Academic Editors: Masatoki Kaneko and Michael H. Dahan
Background: There are no detailed reports in the literature on
maternal cytomegalovirus antibody screening for universal newborn hearing
screening (UNHS) referral patients. We examined maternal cytomegalovirus antibody
screening results and estimated the incidence of maternal primary cytomegalovirus
infection among UNHS referral patients. Methods: During
September 2013–March 2021, fresh urine samples were collected in the first week
after birth from 98 neonates with UNHS referral results at 15 obstetrical
institutions in Mie, Japan (the first hearing screening). We performed a
real-time polymerase chain reaction analysis to detect cytomegalovirus DNA in the
samples. Infants with
Cytomegalovirus (CMV) is the most common pathogen in transplacental infection during pregnancy and subsequent congenital anomalies in both developed and developing countries. Congenital CMV (cCMV) infection in trans-placentally-infected infants can only be diagnosed in the first few weeks of birth because newborns develop CMV antibodies due to infection with harmless CMV through breastfeeding. Thus, neonatal urine and saliva specimens are usually tested for CMV DNA. Symptoms at birth in patients with severe cCMV infection include hepatosplenomegaly, petechia, pneumonia, retinitis, small-for-gestational-age, cerebral calcification, and ventriculomegaly [1]. Complications, such as abnormal fetal heart rate patterns or fetal distress, during delivery have been reported in patients with severe symptomatic cCMV infection [2]. Moreover, the presence of persistent or late-onset neurological symptoms after birth has been observed in both patients with severe symptomatic and those with asymptomatic cCMV infection at birth. Among the neurological symptoms, sensorineural hearing loss (SNHL) is considered the most common. cCMV infection is the most prevalent cause of congenital SNHL at birth in 8–21% of patients [1].
Universal newborn hearing screening (UNHS) is performed for the early detection of congenital SNHL. The otoacoustic emission test is considered to have inferior sensitivity compared with the automated auditory brain-stem response (AABR) test [3]. Approximately 4–5 of 1000 neonates fail in the UNHS (using AABR test) and have to be sent to the otorhinolaryngologists for further examinations of congenital hearing loss, while the remaining pass based on the UNHS. Approximately 50% of those sent to the otorhinolaryngologists are diagnosed with congenital SNHL after further examinations [4]. In addition to the otoacoustic emission or AABR test in UNHS, auditory brain-stem response and auditory steady-state response tests, as well as temporal bone computed tomography, are used to perform detailed examinations for congenital SNHL [5]. Patients with symptomatic cCMV infection with SNHL at birth can be detected by UNHS. However, nearly 15% of children with asymptomatic cCMV infection develop SNHL later in life and, therefore, remain undetected according to the UNHS [6]. Minami et al. [7] reported a high risk of missing patients with cCMV infection with SNHL in UNHS (13 of 44 patients with cCMV infection passed UNHS bilaterally).
Maternal CMV antibody screening can be performed to identify women with primary CMV infection during pregnancy with a high risk of subsequent cCMV infection. In maternal antibody screening, CMV-specific immunoglobulin (Ig) G and IgM antibodies and IgG avidities are measured in pregnant mothers. Some previous studies have reported on maternal primary CMV infection in Japan, including ours [8, 9, 10, 11]. In these reports, low CMV IgG avidity or IgG seroconversion to positive results was found in all pregnant women with primary CMV infection. Even in mothers with CMV IgG seroconversion during pregnancy, with the highest rate of in-utero CMV transmission, the rate of cCMV infection in infants is nearly half.
To the best of our knowledge, no previous study has provided detailed results of maternal CMV antibody screening for UNHS referral patients. In this study, we examined maternal CMV antibody screening results and identified the incidence rate of maternal primary CMV infection among UNHS referral patients for the first time.
In Mie, Japan, UNHS (using the AABR test) is performed at about 4 days after
birth (the first hearing screening) among almost all neonates, except when the
mothers do not wish to accept that the test be performed. We prospectively
enrolled neonates with referral results in the first hearing screening at each
obstetrical institution in Mie, Japan and collected their fresh urine samples
during the first week after birth. We performed a real-time polymerase chain
reaction analysis to detect CMV DNA from the samples at Mie University Hospital
in Mie, Japan, as previously described [9, 10]. Infants with
While the urine tests were being performed, the second hearing screening was being performed in neonates with the first hearing screening referral results on the same day or the next day after that. Patients who were diagnosed with or without cCMV infection, but had the second hearing screening referral results, were sent to the otorhinolaryngologists for further examination of congenital hearing loss, comprising auditory brain-stem response and auditory steady-state response tests, as well as temporal bone computed tomography. Congenital hearing loss was diagnosed within the first 3 months of their birth, whereby the audiological test results were abnormal. Even in patients with congenital hearing loss and cCMV infection, the temporal bone computed tomography test was performed to identify bony malformations as a cause of SNHL.
