk sacs during the first trimester of pregnancy. It was first identified in human fetal serum but not adult serum in 1956 [17]. AFP has a high concentration in fetal blood circulation and peaks at 12–16 weeks of gestation but decreases after birth. The content of AFP drops to normal by the age of two years and remains low in adult serum in normal physiology [18]. AFP has many important physiological functions, including transport function, bidirectional regulation function as a growth regulator, immunosuppression, apoptosis induction, etc. [19]. AFP is closely related to the occurrence and development of hepatocellular carcinoma (HCC) and is mainly used as a serum marker for primary HCC in clinical diagnosis, prognosis, and transplant selection [20]. Elevations in serum AFP levels have also been reported in many other non-neoplastic diseases such as germ cell tumors, colitis, gastric cancer, ataxia telangiectasia, acute hepatitis, chronic liver diseases and cirrhosis [21]. The serum levels of AFP have also been found to be altered in some ovarian lesions. Ting Tang et al. [22] observed that the serum levels of AFP in 190 patients with ovarian endometriosis were obviously downward in comparison with 103 healthy subjects. Morever, the value of AFP, CA125, CA199, and HE4 can be combined to distinguish the two groups, with high sensitivity and specificity [22]. A literature review revealed that serum AFP increased in ovarian sertoli-leydig cell tumors [23].
Carcino-embryonic antigen (CEA) was first described in human colon cancer tissue extracts in 1965 [24]. It is a glycoprotein with a molecular weight of approximately 200 kilodalton (kDa). CEA was used as a specific marker for the early diagnosis of colon cancer and rectal cancer. Through a large number of clinical practices, in addition to gastrointestinal malignancies, an increasing trend of CEA was also found in serum of breast cancer, carcinoma of the lungs and other malignant tumors [25, 26]. What’s more, CEA levels could be influenced by some other factors such as smoking, sex gender, age and obesity [27, 28]. Therefore, CEA is usually not used as a specific index forcertain malignant tumors, but as a broad-spectrum tumor marker, which plays an important clinical value in the differentialdiagnosis, disease surveillance and efficacy evaluation of malignant tumors. Previous reports supported that CEA was a useful marker in the diagnosis and assessment of cervical carcinoma [29, 30]. CEA can function as cell adhesion molecules when expressed on the tumor cell surface [31, 32]. CEA may also play a part in innate immune defense, protecting colon as well as the upper digestive tract, bladder and skin sweat glands from microbial attack [33].
Very little was found in the literature on the relationship between tumor markers CA125, AFP and CEA and PCOS. This research aims at exploring the association of tumor markers CA125, AFP and CEA with total testosterone of PCOS based on a large population-based cohort.
The study population consisted of the patients who attended the Outpatient Department of Women’s Hospital, School of Medicine, Zhejiang University between January 2010 and May 2020. The study was approved by Institutional Review Board of Women’s Hospital, School of Medicine, Zhejiang University. Four hundred and eighty patients with PCOS aged 18 to 40 years were included in the study. PCOS was diagnosed in accordance with the Rotterdam criteria (ESHRE/ASRM criteria) [2]. A group of healthy women (n = 890) who came to our hospital for physical examination during the same period were matched for age and included as controls. Patients who had 21-hydroxylase-deficient non-classical adrenal hyperplasia; hyperandrogenism and acanthosis nigricans syndrome; hyperprolactinemia; Cushing syndrome; pregnancy; oncological diseases, and those using any medication (e.g., oral contraceptive pills, antilipidemic drugs and steroid medications) within 6 months of their initial visit were excluded.
The venous blood was collected from all the included women after an overnight fasting period of 12 hours from the 3rd to 5th days of the menstrual cycle or random time of the irregular menstrual cycle.
The hormones including total testosterone (TTE), follicle stimulating hormone (FSH), LH, E2, PGN and prolactin (PRL) were measured with the use of an electrochemiluminescence immunoassay method (Cobas 8000 e-602 analyzer, Roche Diagnostics Ltd, Mannheim, Germany). CA125, CEA and AFP were estimated by electrochemiluminescence immunoassay method (Cobas 8000 e-602 analyzer, Roche Diagnostics Ltd, Mannheim, Germany).
