Academic Editor: Ugo Indraccolo
Background: To identify endometrial mesenchymal stem cells (eMSCs) in
retrograde menstruation, in various endometriosis lesions, in normal control
tissues, and to investigate the association between eMSCs and endometriosis. We
also plan to evaluate the effect of gonadotropin-releasing hormone agonists
(GnRH-a) on eMSCs. Methods: Patients diagnosed with endometriosis were
included if they had experienced surgery during the time frame 1 January 2015 to
31 December 2019 in West China Second Hospital, Sichuan University.
Immunofluorescence was performed to identify eMSCs in those tissues with cell
surface markers PDGFR-
Chronic pelvic pain (CPP) and dysmenorrhea are the most common health problems that affect women of childbearing age [1]. There is a variety of causes for CPP which include endometriosis, adenomyosis, chronic infection, vulvodynia, irritable bowel syndrome, and bladder pain syndrome. Endometriosis is the most common cause of CPP [2], accounting for 24–40% of all CPP diagnoses [3]. For the management of endometriosis, surgery is a frequent choice since the efficacy of medical treatment alone is either poorly documented or of limited efficacy. However, owing to the unclear etiology of endometriosis, its recurrence rate following surgery remains high. Reoperation occurs in 51% of patients with endometriosis, often resulting in damage to ovarian reserve [4]. The risk for reoperation, coupled with uncertainty for results and the presence of continued pelvic pain, makes endometriosis a chronic disease.
Reya et al. [5] proposed a cancer stem cell (CSC) theory that some rare cell populations that exist in cancer tissues, had the capacity for self-renewal, multipotential differentiation, tumorigenesis, metastasis, relapse and treatment-resistance. Evidence exists that endometrial mesenchymal stem cells (eMSCs) are located within the endometrium [6]. eMSCs are believed to contribute to cyclical changes of human endometrium, including proliferation, differentiation, tissue breakdown and shedding under the influence of estrogen and progesterone during the menstrual cycle [7]. The migration of eMSCs is similar to that of CSCs, with endometriosis possessing biological behaviors of local aggressiveness, distant metastasis and high disease recurrence. eMSCs may play a key role in the development and relapse of endometriosis. A suggested hypothesis is that eMSCs are abnormally shed during menses, present in amniotic fluid and blood. They are capable of gaining access to the peritoneal cavity or an abdominal wall scar, where they establish ectopic implants in those women who develop endometriosis.
This work aimed to identify eMSCs in retrograde menstruation, various endometriosis lesions, and normal control tissues in order to investigate the association between eMSCs and endometriosis. The effectiveness of gonadotropin-releasing hormone agonists (GnRH-a) on endometriosis was also compared using percent of eMSCs between the groups.
Patients with the following criteria were enrolled in this study. Criteria included: (1) All subjects aged 20 to 45 underwent surgery after menstruation during the time frame 1 January 2015 to 31 December 2019 at West China Second Hospital, Sichuan University; (2) Diagnoses were endometriosis including ovarian endometriosis, adenomyosis, abdominal wall scar endometriosis (AWSE) and deep endometriosis (DE), all confirmed by experienced pathologists; (3) All subjects had regular menstrual cycles (25–35 days) and were documented as not being pregnant. Patients who received any kind of hormonal therapy (levonorgestrel-releasing intrauterine system or oral contraceptives) for endometriosis before surgery, or suffered from any severe medical or surgical complications were excluded. For postoperative management, all patients were to receive 6 cycles of GnRH-a. Following the treatment, patients were recommended to attempt pregnancy, take oral contraceptives or receive a levonorgestrel-releasing intrauterine system. All patients underwent follow-up for at least 2 years. Relapse of endometriosis-associated dysmenorrhea, dyspareunia, non-menstrual pelvic pain, or a cyst more than 2 cm detected by ultrasound was considered a recurrence. For patients with adenomyosis, postoperative hormonal therapy or follow-up was not required as they underwent a total hysterectomy. To evaluate the effectiveness of GnRH-a on eMSCs, we selected patients who suffered from dysmenorrhea or DE. These patients had indications for preoperative GnRH-a treatment. Before surgery, they received a minimum of 3 cycles of GnRH-a (leuprorelin or triptorelin acetate 3.75 mg subcutaneous injection every 4 weeks). We selected matched control patients who had similar clinical manifestation and were diagnosed with adenomyosis or DE. These patients preferred surgery to GnRH-a and they were matched (1:1) with treatment patients on age, body mass index (BMI), fertility desire and operative approach. Informed consent was obtained from all patients in the study. This study was approved by the ethics committee on human research at West China Second Hospital, Sichuan University.