We have been conducting maternal CMV antibody screening at 24 obstetrical institutions in Mie, Japan since 2013 as part of the “Cytomegalovirus in Mother and Infant-Engaged Virus Serology (CMieV)” program. In this program, all pregnant women are screened for CMV IgG and IgM antibodies (Denka Company Limited, Tokyo, Japan) during early pregnancy. We also conducted CMV IgG antibody avidity tests in pregnant women with positive CMV IgG and IgM antibody results. IgG antibody avidity tests were performed at Aisenkai Nichinan Hospital in Miyazaki, Japan, as previously described [9, 13]. Alternatively, we repeated the tests for CMV IgG and IgM antibodies during the late pregnancy in pregnant women with negative CMV IgG antibody results during the early pregnancy. We considered the results as follows: low CMV IgG avidity or IgG seroconversion, positive IgG and negative IgM, high IgG avidity, and no IgG seroconversion as maternal primary infection, a past infection, non-primary infection, and no infection (uninfected), respectively.
We calculated the incidence rates (%) with 95% confidence intervals (CIs) for cCMV among all patients with referral results in UNHS (the first hearing screening) and maternal primary CMV infection among patients who underwent maternal CMV antibody screening. SPSS software version 27 (IBM Corp., Armonk, NY, USA) was used for statistical analyses.
During September 2013–March 2021, 98 neonates with referral results in UNHS (the first hearing screening) at 15 obstetrical institutions (1 university hospital, 4 general hospitals, 3 private hospitals, and 7 private clinics) in Mie, Japan, were enrolled and fresh urine samples were collected. The median number of gestational weeks at birth was 38 (range: 25–41) weeks, and the median birth weight was 3039 (range: 534–4453) g. The admission rate in the neonatal intensive care unit was 31.6% (31 of 98 patients). Thirty-nine and 59 patients had bilateral and unilateral referral results in the first hearing screening, respectively.
Among the 98 referred patients in the first hearing screening, five had
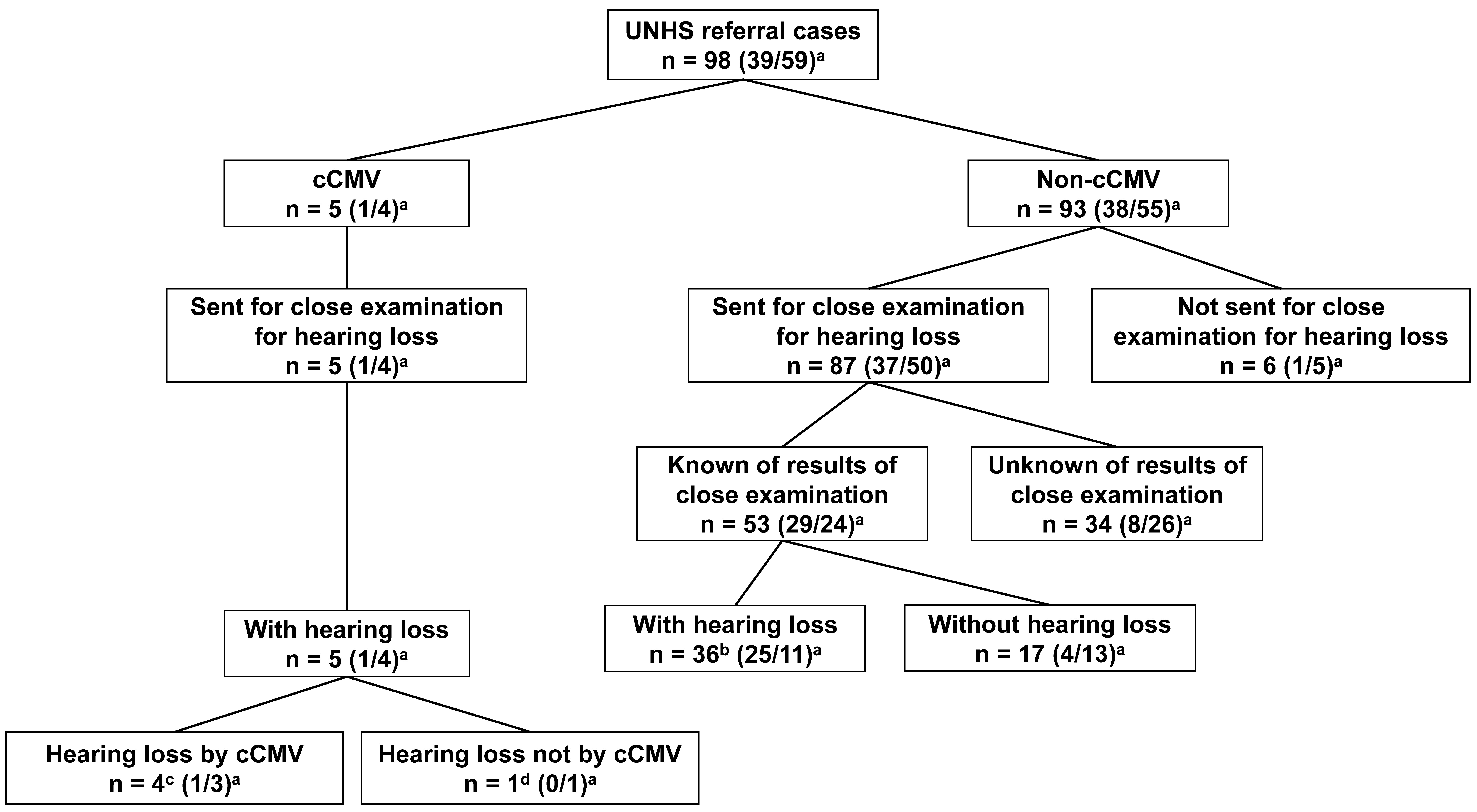 Fig. 1.
Fig. 1.Presence or absence of congenital cytomegalovirus infection and
congenital hearing loss in participants. UNHS, universal newborn hearing
screening; cCMV, congenital CMV; CMV, cytomegalovirus.