Statistical analysis of the data was performed using the SPSS statistical
software package, version 26.0 (IBM, Armonk, NY, USA). Continuous variable data
were analyzed by the Kolmogorov Smirnov test to determine whether they were
distributed normally. The data was expressed as medians with 25% and 75%
quartiles as all variables were not normally distributed. Age and tumor markers
were compared between PCOS and control groups using the Mann-Whitney U test. The
Kruskal–Wallis H test was performed among four subgroups divided by the
quartiles of TTE. To further explore the association between TTE and tumor
markers, the Spearman’s correlation analysis was conducted and the scatter
diagram was drawn considering AFP, CA125 and CEA as dependent variables and age
as well as TTE as the independent variables. All of the tests were two-sided, and
a p-value
A total of 1370 blood samples was examined, of which 480 were from PCOS, and 890 were from healthy women. As shown in Table 1, there was no significant difference in age between the two groups. The serum levels of AFP and CEA were significantly higher, yet the level of CA125 was significantly lower in PCOS women than the control group (p = 0.015, p = 0.001 and p = 0.000, respectively).
| Terms | PCOS (N = 480) | Control (N = 890) | p-value |
| Age (Years) | 28 (26–30) | 28 (26–31) | 0.388 |
| AFP (ng/mL) | 2.20 (1.50–3.10) | 2.00 (1.40–2.80) | 0.015 |
| CA125 (U/mL) | 12.30 (9.13–16.70) | 15.70 (11.58–20.20) | 0.000 |
| CEA (ng/mL) | 1.10 (0.80–1.60) | 1.00 (0.70–1.50) | 0.001 |
| Note: Data were presented as medians with 25% and 75% quartiles. PCOS, polycystic ovary syndrome; AFP, alpha fetoprotein; CA125, carbohydrate antigen 125; CEA, carcino-embryonic antigen. Comparisons were made using Mann-Whitney U test. | |||
To analyze the association between TTE and tumor markers as well as metabolic parameters in PCOS patients. The included PCOS women were divided into four subgroups according to the quartiles of TTE and analyzed their differences. As shown in Table 2, age, E2, LH, LH/FSH and CA125 levels demonstrated significant differences among the subgroups (p = 0.002, p = 0.003, p = 0.000, p = 0.000 and p = 0.017, respectively). The results showed that in lower TTE quartile, age and CA125 were higher, while E2, LH and LH/FSH were lower.
| Items | TTE |
1.0 |
1.4 |
TTE |
p-value |
| Age (Years) | 29 (26–31) | 28 (26–30) | 28 (26–31) | 27 (25–29) | 0.002 |
| E2 (pmol/L) | 129.60 (93.62–187.50) | 139.50 (100.15–174.30) | 149.20 (110.65–176.65) | 164.30 (116.70–212.90) | 0.003 |
| LH (IU/L) | 7.68 (4.54–12.19) | 11.00 (6.90–16.24) | 13.92 (8.71–18.03) | 13.16 (8.88–17.25) | 0.000 |
| FSH (IU/L) | 5.98 (4.56–6.94) | 5.87 (5.03–6.99) | 6.09 (5.33–6.76) | 5.59 (4.83–6.48) | 0.234 |