Retrograde menstruation and tissue sections were collected to detect eMSCs by
utilization of immunofluorescence. For immunofluorescence staining to identify
eMSCs, primary antibodies included mouse anti-human anti-CD146 and rabbit
anti-human anti-PDGFR-
Data was presented as mean
In the period from 1 January 2015 to 31 December 2019, a total of 508
patients, which included 270 cases of ovarian endometriosis, 129 cases of
adenomyosis, 37 cases of AWSE, and 72 cases of DE, were enrolled. The age (mean
| Group | OE (n = 270) | AM (n = 129) | AWSE (n = 37) | DE (n = 72) |
| Age (year) | 32.7 |
39.2 |
36.8 |
33.4 |
| BMI (kg/m |
20.1 |
20.8 |
21.1 |
20.8 |
| Nulliparity | 175 | 48 | 0 | 41 |
| Laparotomy | 91 | 77 | 37 | 37 |
| 2-year recurrence | 7.4% | NS | 2.7% | 6.9% |
| OE, ovarian endometriosis; AM, adenomyosis; AWSE, abdominal wall scar endometriosis; DE, deep endometriosis; BMI, body mass index; NS, not statistics. | ||||
For evaluation of GnRH-a, a treatment group consisted of 50 adenomyosis patients
and 16 DE patients, with a similar control group. There was no significant
difference from baseline between the treatment group and the control group
(p
| Treatment group (n = 66) | Control group (n = 66) | p | |
| Adenomyosis, n (%) | 50 (75.76%) | 50 (75.76%) | - |
| DE, n (%) | 16 (24.24%) | 16 (24.24%) | - |
| Age (years) | 36.0 |
37.5 |
0.14 |
| BMI (kg/m |
20.6 |
20.7 |
0.92 |
| Nulliparity, n (%) | 31 (46.97%) | 32 (48.48%) | 0.99 |
| Laparotomy, n (%) | 29 (43.94%) | 33 (50.00%) | 0.78 |
| Laparoscopy, n (%) | 37 (56.06%) | 33 (50.00%) | 0.78 |
| 2-year recurrence* | 2 | 3 | 0.62 |
| GnRH-a, gonadotropin-releasing hormone agonists; DE, deep endometriosis; BMI, body mass index; * only statistics for DE group. | |||
The operation was carried out after menstruation. Twenty-three cases of
retrograde menstruation were found during surgery and gathered for analysis. We
detected eMSCs in retrograde menstruation material. As shown in Fig. 1, the eMSCs
expressing PDGFR-
 Fig. 1.
Fig. 1.
The eMSCs in retrograde menstruation (
eMSCs were detected in various endometriotic lesions. However, eMSCs could not
be identified in normal ovary, myometrium, adipose tissue or peritoneal membrane.
