† These authors contributed equally.
Background: To evaluate the
efficacy of transvaginal sonography (TVS) in the management of cesarean scar
pregnancy (CSP). Methods: In this retrospective study conducted at
Beijing Friendship Hospital of Capital Medical University, 142 CSP patients were
collected from January 2015 to September 2019. Patients were divided into two
groups, laparoscopy use group (Lap) and no laparoscopy use group (non-Lap)
determined by the use of laparoscopy. The ultrasound parameters analyzed between
these groups included maximal diameter of gestational sac, presence of fetal
heartbeat, local myometrial thickness and grading of color Doppler signals.
Results: The maximal diameter of gestational sac in the non-Lap and Lap
groups was 2.330
Cesarean scar pregnancy (CSP) is defined as a peculiar and rare ectopic pregnancy following a previous cesarean delivery (CD) with the implantation of the gestational sac in the hysterotomy scar [1]. Within the last decades, CSP have become increasing common for the increase of cesarean deliveries. The current estimated incidence of CSP is 1 in 1688 pregnancies [2, 3]. Mothers with CSP are at increased risk of massive hemorrhage, uterine rupture and maternal mortality during pregnancy or at the time of surgical curettage [4, 5]. Following the diagnosis, termination of pregnancy is recommended to avoid severe complications. Dilatation and curettage (D&C) and uterine artery embolization (UAE) are common treatments for CSP to minimize the blood loss, but massive bleeding and uterine rupture can still be encountered [6, 7, 8]. In some severe cases, laparoscopic local resection/hysterectomy (LLR/H) is required.
Early recognition and timely management are important for optimization of therapy and improved CSP patient outcomes. CSP is difficult to diagnose without the use of ultrasound, due to its atypical clinical signs and symptoms. Laparoscopic excision of CSPs is associated with a success rate of 97% and the combined use of laparoscopy and D&C decreases the risk of hemorrhage and hysterectomy [9].
Because the diagnosis of CSP relies on sonographic diagnostic criteria [10], a systematic method to classify the severity of CSP is needed [11].
In this study, we retrospectively analyzed 142 CSP patients in our hospital and divided them into two groups differentiated by the use of laparoscopy. We compared different ultrasound parameters between the two groups in order to determine whether the ultrasound examination could guide the selection of the surgical approach and improve maternal outcome.
We performed a retrospective analysis of CSP patients using records from the Department of Ultrasound, Beijing Friendship Hospital, Capital Medicine University (Beijing, China) for the period between January 2015 and September 2019. During this 5-year interval, there were 142 CSP patients identified with ages ranging from 25 to 45 years (Median = 35, Interquartile range = 6). This study was approved by the Ethics Committee of Beijing Friendship Hospital, who deemed written informed consent was not necessary because of the retrospective nature of the study and that all patient data was de-identified.
All patients included in this study had confirmation of the diagnosis of CSP
according to the criteria published by Godin et al. [12] which included:
(1) a history of lower segment cesarean section; (2) a positive serum
Treatment options of CSP included UAE, curettage with ultrasound guidance, methotrexate (MTX), hysteroscopy or laparoscopic local resection/hysterectomy (LLR/H). The treatment flow at our institution is shown in Fig. 1.