| LH/FSH | 1.35 (0.91–2.12) | 1.93 (1.30–2.52) | 2.24 (1.57–2.88) | 2.23 (1.63–3.16) | 0.000 |
| PGN (nmol/L) | 0.99 (0.67–1.70) | 1.09 (0.77–1.65) | 1.14 (0.73–1.48) | 1.24 (0.82–2.05) | 0.054 |
| PRL (ng/mL) | 15.10 (10.60–21.10) | 14.90 (10.85–20.90) | 15.10 (11.00–19.90) | 14.30 (11.40–18.90) | 0.905 |
| AFP (ng/mL) | 2.20 (1.50–3.20) | 1.90 (1.35–2.80) | 2.20 (1.60–3.00) | 2.30 (1.40–3.30) | 0.188 |
| CA125 (U/mL) | 12.60 (9.80–16.90) | 13.20 (9.85–17.70) | 12.40 (9.65–15.20) | 10.30 (8.40–16.60) | 0.017 |
| CEA (ng/mL) | 1.00 (0.70–1.50) | 1.10 (0.70–1.50) | 1.20 (0.80–1.70) | 1.10 (0.80–1.70) | 0.246 |
| Note: Data were presented as medians with 25% and 75% quartiles. PCOS, polycystic ovary syndrome; TTE, total testosterone; E2, estradiol; LH, luteinizing hormone; FSH, follicle stimulating hormone; PGN, progesterone; PRL, prolactin; AFP, alpha fetoprotein; CA125, carbohydrate antigen 125; CEA, carcino-embryonic antigen. Comparisons were made using Kruskal–Wallis H test. | |||||
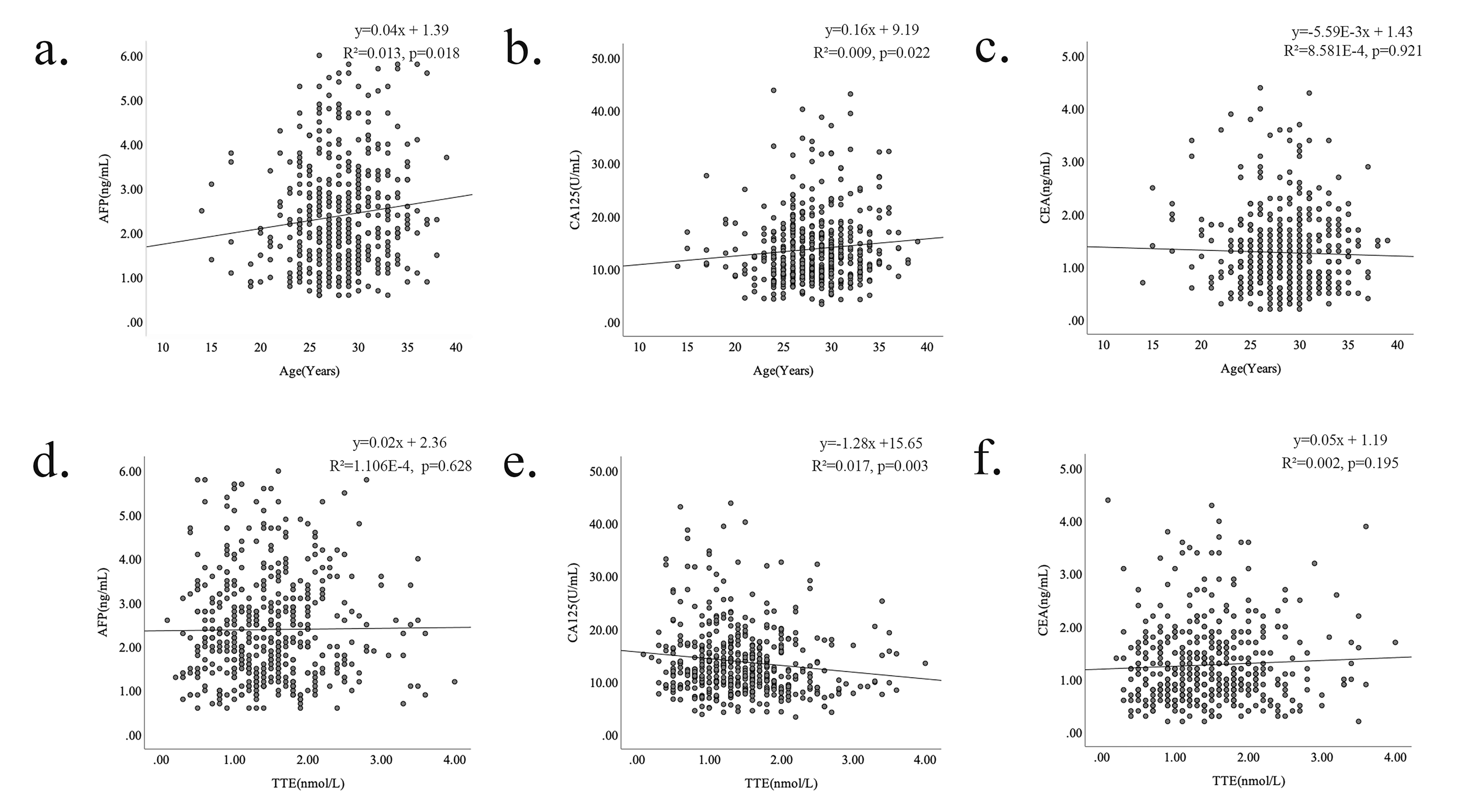
To further analyze the association between TTE and tumor markers in PCOS
patients. The Spearman correlation analysis was performed in PCOS (Fig. 1). The Spearman
correlation analysis revealed that CA125 was significantly and positively
correlated with age (p = 0.003, R
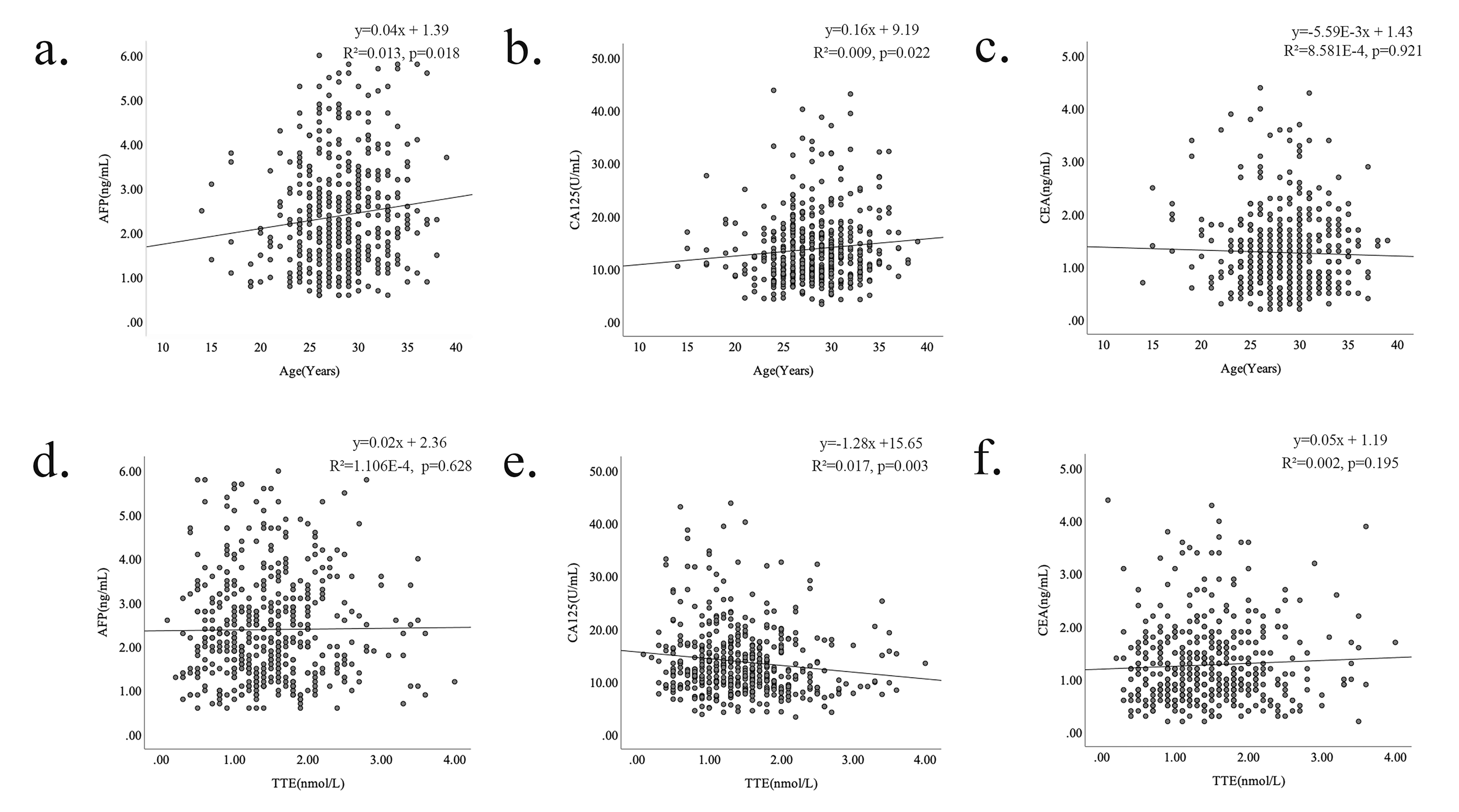 Fig. 1.
