Academic Editor: Masatoki Kaneko
Background: We analyzed both early
and late (persistent) phases of each cytomegalovirus (CMV) antibody in mothers
with primary CMV infection during pregnancy and subsequent congenital CMV
infection for a long period from late pregnancy to after delivery using our
stored serum samples. Methods: We used stored serum samples
obtained during pregnancy to after delivery from mothers with CMV immunoglobulin
(Ig) G seroconversion and subsequent infant congenital CMV infection. CMV
antibodies, including CMV IgG titer, IgM titer, and IgG avidity, were assessed
using the Denka IgG assay, Denka IgM assay Ver.1 and Ver.2, and Enzygnost IgG
assay and Denka IgG avidity assay, respectively. We analyzed the dynamics of each
CMV antibody for a long period from late pregnancy to after delivery and
correlations of each antibody, calculating Pearson’s correlation coefficients
(R
Cytomegalovirus (CMV) is the leading cause of transplacental infection worldwide. Infants having congenital CMV (cCMV) infection may be symptomatic (e.g., microcephaly, chorioretinitis, abnormal auditory brainstem response, abnormal brain magnetic resonance image, hepatosplenomegaly, petechia, or thrombocytopenia) or asymptomatic at birth. Both symptomatic and asymptomatic infants with cCMV infection are at risk of neurological sequelae (e.g., sensorineural hearing loss, amblyopia, developmental delay, or cerebral palsy). Maternal primary CMV infection during pregnancy is associated with the occurrence of infant cCMV infection, both symptomatic and asymptomatic. Both non-primary and primary maternal CMV infections during pregnancy have been proposed as risk factors for long-term neurological sequelae in infants with cCMV infection [1].
CMV antibodies used in maternal serological tests generally involve
immunoglobulin (Ig) G, IgM, and IgG avidity. Maternal CMV serological tests aim
to diagnose primary infection during pregnancy. The gold standard for diagnosing
primary infection is the seroconversion (negative to positive) of CMV IgG during
pregnancy. Furthermore, a set of positive IgG, positive IgM, and low IgG avidity
during pregnancy is not a confirmatory diagnostic tool but a realistic one in
mothers in whom IgG seroconversion cannot be detected. CMV IgM is detectable at
0–3 weeks, with peak antibody titers observed at 4–12 weeks after primary CMV
infection. CMV IgM in mothers is not always specific for primary infection. CMV
IgM may persist for months or years after primary infection or occur due to assay
cross-reactivity of antibody tests. Additional measurements of IgG avidity are
recommended in mothers with positive IgG and IgM to identify primary infection.
Performance parameters for CMV IgM for primary infection may vary slightly, such
as sensitivity, specificity, and positive and negative predictive values. Recent
studies on different CMV IgM assays showed that the concordance of the outcomes
for CMV IgM between those assays is
We have performed the maternal CMV antibody screening “Cytomegalovirus in Mother and infant-engaged Virus serology (CMieV)” program in Mie, Japan, since 2013. We identified mothers with primary infection during pregnancy and tested their infants for cCMV. We recorded that high CMV IgM titer was correlated with cCMV occurrence in mothers with primary infection [16]. Alternatively, we demonstrated that the young age of mothers was a high-risk factor for primary infection and that multiparity combined with the young age of mothers was a high-risk factor for cCMV occurrence [17, 18].
In this study, using our stored serum samples in the antibody screening program, we analyzed each CMV antibody in mothers with primary infection during pregnancy and subsequent cCMV infection in both early and late phases after primary infection for a long period from late pregnancy to after delivery.
After diagnosing infant cCMV infection in the CMieV program, we collected and stored serum samples from mothers for as long as possible after delivery. In this study, we used stored (frozen to –80 ℃) serum samples at Mie University Hospital in Mie, Japan, obtained during pregnancy to after delivery from mothers with CMV IgG seroconversion (negative to positive) during pregnancy and subsequent infant cCMV infection. As previously reported, infant cCMV infection was diagnosed by a quantitative CMV DNA test in fresh neonatal urine samples using a real-time polymerase chain reaction method at Mie University Hospital [16].