Four of the five patients with cCMV infection underwent maternal CMV antibody screening. All four patients with cCMV infection had maternal primary CMV infection during pregnancy. Of the four patients, three (Cases 1–3) were positive for CMV IgG and IgM antibodies in maternal blood with low CMV IgG antibody avidity results during early pregnancy; the remaining patient (Case 4) had positive CMV IgG antibody seroconversion results during pregnancy. Detailed information on the five patients with cCMV infection (Cases 1–5) is shown in Table 1.
| No. | Sex | GWs of birth (week) | Birth weight (g) | Side of “refer” in UNHS/Actual SNHL | Degree of SNHL | Cause of SNHL | Amount of CMV DNA in neonatal urine (log10) (copy/mL) | Viral isolation of neonatal urine | Subunit of gB | Abnormality other than hearing loss | Maternal age (years) | Maternal parity (para) | Maternal CMV antibody screening | Subdivision of maternal CMV antibody screening |
| 1 | F | 37 | 2244 | Right/Right | Severe | cCMV | 5 | + | 1 | Brain MRI | 30 | 0 | Primary infection | IgG+, IgM+, Low IgG avidity |
| 2 | M | 37 | 3090 | Left/Left | Severe | cCMV | 4 | + | 3 | Brain MRI | 30 | 0 | Primary infection | IgG+, IgM+, Low IgG avidity |
| 3 | M | 40 | 3482 | Both/Both | Severe (right), moderate (left) | cCMV | 4 | - | NA | Nothing | 24 | 0 | Primary infection | IgG+, IgM+, Low IgG avidity |
| 4 | F | 38 | 2458 | Right/Right | Severe | Not cCMV* | 3 | + | 1 | Nothing | 27 | 1 | Primary infection | IgG seroconversion |
| 5 | F | 38 | 3840 | Left/Left | Severe | cCMV | 4 | + | 1 | Brain MRI | 34 | 1 | No screening | No screening |
| GW, gestational week; UNHS, universal newborn hearing screening; SNHL,
sensorineural hearing loss; CMV, cytomegalovirus; gB, glycoprotein B; cCMV,
congenital cytomegalovirus; MRI, magnetic resonance imaging; Ig, immunoglobulin;
NA, not available; F, female; M, male. *Stenosis of cochlear nerve canal and narrow internal auditory canal. | ||||||||||||||
Of the 98 patients, 93 were not diagnosed with cCMV infection. Among the 93 patients not diagnosed with cCMV infection, 87 showed positive results in the second hearing screening performed on the same day as the first hearing screening or the subsequent day, and they were sent to the otorhinolaryngologists. We obtained the otorhinolaryngologists’ detailed examination results for congenital hearing loss for 53 of the 87 patients. Of the 53 patients, 36 were diagnosed with congenital hearing loss (Fig. 1 and Supplementary Table 1).