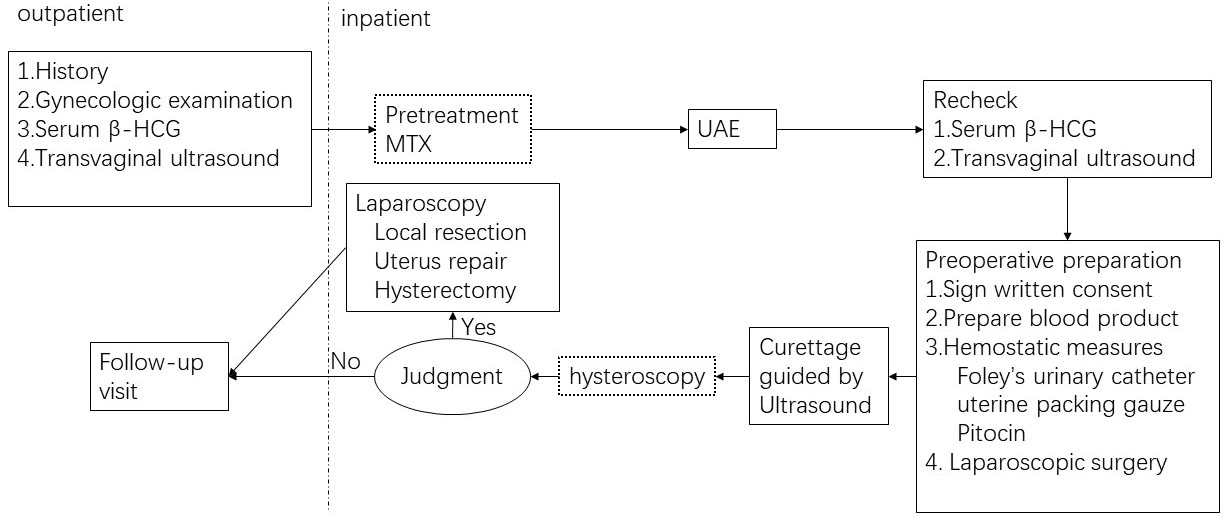 Fig. 1.
Fig. 1.The CSP treatment flow at Beijing Friendship Hospital. Judgment: uncontrollable bleeding, failed removal of enclosed mass or uterine rupture. The dotted box implies that some patients may not receive this kind of procedure in the treatment flow.
UAE is a procedure in which an interventional radiologist uses a catheter to
deliver small absorbable particles to block the uterine artery in order to
decrease the risk of excessive bleeding. MTX treatment was administered to the
patients who were in stable condition, gestational age
Curettage under ultrasound guidance was performed in the operating room in the
out-patient department with an experienced operator within three days after UAE.
Blood products, Foley’s urinary catheter (18F), uterine packing gauze and Pitocin
were prepared prior to surgery in anticipation of massive hemorrhage.
Hysteroscopy was occasionally utilized to check for residual tissue in the
uterine cavity and to observe the cesarean scar. If uncontrollable bleeding
occurred, failure to remove the gestational tissue or evidence of uterine
rupture, laparoscopic local resection with uterine repair or hysterectomy would
be employed as a salvage measure. Discharged patients were followed until the
serum
The total number of patients who met CSP diagnostic criteria and treated with this approach was 142 during these five years of review. None of the CSP patients required a hysterectomy. According to the treatment difficulty level, these patients were divided into two groups: the non-Lap group (the patients who only required UAE and evacuation); the Lap group (the patients with additional laparoscopic local resection and uterine repair following evacuation treatment).
Demographic information (maternal age, gravidity, parity, # of CDs, # of
curettages, interval from last CD), ultrasound parameters and clinical data
(i.e., blood loss and serum
TVS examinations were performed using a GE VOLUSON E8 imaging machine (General Electric Co., Fairfield, CT, USA) or a Phillips iU22 imaging machine (Philips Electronics NV, Eindhoven, Netherlands) by two experienced obstetric ultrasound physicians. The parameters determined by ultrasound were as follows: the location, size, and shape of the gestational sac; the size of the uterus; the thickness of the endometrium; adnexal area and pelvic cavity; myometrium thickness in the incision region; and blood flow surrounding the gestational sac. With moderate bladder filling, the maximum longitudinal diameter and anterior-posterior diameter of the uterus were measured at a longitudinal section of the uterus, and the maximum transverse diameter was at the transverse section of the uterus. The average sac diameter was equal to the average of the maximum longitudinal diameter, anterior-posterior diameter and the maximum transverse diameter. The thickness of the myometrial defect between the bladder and the gestational mass was also recorded (Fig. 2). Blood flow signals were determined according to the International Ovarian Tumor Analysis (IOTA) scoring system: I minimal flow detected; II moderate flow present; III highly vascular with marked blood flow (Fig. 3) [15, 16].
 Fig. 2.
Fig. 2.Schematic measurement of the thickness of myometrial defect.
 Fig. 3.
Fig. 3.Schematic diagram of blood flow signal grading. (A) Grade I. (B) Grade II. (C) Grade III.
