1 Center for Anesthesiology, Beijing Anzhen Hospital, Capital Medical University, 100029 Beijing, China
2 Department of Anesthesiology, Fuxing Hospital, Capital Medical University, 100038 Beijing, China
Academic Editor: Andrea Tinelli
Abstract
Background: The purpose of the study was to compare the safety of local injection of 6 units of pituitrin diluted to 20 mL vs 6 units of pituitrin diluted to 10 mL for laparoscopic uterine fibroid (UF) surgery. Methods: This was a randomized clinical trial of patients scheduled for laparoscopic UF surgery at Fu Xing Hospital, Capital Medical University, Beijing, China. Ninety-six patients were divided into two groups according to the concentration of pituitrin utilized: Group1 (6 units of pituitrin diluted to 20 mL for all injection) 48 cases; Group2 (6 units of pituitrin diluted to 10 mL for all injection) 48 cases. The observation indicators were mean arterial pressure (MAP1) and heart rate (HR1) upon entering the operating room; the lowest mean arterial pressure (MAP2) and the highest heart rate (HR2) within 5 minutes after injecting pituitrin; the highest mean arterial pressure (MAP3) and the lowest heart rate (HR3) within 30 minutes after injecting pituitrin; hemoglobin (Hb1) and hematocrit (Hct1) within one week before surgery; hemoglobin (Hb2) and hematocrit (Hct2) within one day after surgery; and the time for the mean arterial pressure to return to the level of entering the operation room after using pituitrin (Recovery Time). Results: All baseline and observation data showed no statistical difference between the two groups. Conclusions: The safety profile of local injection of pituitrin in the 6 units of pituitrin diluted to 20 mL and 6 units of pituitrin diluted to 10 mL are the same when used for laparoscopic UF surgery.
Keywords
- bleeding
- hemodynamics
- laparoscopy
- uterine fibroid
- pituitrin
- surgery
Approximately 20%–50% of patients with uterine fibroids (UFs) require surgery [1, 2, 3, 4, 5, 6, 7, 8]. Laparoscopic UF surgery can reduce bleeding and facilitate postoperative recovery. It is currently a common surgical method for the treatment of UFs. During laparoscopic UF surgery, to reduce surgical bleeding, the surgeon injects pituitrin into the myometrium surrounding the target myomas. Pituitrin is widely used in gynecologic surgery to constrict blood vessels and reduce blood flow [9, 10, 11]. At present, there are not many studies on the effect of pituitrin on circulation. A small number of studies have focused on the optimal dose of local injection of pituitrin during laparoscopic UF surgery, but there are few studies on its safe and effective concentrations. The present study aimed to investigate the safety and effects of local injection of pituitrin in 6 units diluted to 20 mL and 6 units diluted to 10 mL in laparoscopic UF surgery. The results might provide evidence for the improvement of safety during laparoscopic UF surgery.
This was a randomized clinical trial that included patients scheduled for laparoscopic UF surgery at Fu Xing Hospital, Capital Medical University. This study was approved by the ethics review committee of Fu Xing Hospital, Capital Medical University. All patients signed the informed consent form.
Ninety-six patients were divided into two groups according to the concentration of pituitrin: Group1 (6 units of pituitrin diluted to 20 mL for all injection) 48 cases; Group2 (6 units of pituitrin diluted to 10 mL for all injection) 48 cases.
The inclusion criteria were: (1) 20–55 years of age; (2) American Society of
Anesthesiologists (ASA) grade 1–2; (3) scheduled to undergo laparoscopic UF
surgery. The exclusion criteria were: (1) hypertension; (2) conditions affecting
hemodynamics, such as heart failure; (3) thyroid disease; (4) adrenal disease;
(5) emergency patients; (6) acute or chronic renal dysfunction (abnormal
creatinine and urea nitrogen); (7) impaired liver function (alanine
aminotransferase
The patients were randomly divided into two groups via the random number table method: Group1 (6 units of pituitrin diluted to 20 mL for all injection), and Group2 (6 units of pituitrin diluted to 10 mL for all injection). The patients and surgeons were blind to the assigned group.