Fig. 2 shows the comparison between endometriosis and normal tissues. The
percents (mean
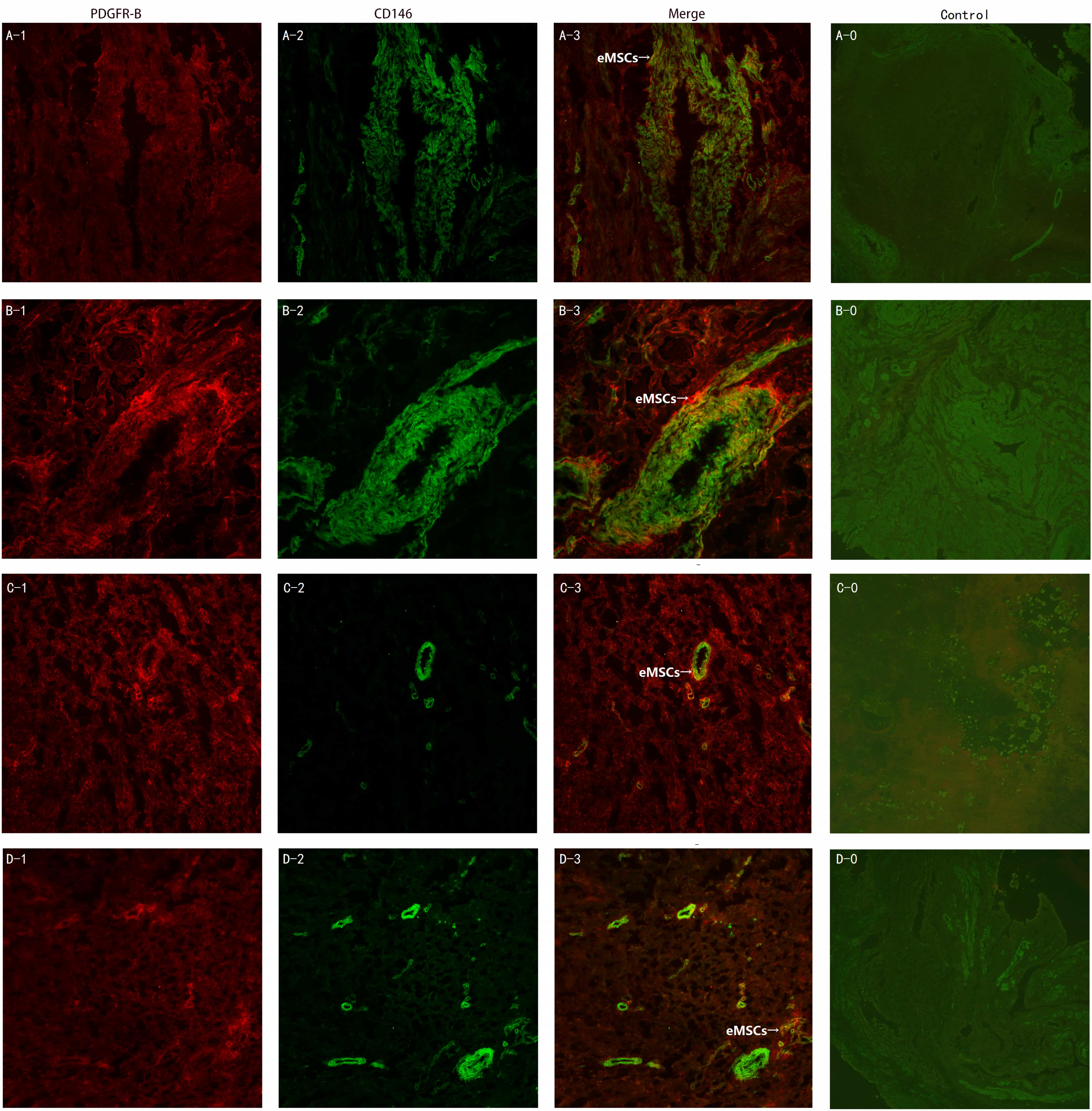 Fig. 2.
Fig. 2.The eMSCs in different endometriosis lesions and control normal
tissues (
 Fig. 3.
Fig. 3.Percent of eMSCs in different endometriosis lesions. (A) Ovarian endometriosis relapse-free. (a) Ovarian endometriosis with relapse. (B) Adenomyosis. (C) Abdominal wall scar endometriosis relapse-free. (c) Abdominal wall scar endometriosis with relapse. (D) Deep endometriosis relapse-free. (d) Deep endometriosis with relapse.