The mothers of 60 of the 98 infants underwent maternal CMV antibody screening. Among the 60 maternal patients, six had primary CMV infection during pregnancy. Among the six maternal patients, four had CMV IgG and IgM antibodies detected in blood with low CMV IgG antibody avidity results during early pregnancy; the remaining two had CMV IgG antibody seroconversion during pregnancy. Three (Cases 1–3) of the four maternal patients, who were positive for CMV IgG and IgM antibodies and had low CMV IgG antibody avidity results, had infants diagnosed with cCMV infection with congenital hearing loss. The infant of the remaining maternal patient (Case 6) was diagnosed with non-cCMV congenital hearing loss. The infant of one (Case 4) of the two maternal patients with CMV IgG antibody seroconversion results had cCMV infection with congenital hearing loss unrelated to cCMV infection (the patient with unilateral cochlear nerve canal stenosis and ipsilateral narrow internal auditory canal mentioned above), and the other (Case 7) had non-cCMV-related congenital hearing loss. In 54 of the 60 patients who underwent maternal CMV antibody screening, 28, 4, and 22 patients had a past infection, non-primary infection, and no infection, respectively. There were no patients with cCMV infection among them (Figs. 2,3).
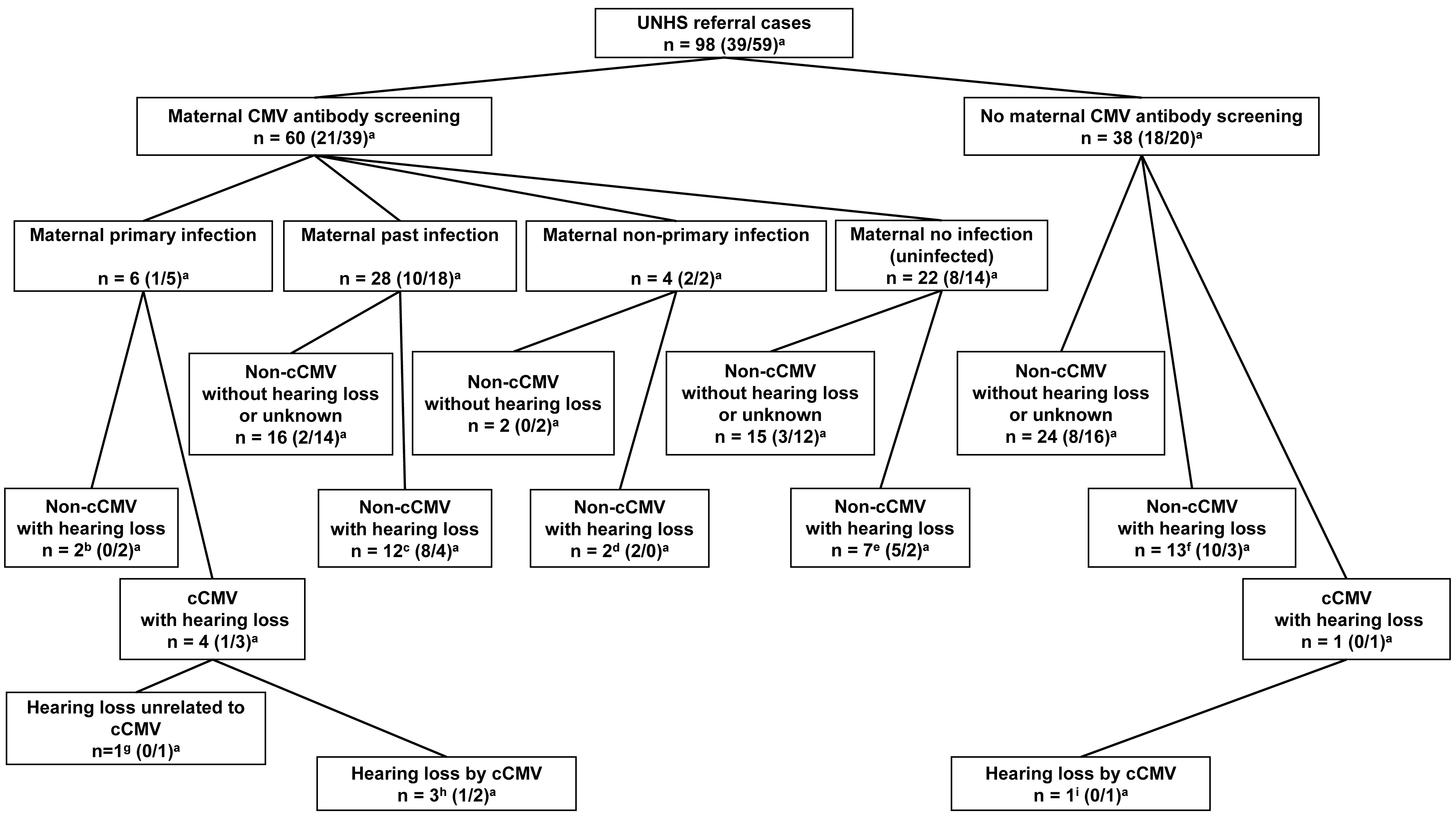 Fig. 2.
Fig. 2.Presence or absence of maternal cytomegalovirus antibody in
participants and results of antibody screening and congenital hearing loss.
UNHS, universal newborn hearing screening; cCMV, congenital CMV; CMV,
cytomegalovirus.
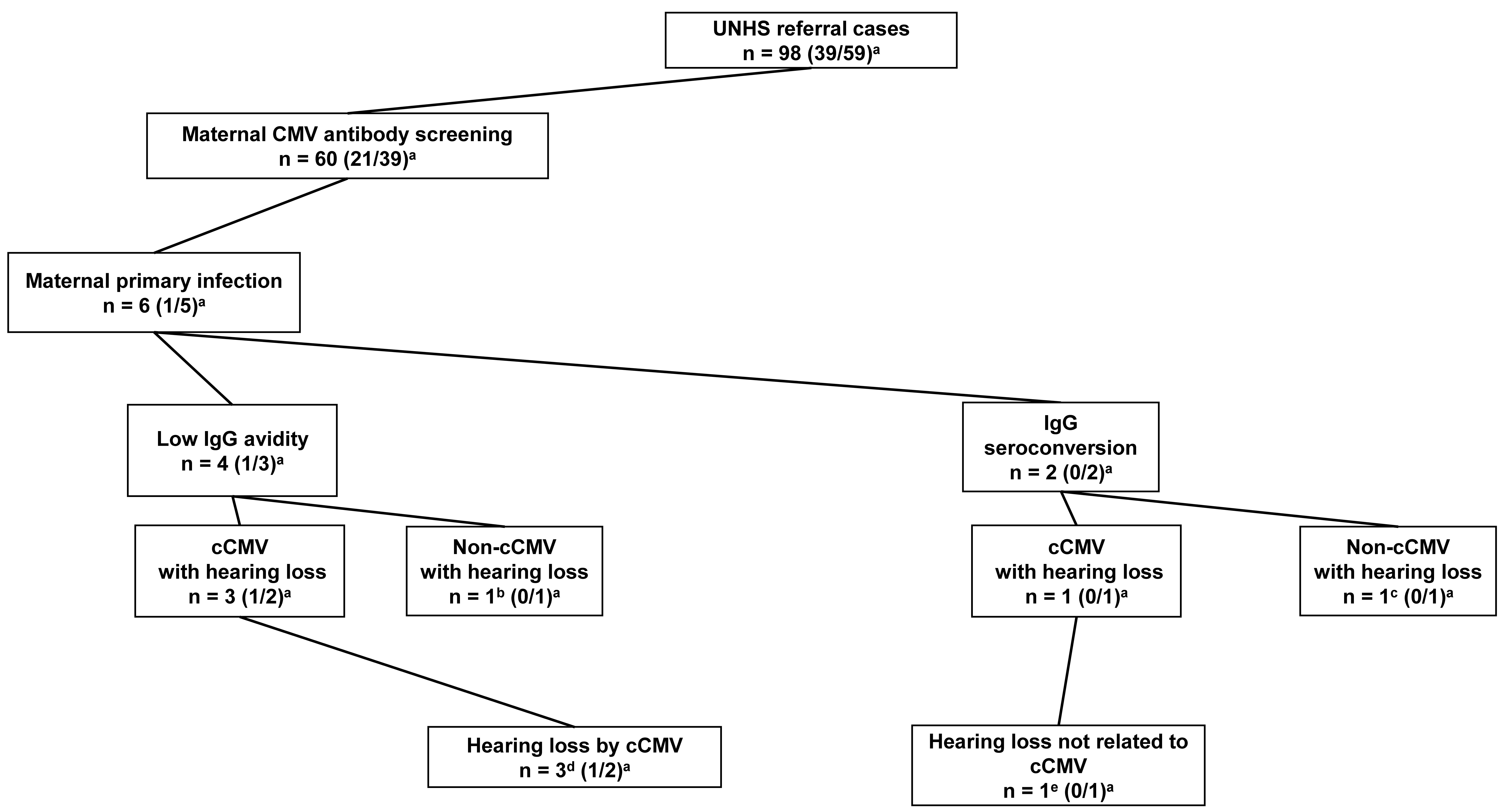 Fig. 3.
Fig. 3.Primary cytomegalovirus infection in maternal cytomegalovirus
antibody screening. UNHS, universal newborn hearing screening; cCMV, congenital
CMV; CMV, cytomegalovirus.
