Academic Editor: Michael H. Dahan
Backrounds: Fetal arrhythmias represent a significant cause of fetal morbidity and mortality and occur in approximately 1–3% of pregnancies. The unknown fetal arrhythmias are the cause of intrauterine fetal demise in as many as 3–10% of cases, as well as the cause of unexplained fetal hydrops or premature births. Methods: A fetal echocardiography test makes it possible to notice structural heart defects at very early stages of pregnancy. The ultrasound heart rate monitoring also involves the use of specific Doppler methods. Heart rhythm disorders may occur in the form of tachycardia (sinus tachycardia (ST), supraventricular tachycardia (SVT) and atrial flutter (AF)) or in the form of bradycardia (sinus bradycardia (SB), atrial bigeminy (AB) and complete AV block). The most frequently diagnosed fetal heart rhythm disorders are isolated extrasystoles with no clinical significance. Results: In this study, we have examined 7863 fetuses (182 were multiple gestations). Out of the total number of examined patients, 572 fetuses (7.23%) had pathological heart features, while 127 (1.61%) had some form of rhythm disorder. Conclusions: The ability to recognize the heart rhythm disorder and commence the treatment in a timely manner increases the treatment success rate.
The heart rhythm disorders occurring in the form of an irregularity or in the form of acceleration and deceleration of heart rate occur in approximately 2% of all pregnancies [1]. The most frequently diagnosed heart rhythm disorders are isolated extrasystoles with no clinical significance. They are only temporary and require regular monitoring without the need for therapeutic procedures [2].
The remaining minor percentage of heart rhythm disorders of approximately 10% is hemodynamically significant and may lead to cardiac insufficiency, dilated cardiomyopathy, development of hydrops and death at fetal or early neonatal period, as well as to the increased risk of neurological morbidity in the future [3, 4, 5].
The cardiac conduction system consists of highly specialized cells capable of generating electrical impulses in the sinoatrial node (SA) and propagating such impulses through atrioventricular node (AV) and His-Purkinje fibers to the chamber myocytes. A correct and synchronized depolarization and repolarization of these cells results in rhythmic contractions of atriums and ventricles [6]. A normal fetal heart rate usually ranges from 120 to 180 beats per minute. The heart rate disorders are categorized as tachyarrhythmias if the heart rate is over 180 bpm, as bradyarrhythmias if the heart rate is below 120 bpm and as ectopic beats usually described as premature atrial contractions [2].
The regular monitoring of pregnancy also includes, inter alia, the ultrasound examination of fetal heart structure and heart rate [7]. A fetal echocardiography test makes it possible to notice structural heart defects at very early stages of pregnancy. The ultrasound heart rate monitoring also involves the use of specific Doppler methods: the M-mode echocardiography, colour and pulse Doppler echocardiography, tissue Doppler. The use of these methods in highly specialized examinations allows for a precise diagnosis and classification of the heart rate disorder into one of the categories.
The heart rhythm disorders may occur in the form of tachycardia (ST), supraventricular tachycardia (SVT) and atrial flutter (AF). These disorders are more complex and may jeopardize the fetus, and for this reason in some cases require drug therapy. The fetal bradycardia includes the disorders such as sinus bradycardia (SB), atrial bigeminy (AB) and complete AV block. While the sinus bradycardia represents an urgent condition in medicine and requires immediate delivery, the atrial bigeminy is a less severe disorder frequently resulting in spontaneous resolution [6].
The complete heart block is the most frequent disorder of the fetal cardiac
conduction system and accounts for one-half of all diagnosed complicated
arrhythmias. The atrial contractions are regular and rhythmic, while the
ventricles contract in an independent rhythm of 40–80 bpm due to a conduction
failure at the AV node level [6]. Etiologically, this type of arrhythmia occurs
as a consequence of structural heart defect or due to the existence of maternal
anti-Ro and anti-La bodies [8]. The comorbidities of the complete heart block
with structural anomalies, such as atrioventricular septal defect and left atrial
isomerism, are frequent. The fetuses without structural heart defects have a more
favorable prognosis. The therapy with dexamethasone and
The goal of this research is to determine the frequency of fetal arrhythmias and to monitor the outcomes of pregnancies complicated by fetal heart rhythm disorders diagnosed via ultrasound, depending on the used therapeutic modality.