CMV antibodies, including CMV IgG titer, IgM titer, and IgG avidity, were measured at two institutions. At Aisenkai Nichinan Hospital in Miyazaki, Japan, CMV IgG avidity was tested using the Enzygnost CMV IgG assay (Siemens Healthcare Diagnostics K.K., Tokyo, Japan), as previously reported [16]. CMV IgG, IgM, and IgG avidity were tested using the Denka CMV IgG, IgM, and IgG avidity assays (Denka Co. Ltd., Tokyo, Japan), respectively, at the Vaccine & Diagnostics R&D Dept. Life Innovation, Denka Co. Ltd. Niigata, Japan [19]. We used two types of the Denka CMV IgM assays: Denka IgM assay Ver.1 (Seiken-CMV IgM kit: Approval No. of the Ministry of Health, Labor and Welfare, Japan; 21600AMZ00201000) (Denka Co. Ltd., Tokyo, Japan) and Denka IgM assay Ver.2 (CMV IgM-Seiken kit: Approval No.; 23100EZX00005000) (Denka Co. Ltd., Tokyo, Japan).
We analyzed the dynamics of each CMV antibody for a long period from late
pregnancy to after delivery and correlations of each antibody, calculating
Pearson’s correlation coefficients (R
We used 67 serum samples obtained between 2013 and 2018 from 12 participants (all Japanese) with CMV IgG seroconversion during pregnancy and subsequent infant cCMV infection (all asymptomatic infections). The clinical and laboratory characteristics of the participants are shown in Tables 1,2.
| Median | Range | ||
| Age (year) | 27.5 | 16–34 | |
| Parity (para) | 0.5 | 0–3 | |
| Weeks |
11.0 | 8–14 | |
| Weeks |
36.0 | 19–50 | |
| Weeks |
38.5 | 37–40 | |
| Serum sample number, per case | 5.5 | 2–11 | |
| Weeks |
123.0 | 38–196 | |
| Months of the last collection of serum samples, per case |
30.8 | 9.5–49.0 | |
| CMV IgG titer at IgG seroconversion (value) | 10.6 | 5.1–24.9 | |
| CMV IgM titer at IgG seroconversion (index) | |||
| Denka IgM assay Ver.1 | 5.71 | 1.50–11.84 | |
| Denka IgM assay Ver.2 | 1.44 | 0.43–4.77 | |
| CMV IgG avidity index at IgG seroconversion (%) | |||
| Enzygnost IgG assay | 24.3 | 0.6–45.9 | |
| Denka IgG avidity assay | 46.9 | 10.3–69.0 | |
CMV, cytomegalovirus; IgG, immunoglobulin G; IgM, immunoglobulin M. | |||
| Case No. | Age (year) | Parity (para) | Weeks of antibody tests at early pregnancy (IgG negative) | Weeks of antibody tests at late pregnancy (IgG seroconversion) | Gestational weeks of delivery (week) | Number of total serum samples used | Number of serum samples used in Denka IgG avidity | Weeks |
Months of the last collection of serum samples |
IgG titer at IgG seroconversion (value) | IgM titer at IgG seroconversion in the Denka assay Ver.1 (index) | IgM titer at IgG seroconversion in the Denka assay Ver.2 (index) | IgG avidity index at IgG seroconversion in the Enzygnost IgG assay (%) | IgG avidity index at IgG seroconversion in the Denka IgG avidity assay (%) |
| 1 | 23 | 0 | 11 | 35 | 39 | 9 | 8 | 196 | 49.0 | 5.1 | 8.34 | 1.83 | 8.2 | 35.6 |
| 2 | 28 | 1 | 8 | 38 | 38 | 7 | 7 | 117 | 29.3 | 10.8 | 5.81 | 1.58 | 22.7 | 43.5 |
| 3 | 17 | 0 | 11 | 34 | 38 | 8 | 7 | 130 | 32.5 | 11.0 | 2.07 | 0.51 | 16.2 | 43.1 |
| 4 | 27 | 3 | 10 | 19 | 39 | 6 | 6 | 120 | 30.0 | 10.4 | 11.22 | 4.09 | 2.7 | 10.3 |
| 5 | 30 | 0 | 9 | 34 | 38 | 11 | 9 | 171 | 42.8 | 24.4 | 10.94 | 3.36 | 45.9 | 60.5 |
| 6 | 25 | 0 | 9 | 37 | 40 | 2 | 2 | 45 | 11.3 | 9.9 | 11.75 | 4.13 | 4.6 | 27.3 |
| 7 | 30 | 2 | 12 | 34 | 38 | 2 | 2 | 38 | 9.5 | 8.7 | 11.84 | 4.77 | 0.6 | 21.1 |
| 8 | 16 | 0 | 14 | 48 | 39 | 4 | 3 | 126 | 31.5 | 24.9 | 1.50 | 0.43 | 32.2 | 66.7 |
| 9 | 21 | 0 | 12 | 39 | 39 | 5 | 4 | 151 | 37.8 | 8.6 | 2.68 | 0.68 | 33.2 | 51.0 |
| 10 | 29 | 1 | 8 | 36 | 38 | 2 | 2 | 38 | 9.5 | 24.4 | 5.60 | 1.30 | 34.8 | 50.2 |
| 11 | 34 | 1 | 11 | 50 | 37 | 6 | 5 | 139 | 34.8 | 18.9 | 1.73 | 0.50 | 29.6 | 69.0 |
| 12 | 31 | 1 | 12 | 36 | 40 | 5 | 3 | 94 | 23.5 | 9.5 | 2.20 | 0.52 | 25.9 | 50.4 |
IgG, immunoglobulin G; IgM, immunoglobulin M. | ||||||||||||||
All CMV antibodies could be measured in 58 of 67 serum samples. However, in the remaining nine serum samples, CMV IgG avidity using the Denka IgG avidity assay could not be measured because of insufficient sample volume.