Fig. 1.Association of tumor markers levels with age and TTE among PCOS women. (a) The correlation between AFP and age. (b) The correlation between CA125 and age. (c) The correlation between CEA and age. (d) The correlation between AFP and TTE. (e) The correlation between CA125 and TTE. (f) The correlation between CEA and TTE. AFP, alpha fetoprotein; CA125, carbohydrate antigen 125; CEA, carcino-embryonic antigen; TTE, total testosterone.
We have for the first time demonstrated that the serum levels of AFP and CEA
were significantly higher, yet the levels of CA125 were significantly lower in
PCOS in comparison with healthy people. Besides, CA125 was significantly
correlated with TTE while no linear relationship was found between AFP, CEA and
TTE. LH was significantly lower as TTE quartile was lower, PGN showed the same
trend, although the statistical difference was less significant. Steroid hormones
production is delicated regulated by LH within the ovarian follicle as the
ovarian steroidogenic response to LH is accelerated in the luteinized
preovulatory follicle, with LH stimulating the rate-limiting conversion of
cholesterol into pregnenolone [34]. Pregnenolone is then metabolized to PGN by
3
Plasma CA125 has been found to be significantly higher in the luteal phase than in the late follicular phase in women with normal menstrual cycles and is significantly associated with E2 levels [36, 37]. The production of CA125 may be related to an increase in luteal function. PCOS and luteal phase deficiency (LPD) have many pathophysiological features in common, such as hyperinsulinemia, elevated AMH levels and angiogenesis deficiency [38]. LPD may be a cause of infertility in PCOS patients. Prenatal TTE-treated female sheep showed a PCOS-like phenotype with luteal dysfunction resulting in reduced pregnancy rates [39]. The lower CA125 level of PCOS in our study may indicate luteal insufficiency in PCOS. As for the relationship between CA125 and androgen, one study assessed CA125, CA199, DHEAS, PRL and E2 in serum of 50 patients with endometriosis, the results demonstrated that significant spearman correlations of CA125 and CA199 with DHEAS [40]. Even though they focused on a different disease and the trends were opposite, there was evidence of a link between serum CA125 and androgens. In fact, the relationship between CA125 and androgen has been studied before. Previous researchers randomly collected 123 follicular fluid (FF) from 28 patients undergoing in-vitro fertilization and embryo transfer (IVF-ET) and assessed E2, PGN and TTE in FF, they found no significant correlation between CA125 and E2, PGN, TTE, oocyte fertilization, embryo quality, and pregnancy rates [41]. A similar study collected 36 FF samples from 12 infertile patients and found no relationship between AFP, CEA and CA125 and IVF outcomes [42]. As there are no similar studies on CA125 of FF from PCOS, whether CA125 plays a different role in PCOS remains to be elucidated.