CMV is the most common viral cause of mother-to-fetus infections, affecting approximately 1% of all live births worldwide, with a prevalence of 0.6–0.7% in developed countries [6]. Congenital hearing loss onset in patients with cCMV infection often occurs after the neonatal period and is therefore not detected by UNHS. The incidence rates of cCMV detected in UNHS referral patients have been reported in previous studies, ranging from 0.9% to 5.0% [14, 15, 16, 17, 18, 19]. Stehel et al. [16] reported an incidence rate of 5.0% (24 of 483 patients), which was closest to that (5.1%) of the current study. The differences in the incidence rates may be attributed to the study population and admission rate in neonatal intensive care units. The rate of neonatal intensive care unit admission affects the detection of congenital hearing loss in UNHS referral patients [6], and the incidence rate of cCMV infection in UNHS referral patients would likely vary according to the study population to some extent. Although the admission rate in this study was nearly one-third of the total, no further comparisons could be made with the previous studies. This needs to be addressed in further studies.
cCMV infection is the most prevalent cause of congenital SNHL at birth in 8–21% of patients [6, 16, 20, 21, 22, 23]. Rates of congenital SNHL secondary to cCMV infection increase to 25% by 4 years of age, owing to patients with late-onset SNHL related to cCMV infection [6, 24]. In the current study, the rate of cCMV infection in congenital SNHL patients at birth was 12.2% (5/41 patients), which is consistent with the previous reports mentioned above, although not all five patients had SHNL due to cCMV infection. This means that even if a UNHS referral patient is positive for cCMV infection, it does not necessarily mean that the SNHL is caused by cCMV infection. Even in patients with cCMV infection with SNHL, a detailed otorhinolaryngological examination is necessary.