The research was designed as a retrospective study carried out at a tertiary center where 7681 pregnant women, i.e., 7863 fetuses have been examined (182 were multiple gestations). In total, 12,274 examinations were carried out, on average 1.6 examinations per patient. The examinations were carried out between 15th and 39th week of gestation, on average in the 25.9 week of gestation. The research has covered a period of 20 years. The subjects included in the study were pregnant women referred from other medical centers where the ultrasound examination of the fetus showed signs of a congenital heart disease. A physician trained in fetal echocardiography examined all patients. All referred patients were examined using various fetal echocardiography methods (2D, M-mode technique, color Doppler or pulse Doppler). Various types of pathological heart features were confirmed in 572 (7.4%) subjects, in the form of structural anomalies, fetal arrhythmias or combined disorders.
The M-mode echocardiography was used to evaluate the fetal arrhythmias by placing the cursor in the ventricle, atrium or AV valve and by simultaneously registering the mechanical activity both of the atrium and of ventricle per unit of time. However, the use of this technique to detect the heart disorder is often difficult, partially due to the position of the fetus which makes proper placement of the cursor impossible, but also due to polyhydramnion, weak myocardial contractility in hydropic fetus or due to mother’s obesity. The use of a pulse Doppler plays a very significant role in the assessment of fetal arrhythmias. For proper assessment of rhythm disorders, the following Doppler positions are being used: foramen ovale and hepatic vein for detection of atrial rhythm, left ventricular outflow for assessment of ventricular rhythm, simultaneous outflow and inflow in any ventricle for AV contraction assessment. The pulse Doppler is better than M-mode echocardiography in assessing atrial flutter due to the hypokinetic movement of the atrial wall, which is an aggravating factor for M-mode control.
Depending on the findings, certain number of pregnancies in our research had to be monitored until delivery, prenatal therapy had to be administered in some pregnancies, while the termination of pregnancy was indicated in some pregnancies.
The patients with prenatally initiated therapy for the correction of fetal heart rhythm disorder had to be under internist supervision before and after the therapy (ECG control, electrolyte balance control).
The demographic data, laboratory data and the results of the prenatal diagnostic methods were obtained from the medical documents.
The serial echocardiograms were done in all fetuses, accompanied by the monitoring of signs of cardiac insufficiency development (Huhta criteria)—determination of heart size, i.e., cardiomegaly development, vein flow control, tricuspid valve flow control, myocardial thickness measuring, umbilical artery and medial cerebral artery flow control and the development of hydrops [9].
A prenatal therapy using oral Dexamethasone was administered to mothers with confirmed existence of anti-Ro and anti-La bodies. A certain number of newborns from pregnancies with prenatally diagnosed heart rhythm disorder of the AV block type had a pacemaker implanted during the first months of life.
A certain number of pregnant women whose fetuses had a rhythm disorder of the tachyarrhythmia type had received a prenatal Digoxin therapy, where necessary supplemented by other antiarrhythmics (Verapamil, Amiodaron). In majority of newborns, the antiarrhythmics therapy continued after birth (mostly with Propranolol and Amiodarone). During the first months of life, a permanent pacemaker was implanted in the majority of children with prenatally detected complete AV block. The pregnancies with congenital heart flaws were monitored until delivery, after which the treatment was continued at the University Children’s Clinic, Tiršova (UDK). The success rate of the applied therapeutic methods and further survival rate have been determined using the data from the medical records with UDK.