Laparoscopic UF surgery is a minimally invasive procedure. Three ports were made in the patient’s abdomen, one of which was in the navel. The lens with an external display was placed into the abdominal cavity through the navel port, and carbon dioxide pneumoperitoneum was induced. The complete uterus could be observed through the display. The two other ports were utilized for surgery. To reduce the bleeding from the surgical wound, the surgeon injected pituitrin in the two concentrations studied into the surgical site and then performed surgery to remove the UFs.
Demographic information, including age, height, weight, number of the UFs, diameter of the largest UF, and location of the largest UF were obtained from the medical records. Surgery-related indicators obtained from the medical records included. MAP1, HR1, MAP2, HR2; MAP3, HR3, Recovery Time were all recorded by the anesthesiologist. Hb1, Hct1, Hb2, Hct2 were obtained from blood tests acquired before and after surgery.
SPSS v26 (IBM, Corp., Armonk, NY, USA) was used for statistical analysis. The
measurement data with normal distribution were represented by mean
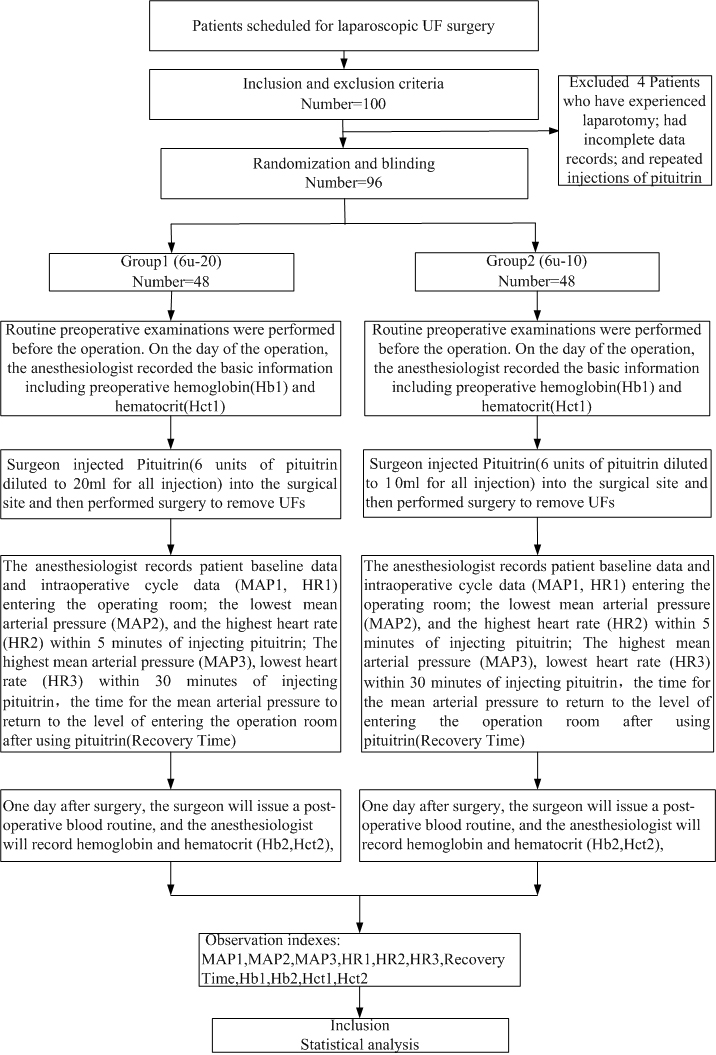
A total of 100 patients were enrolled. an evaluation of 96 patients. The excluded were 4 patients who have experienced laparotomy, had incomplete data records, and repeated injections of pituitrin. There were 48 cases in each group (Fig. 1). All patients underwent successful surgery. There were no significant differences in age, height, weight, number of the UFs the diameter of the largest UF, and location of the largest UF between the two groups. Baseline characteristics are shown in Table 1.