Physiological levels of purified AFP can significantly enhance epidermal growth factor plus insulin-like growth factor I-mediated mitogenic activity in a dose-dependent manner and may be involved in growth factor-mediated cell proliferation regulation in porcine GCs [43]. The same research team later found that AFP significantly inhibited E2 synthesis in a dose-dependent manner mediated by growth factor/FSH in porcine GCs [44, 45]. Combined with these findings, they concluded that AFP may be inhibiting the steroidogenesis of GCs while promoting the proliferation of these cells. Abnormal proliferation of GCs was associated with anovulation of PCOS. Sharron A Stubbs et al. [46] collected whole ovary tissue samples from patients (with/without PCOS, PCOS with/without anovulation) and used minichromosome maintenance deficient 2 (MCM2) as a marker for DNA replication. They found that GCs division didn’t match with oocyte growth in anovulatory PCOS as the quantity of GCs was disproportionately greater than the oocyte diameter in the follicles from anovulatory polycystic ovaries [46]. We did not find relevant studies on the effect of AFP in human ovarian GCs, but another clinical trial in patients with HCC found that AFP functioned to modulate cell proliferation in HCC [47]. This provides evidence that AFP mediates cell proliferation in vivo. In contrast to proliferation, AFP is also involved in apoptosis. Silencing AFP may induce apoptosis in HCC cell line via dysfunction of the p53/Bax/cytochrome c/caspase-3 signaling pathway [48]. The quantity of apoptotic GCs were significantly lower in anovulatory PCOS than in normal ovarian follicles as the expression of apoptotic effectors like activated caspase-3 was significantly lower in GCs of anovulatory PCOS follicles and the expression of cell survival factors like cellular inhibitor of apoptosis protein-2 (cIAP-2) was significantly higher [49]. The role of AFP in apoptosis has also been demonstrated in clinical trials, as Noboru Mitsuhashi et al. [50] found that higher AFP levels were significantly related to lower apoptotic index. They also found a link between AFP and angiogenesis that patients with higher AFP levels had a significantly higher microvessel density [50]. It has been proved that low concentrations of AFP could significantly enhance vascular endothelial growth factor (VEGF)-induced proliferation in placenta and uterus endothelial cells via mitogen-activated protein kinase-dependent pathways [51]. VEGF was the first widely studied angiogenic factor in the ovaries of PCOS patients. VEGF expression levels were found to be high in granulosa, theca and luteal cells in ovarian tissue from PCOS [52]. A later study found that increased stromal vascularization in PCOS ovaries may be related with high serum VEGF induced high ovarian blood flow [53]. Imbalance of angiogenic factors and antiangiogenic factors conduces to abnormal follicular development in women with PCOS, that may further result in accumulation of small follicles and apparent failure to select a dominant follicle, with anovulation and cyst formation [54].
Santa Benchimol et al. [31] considered CEA could be added to the family
of intercellular adhesion molecules as they found that CEA can function as an
intercellular adhesion molecule independent of Ca
We found some evidence for a role of CA125 in PCOS, as CA125 was significantly lower in PCOS and changed with TTE. Combined with our present research results and a number of previous related studies, we have made full explanations and speculations about the results. Our finding may offer new ideas to explore the relationship between PCOS and AFP, CEA and CA125. Further experiments are required to examine the association of tumor markers with the development of PCOS women. It is possible to explore whether AFP, CEA and CA125 in serum/follicular fluid could affect the proliferation, apoptosis of GCs and ovarian vascularization and luteal insufficiency, whether they are related to PCOS anovulation and affect PCOS pregnancy. If the exact mechanism of tumor markers in PCOS can be determined through further experimental studies in the future, tumor markers may be combined with some other indicators to diagnose PCOS. The primary limitation in our research is the potential for confounding. As missing BMI in our study is a factor that may contribute to the levels of tumor markers. In addition, regional and ethnic factors can’t be ruled out as contributing factors as our samples are from a single source. Multicenter studies with larger sample sizes are expected in the future.
The serum level of CA125 is significantly lower and significantly correlated to the hormonal status of PCOS. The lower CA125 level of PCOS may indicate LPD in PCOS resulting in reduced pregnancy rates. AFP and CEA are significantly higher in PCOS. Higher AFP in PCOS may be related with increased proliferation and reduced apoptosis in GCs of PCOS. CEA may function as an intercellular adhesion molecule in PCOS. Further experiments are needed to explore the exact mechanism of tumor markers in the development of PCOS.
CA125, carbohydrate antigen 125; CEA, carcino-embryonic antigen; AFP, alpha
fetoprotein; PCOS, polycystic ovary syndrome; TTE, total testosterone; FSH,
follicle stimulating hormone; LH, luteinizing hormone; E2, estradiol; PGN,
progesterone; GCs, granulosa cells; PRL, prolactin; DHEAS, dehydroepiandrosterone
sulfate; DHEA, dehydroepiandrosterone; A4, androstenedione; DHT,
dihydrotestosterone; AMH, anti-mullerian hormone; HCC, hepatocellular carcinoma;
3
JHZ and FQ designed the research protocol. JHZ and MMP conducted the study. QZ collected data and MMP performed the data analysis. MMP and QZ explained data and wrote the manuscript. FFW provided help and advice on writing review. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University (approval number: IRB-20210101-R).
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. FQ is serving as one of the Guest editors of this journal. We declare that FQ had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to JO.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.