The statistical data processing incorporated the creation of a database with grouping and tabulation of results according to the examined features of pregnant women and fetuses. The data are analyzed using the Microsoft Office Excel (version 2016, Belgrade, Serbia), method of descriptive statistics. The descriptive statistical parameters are expressed through arithmetic mean with measures of dispersion (SD—standard deviation, SE—standard error), median, MOD and relative frequency distribution. The data is shown in the form of a chart and a table.
During the course of the research, a total of 7681 pregnant women, i.e., 7863 fetuses were examined (182 were multiples).
Mother’s age range was between 21 and 38 years and most of them were primiparas. About 80% of included women delivered by cesarean section.
Out of the total number of examined patients, 572 fetuses (7.23%) had pathological heart features, while 127 (1.61%) had some form of rhythm disorder.
This study included 127 fetuses with some form of a prenatally diagnosed heart rhythm disorder, where in 70/127 (55.11%) fetuses the presence of supraventricular extrasystoles was detected during the third trimestre, while in 2/127 fetuses the presence of ventricular extrasystoles (1.6%) had no hemodynamic significance. Some structural heart defect was detected in 6/70 (8.57%) pregnant women whose fetuses had a SVES-type rhythm disorder (Table 1).
| Rhythm disorder type | 127 |
| Supraventricular extrasystoles - SVES | 70 (55.11%) |
| Ventricular extrasystoles - VES | 2 (1.57%) |
| Supraventricular tachycardia - SVT | 27 (21.25%) |
| Atrial flutter - AF | 6 (4.72%) |
| Atrioventricular block - AV block | 22 (17.32%) |
Isolated structural heart defects were detected in 445 fetuses (5.65%), while isolated fetal arrhythmias were detected in 127 fetuses (1.6%). In 12/127 (9.44%) fetuses, the rhythm disorder was accompanied by a structural heart defect (2/12 AV block (1.57%), 4/12 SVT (3.15%), 6/12 SVES (4.72%) (Table 2).
| Rhythm disorder type | No anomalies | With associated anomalies |
| SVES | 64 | 6 |
| SVT | 23 | 4 |
| AF | 6 | 0 |
| AV block | 22 | 2 |
The supraventricular extrasystoles are the most common form of fetal heart rhythm disorder and are benign in character. In our research, 64/127 (50.4%) pregnancies ended successfully by the estimated delivery date.
The analysis of the total number of monitored fetuses-neonates showed that this study included 102/127 live births (80.31%), termination of pregnancy in 10/127 of pregnant women (7.87%), intrauterine fetal death in 7/127 fetuses (5.51%) and death during the neonatal period in 8/127 fetuses (6.3%) (Table 3).
| Outcome | NN | % |
| Alive | 102 | 84.8% |
| Termination of pregnancy | 10 | 7.87% |
| FMU | 7 | 5.6% |
| Death during neonatal period | 8 | 6.4% |
The structural heart anomaly was detected in 12/127 (9.44%) fetuses, while 115/127 (89.76%) fetuses had some of the described heart rhythm disorders without the associated structural flaws.
If we were to exclude the fetuses with registered supraventricular extrasystoles from the total number of monitored fetuses due to their benign character, we would have 55 fetuses with hemodynamically significant heart rhythm or conduction disorder (SVT, AF, AV block).
A heart rhythm disorder of the supraventricular tachycardia type was diagnosed in 27/55 (49%) fetuses, specifically as isolated disorder without associated structural flaws in 23/27 (85.18%) fetuses and with associated structural flaws in 4/27 (14.8%) fetuses, where multiple rhabdomyomas were diagnosed in 3/27 (11.11%) fetuses and a structural flaw in the form of a left heart hypoplasia in one fetus. In 13/27 fetuses with SVT (48.15%), the drug therapy was initiated prenatally and in 2/27 (7.40%) the drug therapy was initiated after birth. Among the 13 fetuses treated prenatally, 8/13 (61.5%) survived, 4/13 (30.7%) died in utero (because the therapy was initiated after the fetal hydrops has developed), while 1/13 (7.7%) pregnancy was terminated due to multiple cardiac tumors.