CMV IgG increased until 61 weeks and did not change significantly afterward, except in in case 8 (Supplementary Fig. 1). CMV IgM decreased until 52 weeks and did not change significantly after that in both Denka IgM assays. Only case 5 showed a continuous decrease after 50 weeks (Fig. 1).
 Fig. 1.
Fig. 1.Dynamics of cytomegalovirus immunoglobulin (Ig) M. (A) Dynamics of IgM in the Denka IgM assay Ver.1. (B) Dynamics of IgM in the Denka IgM assay Ver.2. IgM, Immunoglobulin M.
CMV IgG avidity increased until 64 weeks and did not change significantly after that in both the Enzygnost IgG and Denka IgG avidity assays. The distribution of IgG avidity index values in the Denka IgG avidity assay was narrower than that in the Enzygnost IgG assay (Fig. 2).
 Fig. 2.
Fig. 2.Dynamics of cytomegalovirus immunoglobulin (Ig) G avidity. (A) Dynamics of IgG avidity in the Enzygnost IgG assay (n = 67). (B) Dynamics of IgG avidity in the Denka IgG avidity assay (n = 58). IgG, Immunoglobulin G.
In CMV IgM, a strong positive correlation was found (R
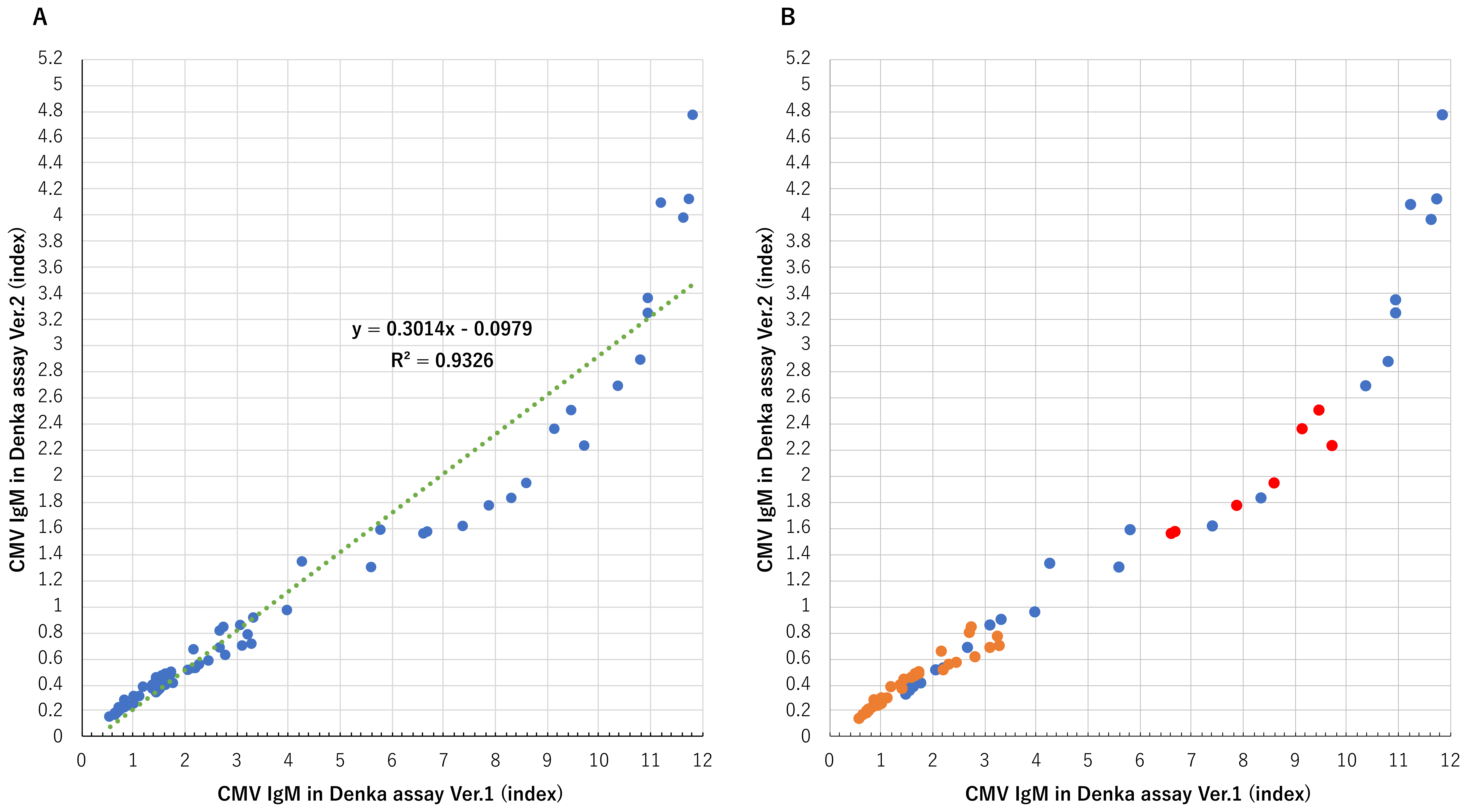 Fig. 3.
Fig. 3.Correlation of cytomegalovirus immunoglobulin (Ig) M between the Denka IgM assays. (A) Correlation of IgM between the Denka IgM assays Ver.1 and Ver.2. (B) Color separation into two phases (early phase: before 60 weeks and late phase: after 60 weeks). In B, blue and orange circles are plots of the early and late phases, respectively. Red circles are plots of late phase in the exceptional case (Case 5). IgM, Immunoglobulin M.