In a maternal CMV antibody screening, low IgG avidity is an indicator of
primary infection, whereas IgG seroconversion confirms primary infection. Thus,
the incidence rate of cCMV infection in mothers with IgG seroconversion (almost
half) is higher than that in those with low IgG avidity (
Both maternal primary and non-primary infections (including past infections) can cause cCMV infection in infants. In countries with a low or intermediate CMV seroprevalence, the occurrence of cCMV infection after maternal primary and non-primary infections is reportedly approximately equal. For example, the maternal CMV seroprevalence as well as the seroprevalence for cases of cCMV infection occurring after maternal primary and non-primary infection (including past infection) is 60%, 52%, and 48%, in France, respectively; whereas in Finland, these were 72%, 47%, and 53%, respectively [1, 26, 27]. There are currently insufficient data on cCMV infection following maternal non-primary infection in Mie, Japan. However, because of the similar rate of CMV seroprevalence in Mie, Japan (66%), a similar ratio of cCMV infection following maternal primary and non-primary infections may exist [8]. Herein, all four UNHS referred neonates with cCMV infection whose mothers underwent CMV antibody screening were positive for maternal primary CMV infection, with no cCMV diagnosis following maternal non-primary infection (including past infection). The reason for this is unclear since the occurrence of congenital hearing loss in patients with cCMV infection is considered the same in maternal primary and non-primary infections [1]. Congenital hearing loss in patients with cCMV infection after non-primary maternal CMV infection may be difficult to detect in UNHS. Further studies are warranted to evaluate this clinically important possibility.