The overall outcome for fetuses/neonates with this type of heart rhythm disorder (SVT) was as follows: 16/27 (59.25%) were live-born, 3/27 (11.11%) pregnancies were terminated during a late stage of gestation (2 rhabdomyomas and left heart hypoplasia), in 6/27 (22.22%) pregnancies an intrauterine death has occurred and 2/27 (7.4%) newborns died during the neonatal period (Fig. 1). The hydrops was detected in 5/27 (18.51%) fetuses that died in utero, while one patient (1/27 = 3.7%) declined the proposed therapy which led to intrauterine fetal death. The Digoxin and Verapamil therapy was initiated prenatally in 13/27 (48%) fetuses. In both cases, the Amjodaron therapy was initiated postnatally (2/27 = 77.4%), while in one case the Amjodaron therapy was initiated during pregnancy (1/27 = 3.7%).
 Fig. 1.
Fig. 1.Outcome of pregnancies for the group of fetuses complicated with SVT.
An atrial flutter type of heart rhythm disorder was detected in 6/55 (10.9%) fetuses. The therapy was initiated prenatally in 2/6 (33.3%) fetuses and postnatally in 4/6 (66.67%) fetuses. The overall outcome was good with 100% survival rate. A Digoxin therapy with Verapamil was initiated prenatally, while the conversion of rhythm after birth was achieved by Amjodaron. A spontaneous conversion to a sinus rhythm occurred in 1/6 (16.66%) fetus during the 34th week of gestation.
A complete AV block was registered in 22/55 (40%) fetuses. In 20/22 (90.9%) fetuses, it was isolated without structural anomalies, but with the presence of immune antibodies (Ro SSA, La SSB), and in 2/22 (9.1%) fetuses it had associated structural anomalies (situs ambiguus with the AV-canal, L-TGA with VSD and st. PA 1). Corticosteroid was administered transplacentally to ten fetuses 10/22 (45.45%), while a pacemaker was implanted in 12/22 (54.54%) during the neonatal period. The overall outcome in the group of fetuses diagnosed with the AV block is shown in the Fig. 2, where we can see that the majority of pregnancies ended in the birth of a liveborn baby, 14/22 (63.63%) were liveborn, one 1/22 (4.54%) pregnancy was terminated due to associated structural flaw of the L-TGA type, and in one 1/22 (4.54%) case an intrauterine fetal death has occurred. The Table 4 shows the structure of neonatal mortality, where we can see that a total of 6/22 (27.27%) neonates have died (2 due to prematurity, 1 due to complex congenital heart disease (CHD), 1 due to sepsis, 1 due to sudden death and 1 due to severe myopatic heart disorder).
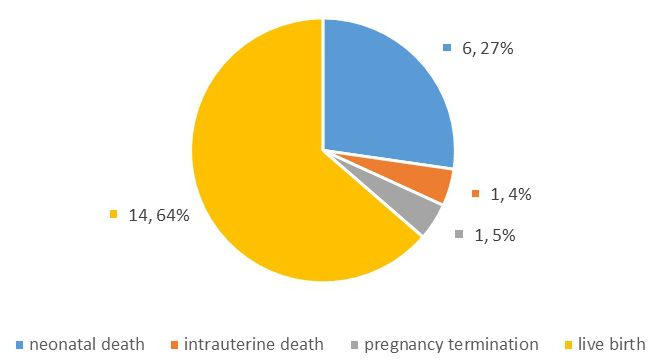 Fig. 2.