Serum results of the late phase (after 60 weeks) were subsumed into the area of low CMV IgM titer except in case 5, which showed an exceptional transition (Fig. 3B). Alternatively, serum results of the early phase (before 60 weeks) were widely distributed. In CMV IgG avidity, no correlation was found between the Enzygnost IgG assay and Denka IgG avidity assay (Supplementary Fig. 2). No correlation was found between the Denka IgM assay Ver.2 and Denka IgG avidity assay (Fig. 4A) and between the Denka IgM assay Ver.2 and Enzygnost IgG assay (Supplementary Fig. 3).
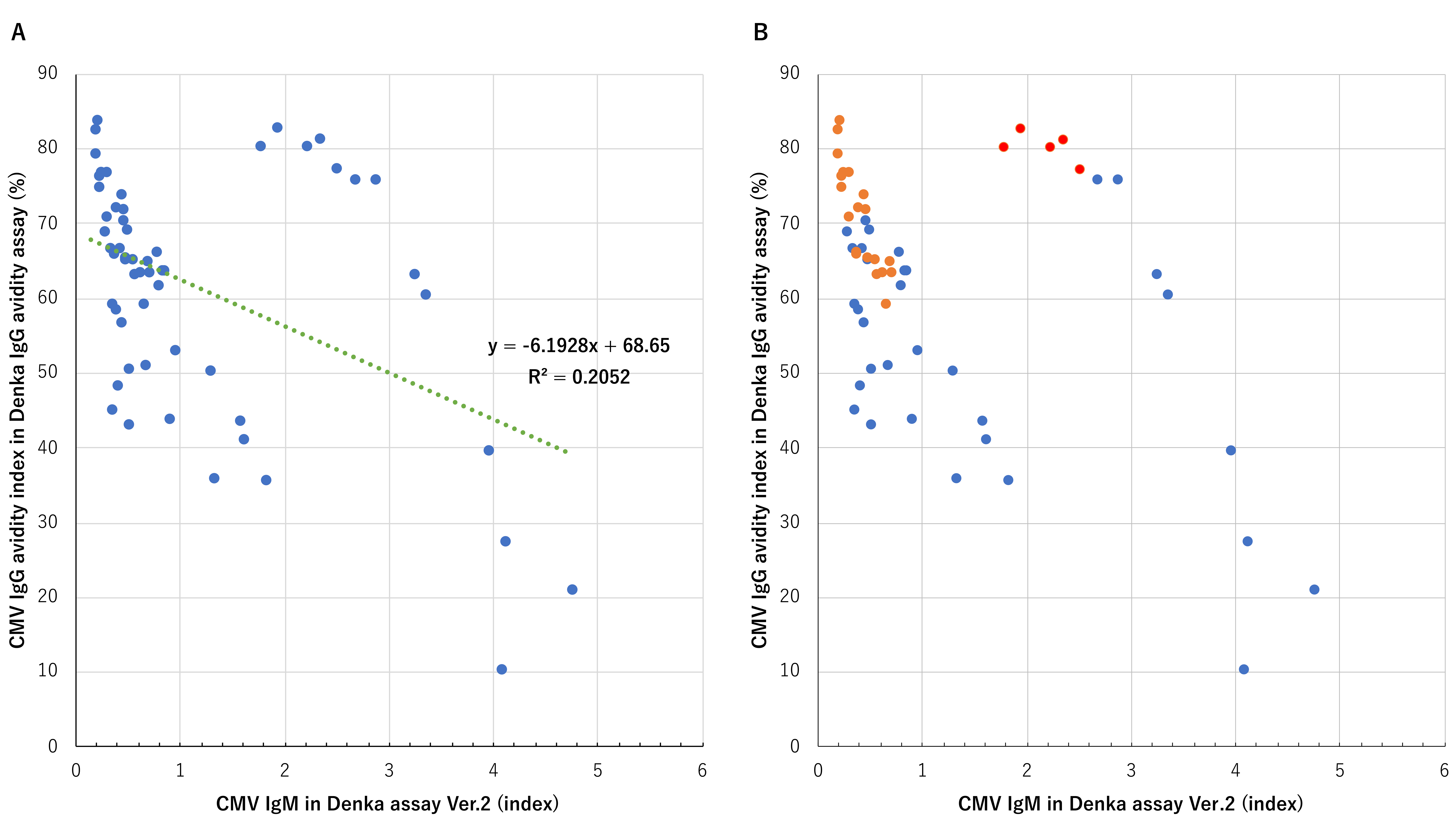 Fig. 4.
Fig. 4.Correlation between cytomegalovirus immunoglobulin (Ig) M and IgG avidity. (A) Correlation between IgM in the Denka IgM assay Ver.2 and IgG avidity in the Denka IgG avidity assay (n = 58). (B) Color separation into two phases (early phase: before 60 weeks and late phase: after 60 weeks) (n = 58). In B, blue and orange circles are plots of the early and late phases, respectively. Red circles are plots of the late phase in the exceptional case (Case 5). IgM, Immunoglobulin M; IgG, Immunoglobulin G.
Serum results of the late phase (after 60 weeks) were subsumed into the area of low CMV IgM titer in assay Ver.2 and high IgG avidity in the Denka IgG avidity assay (Fig. 4B). No correlation was found between the Denka IgM assay Ver.1 and the different IgG avidity assays (Supplementary Figs. 4,5).
CMV IgG antibody and IgG avidity increased until 60 weeks and did not change afterward. In this study, we considered this period as the early phase after primary CMV infection and another period after 60 weeks as the late phase. We focused on the differences in CMV antibodies between the two phases. CMV IgM in case 5 did not easily decrease over time, although the IgG avidity increased. In other cases, serum results of the late phase (after 60 weeks) were subsumed into the area of low CMV IgM titer. This probably means that a low IgM titer represents the late phase after primary infection. Serum results of the early phase (before 60 weeks) were distributed widely compared with those of the late phase. High IgM titer probably represents the early phase after primary infection. Actually, we recorded in the previous study that high CMV IgM titer was correlated both with primary CMV infection during early pregnancy and with cCMV occurrence in these mothers [16]. Alternatively, some patients with low IgM titer in the early phase in this study were possibly in the real late phase after primary infection. For instance, although plots of 45 and 49 weeks in Case 2 and 12 were in early phase, their IgG avidities were as high as others’ IgG avidity in late phase. Since the true timing of primary infection was unknown, we classified patients as being in the early phase; however, they may have been in the late phase.