All the data regarding ultrasound parameters included in our research were collected at diagnosis before the treatment of CSP.
Statistical analyses were performed with SPSS software version 23.0 (SPSS Inc.,
Chicago, IL, USA). Statistical significance tests were two-tailed and accepted at
p
According to treatment difficulty level, 132 (93.0%) patients were included in
the non-Lap and 10 (7.0%) patients in Lap. It should be emphasized that Lap
patients not only received the UAE and evacuation treatments but also the
laparoscopic local resection and uterine repair as a salvage measure due to
massive hemorrhage. Of the 10 cases in the Lap group, 2 patients had
uncontrollable bleeding, 8 patients failed to remove the gestational tissue and
had uterine rupture. In the end, 3 patients underwent temporary ligation of
bilateral uterine arteries + folding and suture of inferior segment of uterus. 7
patients underwent wedge resection of the lesion + uterus repair. The
characteristics of the patients are shown in Table 1. There was no statistically
significant difference in the demographic characteristics between the two groups
including the patients’ age at diagnosis (p = 0.15), the gravidity
(p
| Group | non-Lap | Lap | p-value | |
| Case number | 132 | 10 | ||
| Age |
35 (6) | 38 (7) | 0.15 | |
| Gravidity |
||||
| 21 | 2 | |||
| 111 | 8 | |||
| Parity |
||||
| 126 | 10 | |||
| 6 | 0 | |||
| No. of CDs |
0.51 | |||
| 0–1 | 74 | 4 | ||
| 58 | 6 | |||
| No. of curettages |
0.055 | |||
| 0 | 47 | 1 | ||
| 1 | 52 | 3 | ||
| 33 | 6 | |||
| Interval from last CD (month) |
55 (77) | 46 (44) | 0.32 | |
| Preoperative |
60356 (84469) | 51773 (147985) | 0.92 | |
| Abbreviations: CD, cesarean delivery; | ||||
In order to evaluate the role of TVS in guiding the choice of surgical procedure
and to predict the therapeutic effect for CSP patients, five related variables
were analyzed between the non-Lap and Lap groups. There was no significant
difference in gestational age between the two groups (p = 0.45). The
four ultrasound parameters between the two groups demonstrated significant
differences including the maximal diameter of gestational sac (2.330
| Group | non-Lap | Lap | p-value | |
| Gestational age |
50.5 (14) | 47 (39) | 0.45 | |
| Average diameter of gestational sac (cm) |
2.330 |
2.883 |
0.007 | |
| Fetal heartbeat |
0.026 | |||
| Negative | 81 | 2 | ||
| Positive | 51 | 8 | ||
| Local myometrial thickness (cm) |
0.25 (0.16) | 0.16 (0.073) | ||
| Grading of color Doppler signals |
0.044 | |||
| I | 39 | 0 | ||
| II | 55 | 4 | ||
| III | 38 | 6 | ||
| Abbreviations: Lap, laparoscopy use group; non-Lap, no laparoscopy use group. | ||||
We considered the amount of bleeding as an important reference for evaluating
the difficulty of surgery. As shown in Table 3, the bleeding volume in non-Lap
was 20 (0) mL with 50 (503) mL in the Lap which was a significant difference
between groups (p
| Group | non-Lap | Lap | p-value |
| Amount of bleeding (mL) |
20 (0) | 50 (503) | |
| The percentage of |
67.9% (15.4%) | 68.4% (11.9%) | 0.844 |
| Abbreviations: | |||
Laparoscopic excision of CSP and uterine repair is critical for a complete
removal of the entire scar and content [9]. The laparoscopic excision can reduce
surgical trauma and promote postoperative recovery. However, not all facilities
can carry out laparoscopic excision due to the lack of equipment and/or
experienced surgeons. Early first trimester diagnosis (
Sun QL et al. [19] reported that CSP could be divided into
three types by the depth of basal decidua implanted into the myometrium which
included the superficial type, the partial type and the complete type. The
severity of CSP is determined by a spectrum of sonographic and clinical
characteristics such as the gestational age, the presence of a fetal heartbeat,
the maximal diameter of the gestational sac, the size of the gestational sac,
remnant myometrial thickness, grading of Doppler signals, the location of the
gestational sac and the serum
In this study, we retrospectively analyzed 142 CSP patients and considered the use of laparoscopy as an important indicator for the difficulty of the surgery. For these serious cases, the simultaneous performance of curettage and laparoscopic excision can greatly shorten the operation time, reduce the amount of bleeding and the decrease the length of anesthesia due to excessive operative time. Therefore, we divided these patients into two groups according to whether laparoscopic excision was performed. We found that four ultrasonic parameters were associated with laparoscopy use which included average diameter of gestational sac, fetal heartbeat, local myometrial thickness and grading of Doppler signals.