Fig. 2.Outcome of pregnancies for the group of fetuses complicated with AV block.
| Cause | Prematurity | Complex CHD | Sepsis | Sudden death | Severe myopathy |
| Number | 2 | 1 | 1 | 1 | 1 |
The analysis of all hemodynamically significant heart rhythm disorders observed collectively has shown that in 25/55 (45.45%) fetuses, the therapy was initiated prenatally and in 18/55 (32.73%) the therapy was initiated postnataly (Table 5).
| Treatment | SVT | AF | AV block | Total | ||||
| prenatal | 13/55 | 23.63% | 2/55 | 3.63% | 10/55 | 18.18% | 25/55 | 45.45% |
| postnatal | 2/55 | 3.63% | 4/55 | 7.27% | 12/55 | 21.82% | 18/55 | 32.73% |
The prenatal diagnostic of fetal tachycardia, i.e., heart rhythm disorder became possible after the ultrasound scan became the “golden standard” of modern pregnancy care. The use of various fetal echocardiography methods allows for precise determination of the type of structural heart flaw and for precise measuring of fetal heart rate, separately for the atriums and separately for the ventricles. The therapy used was adjusted depending on the gestational age, presence or absence of hydrops and the presumed tachycardia development mechanism. The ultimate goal of prenatally introduced therapy would be to achieve the rhythm conversion in utero and to prevent the development of hydrops [10].
The occurrence of premature atrial contractions (isolated extrasystoles) during the early second trimester reflects the immaturity of the cardiac conduction system. They typically disappear during the late second and third trimester or during the neonatal period. This type of heart rhythm disorder is associated with structural heart flaws in 1–2% of cases [11]. Sometimes this type of heart rhythm disorder can be related to the existence of atrial septal aneurysm. In approximately 2% of fetuses with detected heart rhythm disorder of the premature atrial contraction type, the heart rhythm disorders of the supraventricular tachycardia type may develop later on [12, 13]. Also, in 2–3% of cases, they may progress to permanent tachycardia while still in utero or during the first 3–4 weeks after birth [14, 15].
In our study, in 64/127 (50.64%) fetuses with detected heart rhythm disorder of the SVES type, the disorder was temporary in nature, did not require any therapy and was not associated with structural heart anomalies, which complies with the data found in literature.
Six out of 127 fetuses with this type of rhythm disorder were diagnosed with a structural heart flaw (4.72%), which complies with the data found in literature [2].
During the third trimester, the ventricular extrasystoles were detected in 2/127 (1.57%) fetuses participating in our study, and the outcome was successful in both cases.
Other heart rhythm disorders that are hemodynamically significant, classified as tachyarrhythmias based on their type, have manifested themselves as supraventricular tachycardia and atrial flutter.
A feature of the heart rhythm disorder of the supraventricular tachycardia type is the sudden increase of heart rhythm frequency between 180–300 bpm (mostly 200–240 bpm), with 1:1 pattern of AV conduction. This rhythm disorder is the most common type of fetal tachycardia and occurs in 66–90% of all cases [15]. It can occur as a consequence of accessory AV pathway or ectopic focus resulting in recurrent tachycardia.
In cases when the tachyarrhythmia develops after the 34th week of gestation, the delivery is planned followed by postnatal drug therapy. In case the heart rhythm disorder is temporary and without effect on fetal circulation, it is recommended to only monitor the condition, without introducing the drug treatment. When the fetal tachyarrhythmia leads to the fetal circulation disorder, it can lead to reduced diastolic filling, venous congestion and reduced cardiac output. The introduction of prenatal therapy is recommended for this type of heart rhythm disorder, where Digoxin is seen as the first line of defense in such cases [16].
The use of Digoxin in treatment of supraventricular tachycardias is particularly successful if the VA interval is short (around 90%) and in cases of atrial flutter without hydrops. The rhythm conversion success rate is approximately 40–60% [17]. Digoxin has a positive inotropic and a negative chronotropic effect resulting in the increase of stroke volume and decrease of the number of beats per minute. Additionally, Digoxin extends the refractory period of the AV node and thus slows down the AV conduction and reduces the ventricular rate.