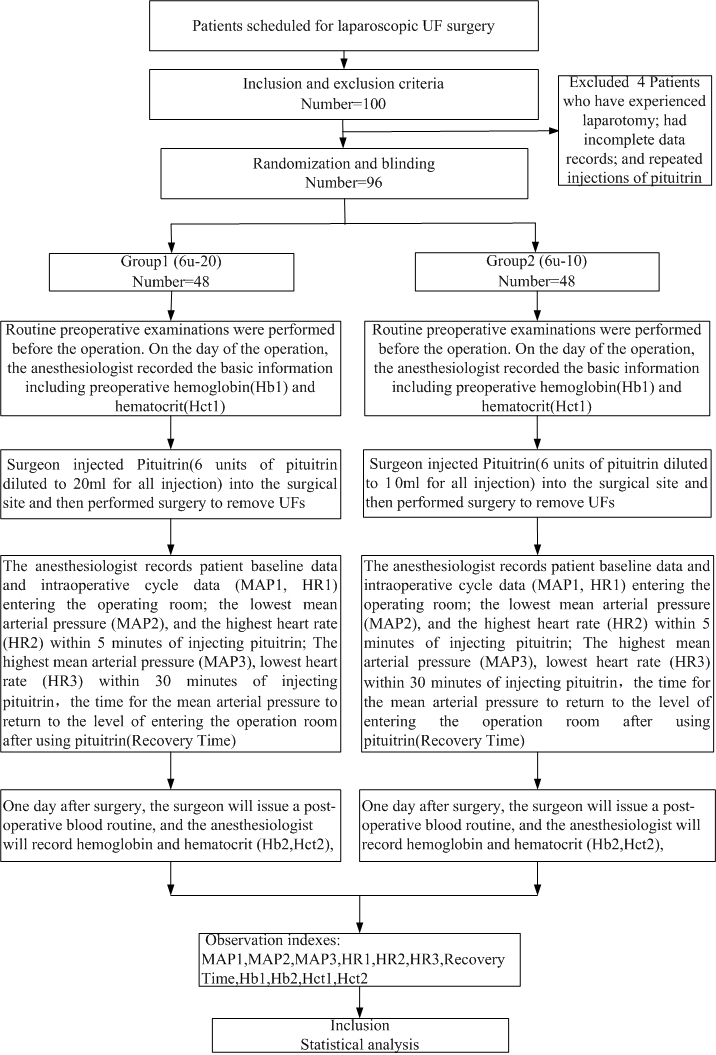 Fig. 1.
Fig. 1.Flow chart.
| Characteristics | 6u-20 Group1 (n = 48) | 6u-10 Group2 (n = 48) | p | |
| Age (years), mean |
39.10 |
39.69 |
0.182 | |
| Height (cm), mean |
163.27 |
161.77 |
0.081 | |
| Weight (kg), mean |
62.031 |
62.542 |
0.054 | |
| Number of UFs mean |
1.54 |
1.65 |
0.765 | |
| Diameter of the largest UF mean |
6.402 |
6.479 |
0.385 | |
| Location of the largest UF | 0.690 | |||
| anterior | 17 (35.4%) | 21 (43.8%) | ||
| posterior | 21 (43.8%) | 19 (39.6%) | ||
| lateral and fundal | 10 (20.8%) | 8 (16.7%) | ||
Table 2 presents the surgery-related data. There were no differences between the groups for MAP1, HR1, MAP2, HR2, MAP3, HR3, Hb1, Hct1, Hb2, Hct2, and Recovery Time between Group1 and Group2.