Herein, we performed a prospective enrollment study of neonatal patients who had referral results in UNHS. Therefore, since there was no enrollment at the time of undergoing UNHS, the exact number and clinical characteristics of neonates who underwent UNHS could not be reported. However, in another study of pregnant women (cytomegalovirus antibodies), along with those included in this study, the number of pregnant women enrolled during the same study period was 44628. Therefore, we assume that approximately the same number of neonates received UNHS. Assuming that approximately 4–5 of 1000 neonates failed at the UNHS [4], about twice as many neonates as the 98 patients in this study would have been considered to have had referral results. The reason for this small number of patients with referral results would be that, UNHS was not performed when mothers did not wish to receive the test, because some patients were sent to the otorhinolaryngologists even after obtaining UNHS referral results without enrollment in this study and their urine samples were not collected. Moreover, in the assessment of the incidence rate of cCMV infection in UNHS referral patients, selection bias to an extent might be present in this study population. We performed neonatal CMV DNA tests from urine samples in all UNHS referral patients; however, we were not able to obtain all of the corresponding CMV antibody screening results from the mothers. Thus, although we identified the incidence rate of cCMV infection among all UNHS referral patients, we showed the incidence rate of maternal primary CMV infection in a limited number of patients (60 of 98 mothers). This could also contribute to a selection bias in this study.
To the best of our knowledge, this is the first study to assess the incidence rate of maternal primary CMV infection using maternal CMV antibody screening in patients with referral results in UNHS. We assessed the incidence rate of cCMV infection in UNHS referral patients at 5.1% and a 10-fold higher risk of the incidence rate of maternal primary CMV infection in the referral patient group (10.0%) as compared with the general population (0.98%).
AABR, automated auditory brain-stem response; cCMV, congenital cytomegalovirus; CI, confidence interval; CMV, cytomegalovirus; Ig, immunoglobulin; SNHL, sensorineural hearing loss; UNHS, universal newborn hearing screening.
AK, KTor, and TI were involved in writing the paper. MH-A was involved in data collection. MI performed CMV DNA tests. TM performed CMV IgG avidity tests. SS performed viral isolation tests. Close examination of congenital hearing loss in infants were performed by MKit, KTak, SU, and SM at the otorhinolaryngology departments. EK, MKih, and FM assisted in operating the maternal CMV antibody screening program. All authors read and approved the final manuscript.
This prospective cohort study was conducted in accordance with the Declaration of Helsinki. We obtained ethical approval (No. 2610) from the Clinical Research Ethics Review Committee of the Mie University Hospital and obtained informed consent from all participants’ mothers.
We appreciate all institutions that partook in the maternal CMV antibody screening program in Mie, Japan, “Cytomegalovirus in Mother and infant-engaged Virus serology (CMieV)” program. Our expressed gratitude goes to the members of the Mie Association of Obstetricians and Gynecologists (A. Terada and Y. Tamaishi) for facilitating collection of neonatal urine samples in UNHS “refer” patients and the directors of that (H. Obata, K. Kanamaru, Y. Kamimoto, T. Kikukawa, T. Takakura, H. Tanaka, K. Nagao, S. Nii, K. Nishimura, Y. Maegawa, T. Maezawa, and H. Minoura) for facilitating the CMieV program. We also thank M. Nakamura and E. Teramoto (Mie University Hospital) for their help with the real-time PCR tests and M. Negoro (Mie National Hospital) for her help conducting the viral isolation tests and subunit analysis of CMV glycoprotein B.
This study was supported in part by the Clinical Research Program for Child Health and Development, awarded by the Japan Agency for Medical Research and Development (AMED) (grant no. 22gk0110061s0501) and JSPS KAKENHI (grant no. 22K15918).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