Furthermore, beta blockers (sotalol) are also used as second drug of choice for tachycardia treatment [16]. Sotalol extends the duration of myocardial cell action potential, which results in the extension of the effective refractory period. Sotalol is recommended as the drug of choice in case of heart rhythm disorders of the supraventricular tachycardia type with long VA interval and without fetal hydrops. Beta blockers are used as monotherapy or in combination with Digoxin. Flecainidin and Amiodaron are used as secondary drugs in treatment of tachycardia, in cases where the use of Digoxin or Sotalol is not adequate. Flecainidin proved to be successful where Digoxin was not adequate to correct the tachyarrhythmia [18]. Flecainide extends the refractory period and slows down the myocardial conduction. It is particularly efficient in the treatment of supraventricular tachycardia complicated by fetal hydrops.
Verapamil belongs to class IV antiarrhythmics from the group of calcium channels antagonists, which reduces myocardial contractility and conductivity through SA and AV node, thus inhibiting the slow influx of calcium ions. Some major studies show that the use of Verapamil together with Digoxin results in the rhythm conversion in more than 50% of fetuses and in hydropic and non-hydropic fetuses [19]. The use in conjunction with beta blockers is not advised due to possible development of an AV block, and also not recommended as a first line of drugs for tachyarrhythmia treatment.
In our study, a total of 15/27 (55.55%) fetuses with heart rhythm disorder of the SVT type has received therapy, which was initiated prenatally in 13/27 (48.15%) fetuses and postnatally in 2/27 (7.4%).
In 13/27 fetuses with SVT (48.15%) with prenatally initiated therapy, the first drug that was introduced was Digoxin, which is fully compliant with the data found in the literature [20, 21, 22, 23]. In majority of patients, Digoxin was administered together with Verapamil. The rhythm conversion was achieved by Amjodaron in 3/27 (11.11%) patients in our study, which represents the second line of defense and complies with the recommendations for SVT treatment (initiated prenatally in one patient and during the neonatal period in two patients). The use of Amiodaron is particularly recommended in case of fetal hydrops development and may be used independently or in combination with Digoxin or Saltol [21, 23].
Both untreated and treated heat rhythm disorders may become additionally complicated with the development of fetal heart insufficiency and fetal hydrops with poor prenatal and postnatal prognosis.
In our studied group of patients diagnosed with SVT, an intrauterine death due to hydrops has occurred in 6/27 (22.22%) patients (5 treated and 1 not treated), which is fully compliant with the data found in the literature [17, 23, 24], while death has occurred in 2/27 (7.40%) fetuses during the neonatal period.
The overall outcome of pregnancies complicated by fetal SVT in our study was as follows: 16/27 (59.25%) were liveborn, 6/27 (22.22%) died in utero, while 2/27 (7.4%) died during the neonatal period.
In the group of patients whose fetuses had a hemodynamically significant heart rhythm disorder, 6/55 (10.9%) fetuses were diagnosed with some associated anomaly, which is fully compliant with the data found in the literature [11, 25].
The second type of disorder of the atrial flutter type is characterized by detection of very high frequencies of the atrial rhythm between 300–500 bpm with variable AV block.
As a consequence of this atrial rhythm disorder with AV block, the ventricular rate ranges from 160 to 250 bpm. In 80% of cases, the AV conduction block occurs in a 2:1 pattern and in a 3:1 pattern in the remaining 20% [17]. This type of rhythm disorder occurs in 10–30% of all tachyarrhythmias [26]. The atrial flutter is associated with structural heart anomalies in more than 30% of cases [17].
In our group of subjects, 6/55 (10.9%) fetuses has an atrial flutter type of rhythm disorder. The treatment success rate was 100% in two fetuses treated neonatally with Dilacor and Verapamil. This therapy in combination with Flecainide is recommended and also administered with high success rate in fetuses with the signs of hydrops [19, 27]. The planning of delivery in this group of patients was initiated based on the gestational age and occurrence of signs of cardiac insufficiency.
The fetal bradycardia is defined as the existence of persistent heart rate below 100 bpm. The transient episodes of fetal bradycardia occur as a consequence of increased vagal stimulation during ultrasound examination due to increased pressure to the upper abdominal wall during examination. The most frequent causes of fetal bradycardia are the sinus bradycardia, blocked atrial bigeminy - trigeminy and AV block [25].