Specimens obtained from the GnRH-a treatment and control groups were tested to
quantitatively compare eMSCs in both groups. As shown in Fig. 4, eMSCs were found
in both adenomyosis and DE in the two groups. There was no statistical difference
in percents of eMSCs between the treatment group and the control group for
adenomyosis patients (1.00%
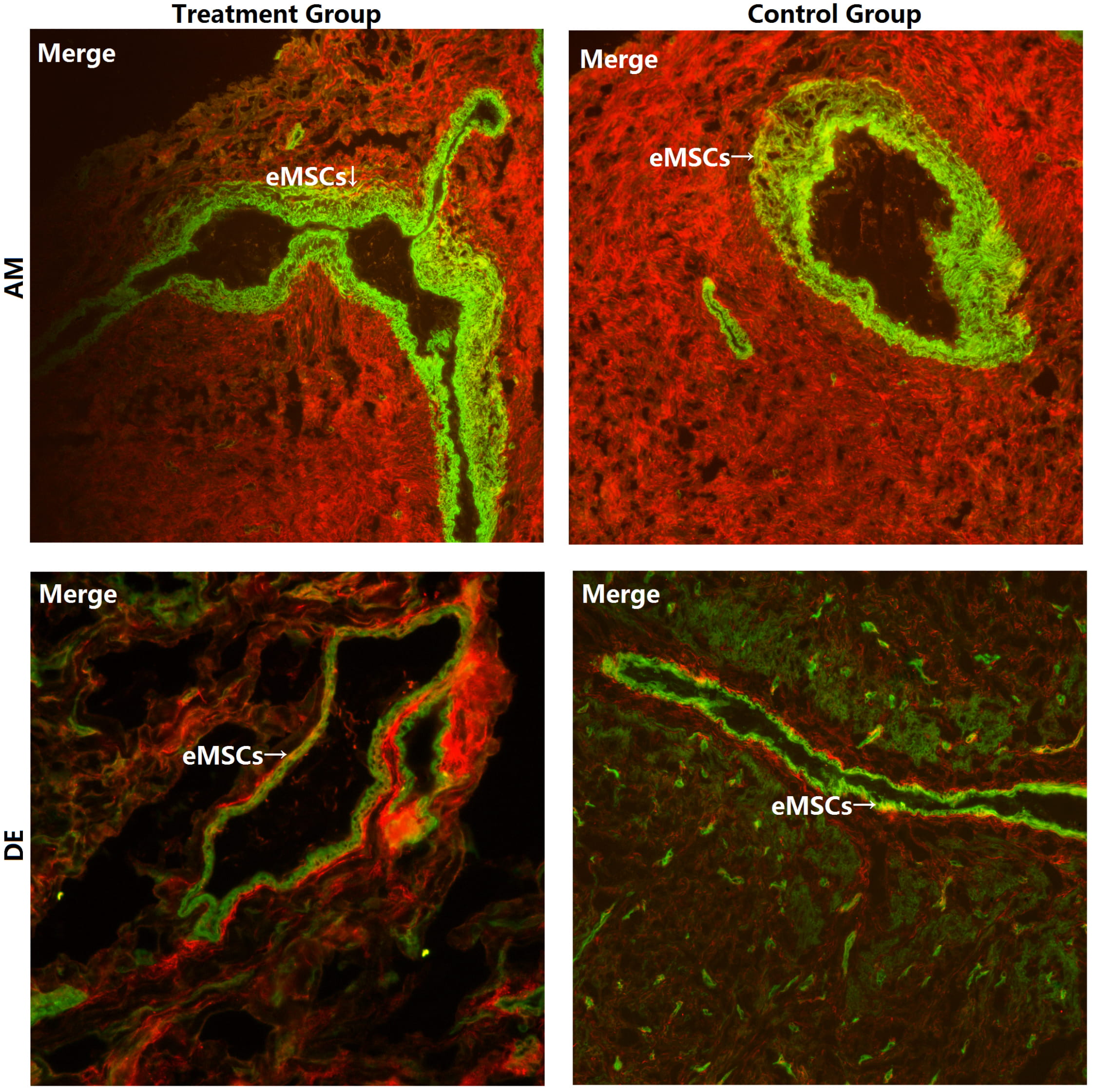 Fig. 4.