It was worth noting that three-dimensional ultrasound would be more precise in order to measure the mass of the gestational sac. Three-dimensional ultrasound can provide more details about the CSP mass such as the lesion volume, vascular index, flow index, blood vessels and blood flow index. Three-dimensional color power angio (CPA) can provide quantitative parameters for peritrophoblastic perfusion and even detect low blood flow without being affected by the angle of the ultrasound wave [22]. Considering that many primary hospitals may be not equipped with this kind of technology, it was more practical to use two-dimensional ultrasound for its wide-spread availability. Contrast-enhanced ultrasound (CEUS) has been developed utilizing two-dimensional ultrasound. By intravenous injection of tiny bubbles, the CEUS could show more details such as the blood flow and surrounding perfusion at the region of interest [23]. Although CEUS can accurately display the location of the gestational sac and thus reduce the risk of severe hemorrhage and uterine rupture [24], the adverse events caused by sonographic contrast agents such hypersensitivity reaction should not be ignored [25]. MRI scans are also important for CSP. Most patients at our institution received an MRI scan to help assess patient’s condition. This research focus solely on the role of ultrasound in CSP.
We believe that the average diameter instead of the maximal diameter of gestational sac better describes the sac size. The statistical analysis demonstrated that a longer average diameter of the gestational sac, a positive fetal heartbeat, a thicker local myometrium thickness and a grade III Doppler signal indicated the need for use of laparoscopy.
During this retrospective analysis, we found 658 cases of ectopic pregnancies including tubal pregnancy, abdominal pregnancy and cervical pregnancy. The percentage of CSP among ectopic pregnancies was 21.6%. We appreciate that this data is much higher than other researchers have reported. It may be due to a higher cesarean section rate in our country, the two-child policy and wide spread use of assisted reproductive technology. Besides, primary hospital would like to transfer their difficult cases to our hospital for better treatment. In order to decrease the incidence of CSP, we recommend contraception for women who have no desire for future childbearing and that women with a history of previous cesarean section have an early ultrasound examination to determine the location of the fetal sac.
We attempted to employ odds radio for the four risk factors in a multivariable logistic regression model to develop a scoring system to make it more practical for the diagnosis by frontline physicians similar to that of Wang et al. [20, 26]. However, the multivariable logistic regression did not demonstrate any statistical significance. More data is needed and a prospective study should be carried out to obtain a more practical and easy-to-use evaluation protocol to help clinicians assess and identify patients who require the use of laparoscopy.
TVS is an important examination method for CSP. A longer average diameter of the gestational sac, a positive fetal heartbeat, a thicker local myometrium and a grade III Doppler signal implicate a more severe CSP which may require laparoscopy as a salvage procedure.
TVS, transvaginal sonography; CSP, cesarean scar pregnancy; CD, cesarean
delivery; D&C, Dilatation and curettage; UAE, uterine artery embolization;
LLR/H, laparoscopic local resection/hysterectomy;
LXQ designed the research study. YL, YS and YY collected the clinical data and Ultrasound examination. FXK analyzed the data. YL and YS wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Beijing Friendship Hospital (2019-P2-227-02, Nov. 28, 2019), who deemed written informed consent was not necessary because of the retrospective nature of the study and that all patient data was de-identified.
We would like to thank Xiangdong Hu for her help in the revision of the manuscript.
This research was funded by the Beijing Excellent Talent Program, grant number 2006000021469G228. This research was funded by Beijing Municipal Administration of the Hospitals’ Ascent Plan, grant number DFL 20180102.
The authors declare no conflict of interest.