CMV IgG titer, IgM titer, and IgG avidity are usually used in maternal CMV serological screening. Typically, a set of positive CMV IgG and IgM, and low IgG avidity means primary CMV infection in pregnant women. Alternatively, a set of positive IgG and IgM, and high IgG avidity or another set of positive IgG and negative IgM (and high IgG avidity) mean non-primary infection during pregnancy [1]. Kaneko et al. [20] reported a highly effective model for predicting high IgG avidity in pregnant women with positive IgM by low IgM titer. However, we could not discover a correlation between CMV IgM titer and IgG avidity in this study. If we could discover a correlation between the two antibodies, we could demonstrate that either CMV IgM titer or IgG avidity is needed in maternal CMV antibody screening. Considering the lack of correlation between the two antibodies, we assumed that IgM titer and IgG avidity are needed in the antibody screening to identify pregnant women with primary CMV infection during pregnancy who had a high risk of subsequent cCMV.
The reason why CMV IgM antibody is not sufficient to identify primary CMV infection is its low specificity regardless of high sensitivity [1]. Low specificity in identifying primary infection is known to occur because of the long persistence of CMV IgM antibody after primary infection. Persistent IgM is a long-lasting positivity of IgM antibody in patients after a viral infection. Besides CMV, the rubella virus is well known as a virus presenting persistent IgM [21]. Unlike strong IgM positivity in the early phase of primary infection, weak positivity is usually present in persistent IgM during the late phase of primary infection. As the titers showing a weak positivity of IgM antibody in patients with persistent IgM differ between IgM assays, a diagnosis of persistent IgM is not made using IgM titers alone. Therefore, IgG antibody avidity testing is useful in diagnosing persistent IgM. A weak, long-lasting IgM positivity concurrent with a high IgG antibody avidity is essential in diagnosing persistent IgM. In this study, serum results of the late phase (after 60 weeks) were subsumed into the area of high CMV IgG avidity and low CMV IgM titer. That area probably was equivalent to persistent IgM, lasting more than one year after primary infection and delivery. As the titers of the Denka IgM assay tended to be low in Ver.2, the persistence of IgM antibody was considered to be less in the Denka assay Ver.2 than in Ver.1. From the viewpoint of the clinical use of IgM assay, Ver.2 might be useful in identifying primary CMV infection.
This study had some limitations. First, the interval between serum sampling and the serum sample number varied with each case. The serum samples we used were not collected at regular but discrete times and were not equally collected from all cases. The longer the interval, the lower the accuracy of the CMV antibody changes recorded might be in this study. Second, we did not obtain serum samples during the middle stage of pregnancy. Since we obtained serum samples in the late stage and afterward, we could not confirm patients with IgG seroconversion in the middle stage of pregnancy. Even if a patient was seroconverted in the middle stage, she was likely included in the analysis with those who were seroconverted in the late stage of pregnancy.
Even with some exceptions, CMV IgM antibody and IgG avidity in pregnant women with primary CMV infection reached their extremes until 50 and 60 weeks, respectively, and did not change considerably after. CMV antibodies in mothers during the late phase (after 60 weeks, assuming the expectant weeks of delivery as 40) showed high IgG avidity and low IgM titer, which probably was equivalent to the persistent IgM. This study implied that CMV IgM was less persistent in the Denka assay Ver.2 than in Ver.1. The Denka assay Ver.2 might be useful in identifying primary CMV infection.
Not available for data and materials other than those in the manuscript.
KT and AK designed the research study and performed the research. TM performed CMV IgG avidity tests. KT and MH-A analyzed the data. KT and HT wrote the manuscript. EK, MK and FM assisted in operating the maternal CMV antibody screening “Cytomegalovirus in Mother and infant-engaged Virus serology (CMieV)” program. TI supervised this work. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
This observational study was conducted in accordance with the Declaration of Helsinki. We adopted an opt-out approach and obtained ethical approval (No. 1703) from the Clinical Research Ethics Review Committee of the Mie University Hospital, Mie, Japan.
We appreciate all obstetrical institutions that partook in the maternal CMV antibody screening “CMieV” program in Mie, Japan. Our expressed gratitude goes to the directors of the Mie Association of Obstetricians and Gynecologists (H. Obata, K. Kanamaru, Y. Kamimoto, T. Kikukawa, T. Takakura, H. Tanaka, K. Nagao, S. Nii, K. Nishimura, Y. Maegawa, T. Maezawa, and H. Minoura) for facilitating the CMieV program. We also thank M. Yamazaki and T. Bessho (the Vaccine & Diagnostics R&D Dept. Life Innovation, Denka Co. Ltd. in Niigata, Japan) for their help with antibodies tests.
This study was supported in part by the Clinical Research Program for Child Health and Development, awarded by the Japan Agency for Medical Research and Development (AMED) (grant no. 22gk0110061h0001) and JSPS KAKENHI (grant no. 22K15918).
The authors declare no conflict of interest.