Fig. 4.The eMSCs in the GnRH-a treatment group and the control group
(
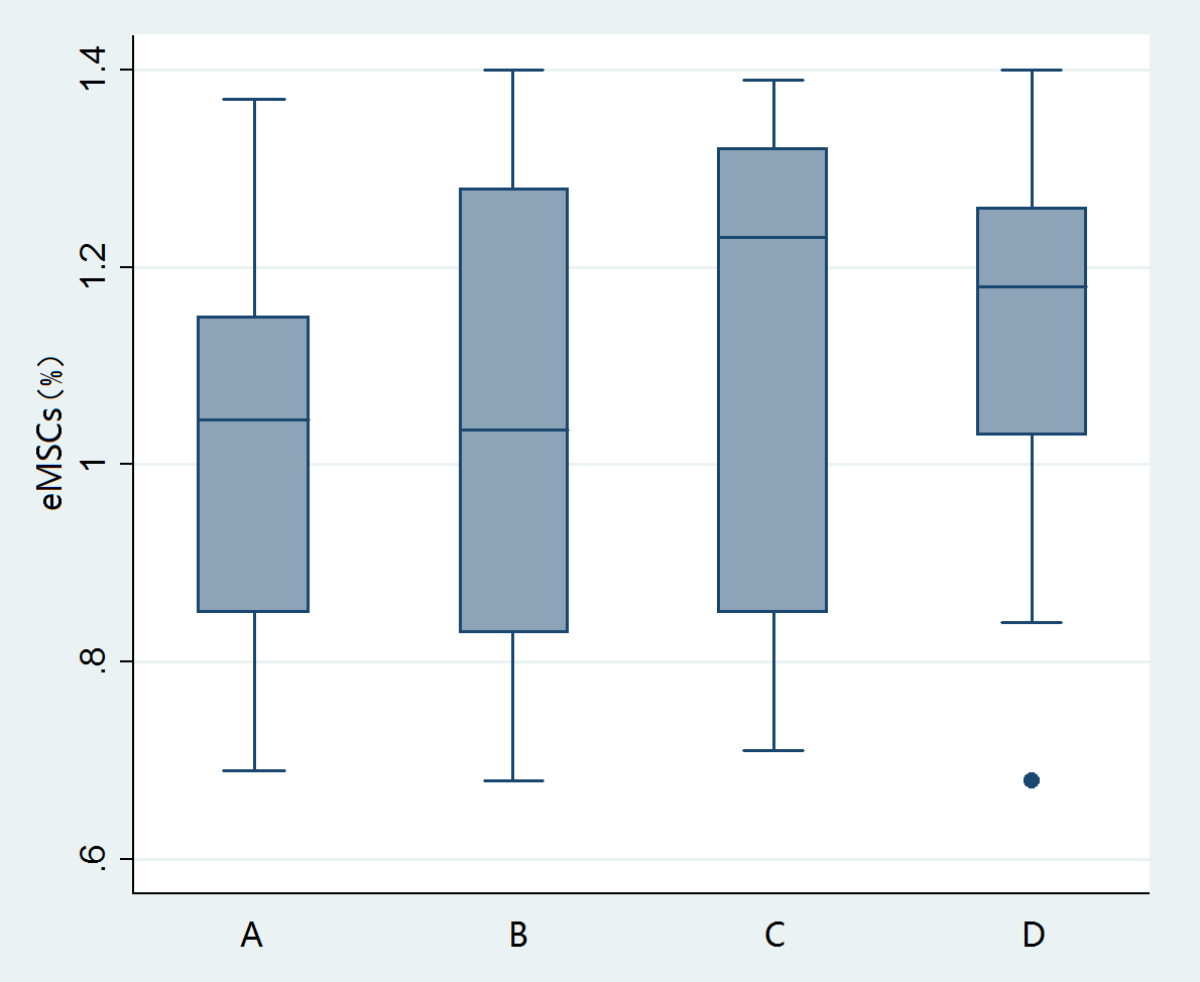 Fig. 5.
Fig. 5.Percents of eMSCs in the GnRH-a treatment group and the control group. (A) Treatment group of adenomyosis. (B) Control group of adenomyosis. (C) Treatment group of deep endometriosis. (D) Control group of deep endometriosis.