| Characteristics | 6u-20 Group1 (n = 48) | 6u-10 Group2 (n = 48) | p |
| MAP1 mean |
89.17 |
91.99 |
0.492 |
| MAP2 mean |
77.08 |
80.88 |
0.280 |
| MAP3 mean |
97.83 |
102.12 |
0.948 |
| HR1 mean |
74.91 |
80.25 |
0.248 |
| HR2 mean |
66.13 |
67.02 |
0.722 |
| HR3 mean |
63.69 |
65.31 |
0.292 |
| Recovery Time (min), mean |
29.58 |
31.77 |
0.202 |
| Hb1 mean |
130.19 |
128.81 |
0.308 |
| Hb2 mean |
113.31 |
115.83 |
0.901 |
| Hct1 mean |
38.49 |
38.34 |
0.339 |
| Hct2 mean |
33.56 |
34.61 |
0.762 |
| Note: MAP1 and HR1 were recorded when entering the operating room; MAP2 and HR2
were recorded within 5 minutes after injecting pituitrin; MAP3 and HR3 were
recorded within 30 minutes after injecting pituitrin; Hb1 and Hct1 were recorded
within one week before surgery; Hb2 and Hct2 were recorded one day after surgery. Recovery Time: the time for the mean arterial pressure to return to the level of entering operating room after using pituitrin. | |||
The present study evaluated local injections of two concentrations of pituitrin that have similar effects on the hemodynamic fluctuations during surgery.
Currently, vasopressin is widely used in gynecologic surgery to reduce bleeding, make the operative field clear and shorten the operative time. As not all hospitals have access to vasopressin, pituitrin is a visable alternative. However, there are some differences between pituitrin and vasopressin.
Pituitrin contains oxytocin and vasopressin and is commonly used in laparoscopic UF surgery. Oxytocin activates vascular endothelial receptors [12], increases intracellular calcium ion concentration, promotes the release of nitric oxide, causes vasodilation, and reduces blood flow in UFs [13, 14, 15]. The vasodilation caused by oxytocin can lead to a decrease in blood pressure and an increase in heart rate. Vasopressin acts on vasopressin V1 receptors to cause contraction of vascular smooth muscle and myometrium, thereby reducing bleeding during surgery [16, 17, 18] and shortening operative time. Contraction of vascular smooth muscle and myometrium can cause increased blood pressure and slower heart rate. Pituitrin has a bidirectional effect on circulation. Since the half-life of oxytocin is 3–4 minutes [9, 19] and the half-life of vasopressin is 4–20 minutes [20], the observation time point set in this study is the lowest blood pressure and the highest heart rate within 5 minutes after local injection of pituitrin as well as the highest blood pressure and lowest heart rate within 30 minutes after injection.
The study by Cohen et al. [21] indicates both dilute and concentrated vasopressin solutions that use the same drug dosing demonstrate comparable safety and tolerability when used for minimally invasive myomectomy; however, higher volume administration of vasopressin does not reduce blood loss. These results were consistent with the result of our study.
Our study concluded that the safety profile of local injection of pituitrin in the 6 units of pituitrin diluted to 20 mL and 6 units of pituitrin diluted to 10 mL are the same when used for laparoscopic UF surgery. Pituitrin can reduce bleeding during surgery, theoretically make the surgical field clear, and shorten the operative time. However, pituitrin also has adverse reactions and affects circulation [20]. Therefore, the dosage should be strictly controlled.
The present study had several limitations. First, this study is a single-center study. Some multi-center studies can be carried out for different regions, different races, and groups of people. Second, the sample size can be further expanded for research in the future.
The safety effects of local injection of pituitrin in the 6 units diluted to 20 mL and 6 units diluted to 10 mL are the same when used for laparoscopic UF surgery.
XC—data collection, extraction, drafting of the manuscript, analysis of data, manuscript revision; JM—design and revision, statistical analysis.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Fu Xing Hospital, Capital Medical University (approval number: 2020FXHEC-KY036 ClinicalTrials ID: NCT03524950).
We would like to express my gratitude to all those who helped me during the writing of this manuscript.
This study was supported by the Beijing Municipal Administration of Hospitals’ Clinical Medicine Development of Special Funding Support (#ZYLX201810). The work was supported by grants from the National Natural Science Foundation of China (No. 81871592).
The authors declare no conflict of interest.