The sinus bradycardia is a physiological phenomenon occurring as a consequence of vagal stimulation, prolonged pressure to the fetal head during ultrasound examination. Occasionally, it can be associated with long QT syndrome [28].
There is practically no treatment for the sinus bradycardia during the Intrauterine fetal life.
In case of development of a complete AV block associated with a structural heart flaw, it is not treated in utero. If the heart rate is below 50 bpm, the risk of fetal hydrops development increases. Betamethasone or dexamethasone are used to treat the immune-mediated AV block. The use of corticosteroids reduces the inflammatory reaction that causes the development of the AV block. Furthermore, it is recommended to use the beta sympathomimetic drugs (Terbutalin, Salbutamol, Isoprenalin) to accelerate the heart frequency (if the heart rate is below 55 bpm) [29].
In more than 40% of cases, the congenital AV block occurs in comorbidity with congenital heart anomalies, in particular heterotaxy (left atrial isomerism) and congenitally corrected transposition of large blood vessels. The remaining 60% is associated with the diseases of the mother’s connective tissue. This disorder is fairly common with incidence of 1:11.000 to 1:22.000 live births in the general population and 1–2% of live births with present RoSSA antibodies and the risk of reoccurrence of 14–17% [30, 31]. The immune-mediated AV block is the result of an inflammatory response and damage to the myocardium and fetal cardiac conduction system. The immune-mediated heart damages include various disorders, such as myocardial dysfunction, cardiomyopathy, endocardial fibroelastosis and conduction disorders [32, 33], as well as the tricuspid regurgitation and ventricular tachycardia [34]. An isolated AV block that is not a consequence of the presence of RoSSA and SSB antibodies has the best prognosis in the long run. The Baruteau study shows 100% survival rate in 120 children who had an isolated AV block, of which 23 were detected in utero or during the first months of life [35].
Among our subjects, this type of rhythm disorder was connected to complex heart flaws in 2/22 (9.09%) cases. An isolated complete AV block that is not associated to a heart condition is usually immunomediated and occurs as a consequence of presence of Ro (SSA) and La (SSB) antibodies passing through placental barrier and performing the fibrosing of conducting fibers [36].
Treatment outcome is poor, in particular if the ventricular rate is below 55 bpm and there are signs of hydrops. Lately, it is recommended to use the beta sympathomimetics (Terbutalin) to increase the ventricular frequency, but this has had no significant effect on the survival rate [37]. The survial is also limited by prematurity complications. The survival rate in our group of patients was around 14/22 (63.6%), where corticosteroids were used for treatment in 10/22 (45.45%) patients, while in 12/22 (54.54%) patients the treatment was continued and completed by the implantation of a pacemaker during the first months of life.
The fetal rhythm disorder is a significant cause of fetal morbidity and mortality. The ability to recognize it and commence the treatment in a timely manner increases the treatment success rate. Numerous protocols for the treatment of heart rhythm disorders of the tachyarrhythmia type have been defined to date, leading to successful prenatal conversion to sinus rhythm.
Furthermore, the heart rhythm disorder treatment protocols have been defined for different types of bradycardia, in particular in case of complete AV block development. Due to the fact that the heart rhythm disorders may occur during the early second trimester of gestation, an adequate choice of drugs and the ability to recognize possible complications allow for the extension of pregnancy and reduction of prematurity complications. On the other hand, an unknown rhythm disorder may cause intrauterine fetal death, non-immune hydrops or premature delivery.
JS, VP, OKV, MT, IS, AL designed the research study. JS, VP, OKV, AL, IJ, MS performed the research. MS, MT, IJ, IS analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All subjects gave their written consent for inclusion before they participated in the study. The study was conducted with accordance with the Helsinki Declaration and and the protocol was approved by the Ethics Committee of University Children’s Clinic, Belgrade (protocol number 017, 16/32).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