CPP and dysmenorrhea are common health problems and affect women of reproductive age. Women with CPP, regardless of a diagnosis of endometriosis, experience significant negative impact across a range of life issues including education, work, social, and sexual relationships [8]. Although endometriosis is one of the most common gynecological disorders, the etiology of endometriosis still remains unclear, resulting in unsatisfactory management and high risk of recurrence after surgery. Theories include implantation theory, metaplasia theory of coelomic epithelium and induction theory, none of which clearly explain the etiology of endometriosis resulting in non-ideal treatments [9, 10, 11]. To improve therapeutic effect, the etiology of endometriosis needs to be clarified. Previous studies have suggested that the recurring endometriotic lesions arise from lesions or cells not completely removed during the primary surgery [12]. Thus, the recurrence may be unavoidable until the etiology is clarified and targeted therapy developed. The CSCs theory proposes that both tumor development and progression are driven by undifferentiated stem cells capable of self-renewal and tumor-initiation. Considering the migration of eMSCs being similar to that of CSCs, and with endometriosis possessing biological behaviors of local aggressiveness, distant metastasis and high disease recurrence, similar to those of ovarian cancer, eMSCs may play a key role in development and relapse of endometriosis.
In 2007, Schwab et al. [6] harvested CD146
GnRH-a, a man-made gonadotropin-releasing hormone, can suppress adenohypophysis and reduce estradiol. GnRH-a could be an adjuvant treatment for endometriosis after surgery [20]. However, the effect of GnRH-a is reversible and recurrence may occur if treatment ceases [21]. A multicenter randomized controlled trial demonstrated that compared with surgery alone, postoperative GnRH-a could prolong the period of recurrence but not effect fertility or reduce recurrence rate (p = 0.08) [22]. In our research, percents of eMSCs showed no statistical difference between the GnRH-a treatment group and the control group, whether adenomyosis (p = 0.27) or DE (p = 0.82) was present. We postulated that GnRH-a may only affect mature endometrial cells except for eMSCs. When treatment is completed or interrupted, the patients’ hormone concentrations return and eMSCs are activated, resulting in recurrence. This suggests that the effect of GnRH-a may be limited for endometriosis.
CSCs also have the capacity to affect treatment-resistant tumors [5]. The traditional therapy might not affect CSCs, since residual cells developed homologous ovarian cancer lesions near the primary site [23, 24]. Evidence has revealed that CSCs are not only responsible for primary tumor growth, metastasis and relapse of disease, but also for the development of chemoresistance [25]. Considering that the migration of eMSCs is similar to that of CSCs and that endometriosis possesses biological behaviors of local aggressiveness, distant metastasis and high disease recurrence similar to those of ovarian cancer, eMSCs may also play a leading role in the limited efficacy of various drug treatments. Effects of drugs and conservative surgery have a limited role for endometriosis [4]. More than half of patients who underwent conservative surgery required a second surgical procedure. Repeat surgery may damage the patient’s ovary harming fertility as well as endocrine function [26]. Recurrent dysmenorrhea and infertility also reduce the patient’s quality of life. Our results have demonstrated that peritoneal endometriosis (ovarian endometriosis and DE) might be associated with eMSCs in retrograde menstruation while AWSE is associated with their presence in amniotic fluid at the time of caesarean section. Regardless of whether patients received postoperative GnRH-a, the recurrence rate of AWSE is far below that of peritoneal endometriosis following complete removal of lesions [27]. This may suggest that retrograde menstruation is closely related to the inevitable recurrence of endometriosis within the pelvic cavity. Hence, we postulate that even though surgery could remove every lesion, recurrence cannot be prevented. We suggest that gynecologists regard endometriosis as a chronic disease and develop an individual lifetime management plan for patients based on age, clinical manifestations, fertility desire and quality of life.
Our study demonstrated that eMSCs played a critical role in the development and recurrence of endometriosis and that GnRH-a did not affect eMSCs. Gynecologists may regard endometriosis as a chronic disease resulting in lifetime management, especially for patients with CPP.
JZ—protocol development, data collection, data analysis, manuscript writing. XL—data analysis, manuscript editing. TY—protocol development, manuscript editing. AT—data collection, data analysis, manuscript writing. RP—data collection, data analysis, manuscript writing. GZ—data analysis, manuscript writing. SL—data analysis, manuscript writing. XZ—protocol development, manuscript editing. CB—protocol development, manuscript editing. GS—protocol development, manuscript editing. All authors read and approved the final manuscript.
This study was approved by the ethics committee on human research at West China Second Hospital, Sichuan University (No. 2020074). All subjects gave their informed consent for inclusion before they participated in the study.
Not applicable.
This work was supported by the Key Research Projects of Sichuan Province (grant numbers S15059).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
