Background: This study aimed to evaluate the clinical outcomes
and dose-volume parameters of computed tomography CT-based brachytherapy for the
vaginal recurrence of uterine cancer after hysterectomy. Methods: We evaluated 22 uterine cancer patients treated with CT-based
brachytherapy for vaginal recurrence between December 2010 and August 2015.
Interstitial brachytherapy (ISBT) was used when the vaginal tumor was thicker
than 5 mm and/or located at extended extravaginal tissue, whereas intercavitary
brachytherapy was performed if it was 5 mm or thinner. Results: Overall,
11 patients had cervical cancer, and 11 had endometrial cancer. The median
pretreatment tumor size on magnetic resonance imaging was 17 mm (range, 0–45
mm). Four patients had vaginal recurrence recognized only in the gynecological
examination. The primary location of recurrence was the vagina, with extravaginal
extension observed in 9 patients. Seventeen patients (77%) received external
beam radiotherapy and brachytherapy. ISBT was performed in 12 patients (55%).
The median clinical target volume (CTV) D90 was 69.2 Gy (62.6–72.8 Gy). The
median D2cc of the bladder, sigmoid, and rectum were 70.2 (63.8–77.6), 37.4
(30.0–43.6), and 52.8 Gy (38.6–63.5 Gy), respectively. Complete response was
reached in all patients. The 5-year overall survival rate and local control rate
(LC) were 84.8 and 95.5%, respectively. No patient experienced grade
Patients with uterine cancer, other than early-stage disease, such as stage IA (G1), often experience a recurrence in their pelvic region after radical surgery, with the most frequent being vaginal recurrence [1]. Several studies have reported that salvage 2-dimensional (2D) brachytherapy for uterine cancer patients with vaginal recurrence leads to good local control (LC). However, the incidence of late complications was high in these cases [2, 3]. Computed tomography (CT)- or magnetic resonance imaging (MRI)-based image-guided adaptive brachytherapy (IGABT), which could enable dose evaluation for the clinical target volume (CTV) and organs at risk (OARs), has been increasingly used in recent years [4], and has led to improved LC and low incidence of late complications among patients with locally advanced cervical cancer [5]. However, few studies have reported on the outcomes of IGABT for uterine cancer patients with vaginal recurrence [6, 7].
Thus, this study aimed to evaluate the outcomes and feasibility of IGABT in uterine cancer patients with vaginal recurrence after being managed by surgery.
This retrospective study evaluated 22 uterine cancer patients treated with CT-based IGABT between December 2010 and August 2015 for vaginal recurrence confirmed by biopsy after being primarily managed by surgery. Patients receiving postoperative radiotherapy were excluded from analysis. As an adjuvant treatment after surgery, chemotherapy was administered to patients with either International Federation of Gynecologists and Obstetricians (FIGO) stage III endometrial cancer, FIGO Ib2 or greater cervical cancer. Patients with distant metastases at the time of vaginal recurrence diagnosis were excluded. Vaginal recurrence was defined according to the following criteria: (1) tumor recurrence at the vaginal stump and/or wall without extravaginal extension and (2) tumor recurrence with extravaginal extension, with the tumor continuously invading extravaginal tissue. The extension of the vaginal recurrence was determined by the gynecological examination findings and/or imaging techniques, such as CT and MRI.
All patients received high-dose-rate (HDR) brachytherapy. If the patients (1) did not undergo lymph node dissection at the initial surgery, (2) had the tumor invading their extravaginal tissue, or (3) had pelvic lymph node metastasis at the time of vaginal recurrence, radiotherapy consisted of a combination of external beam radiotherapy (EBRT) and HDR brachytherapy.
EBRT was delivered using the three-dimensional conformal technique with a linear accelerator (Clinac iX; Varian Medical System, Palo Alto, CA) with a 10 MV photon beam. The EBRT field included pelvic lymph node regions, such as common, internal and external iliac lymph nodes, as well as obturator lymph nodes. Whole pelvic EBRT was initially administered at 30.0–50.0 Gy in 15–25 fractions using the four-field technique, and a further 0–20 Gy in 0–10 fractions was administered along with the insertion of a 3-cm wide midline block (MB) using the anterior-posterior/posterior-anterior technique.
The first HDR-ICBT or ISBT was performed within 7 days after MB insertion. Brachytherapy was performed using an iridium 192 (192 Ir) remote afterloading system (MicroSelectron HDRTM; Nucletron, Veenendaal, the Netherlands). For all patients, planning was based on CT images of 2.5-mm slice thickness, and dose calculations were performed using Oncentra (Nucletron, Veenendaal, the Netherlands).
The type of brachytherapy was selected according to the initial extent of the
disease and the tumor thickness, measured via MRI, CT, or gynecological
examination just before brachytherapy. For patients with vaginal stump and/or
wall recurrence, intracavitary brachytherapy (ICBT) was selected if the tumor
thickness just before brachytherapy was
 Fig. 1.
Fig. 1.Dose distributions of (A) ICBT using cylinder application, (B) ICBT using ovoid application, and (C) of ISBT. Isodose line: blue line: 25% of prescribed dose; light blue line: 50%; green line: 75%; red line: 100%; brown line: 150%; orange line: 200%; yellow line: 250%; white line: 300%.
A weekly regimen of cisplatin (40 mg/m
Treatment responses were assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST) at 2–3 months after radiotherapy completion using histopathology and/or MRI [8]. Late adverse events were graded based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0. The follow-up comprised of gynecological examinations, cytology, blood tests, and imaging by CT or MRI, every 2–6 months for 5 years.
For the dose summation of brachytherapy plus EBRT prior to MB insertion, the
equivalent dose in 2 Gy fractions was calculated based on the linear-quadratic
model [9]. The tumor dose was calculated using an
The patient characteristics are shown in Table 1. All patients were operated by
laparotomy. Initial International Federation of Gynecologists and Obstetricians
stage III endometrial cancer was observed in 2 patients who received total
abdominal hysterectomy and bilateral salpingo-oophorectomy as initial surgery and
adjuvant chemotherapy. The median tumor size at the time of vaginal recurrence
diagnosis and as measured by MRI was 17 mm (range, 0–45 mm). Vaginal recurrence
was identified through gynecological examination and was not detected using MRI
in 4 of the 22 patients. Overall, 15 patients received radiotherapy alone, and 7
patients received CCRT. There were 12 patients (55%) treated with ISBT and 10
patients (45%) with ICBT. ISBT was administered to all 9 patients with
extravaginal recurrence and to 3 of the 13 patients with recurrence at the
vaginal stump and/or wall. At the time of recurrence, 3 patients had pelvic node
metastasis detected by CT and/or MRI. Table 2 shows the clinical and treatment
features of the ICBT and ISBT groups. The size of vaginal recurrence was
significantly larger in patients treated with ISBT than those treated with ICBT
(p
| Characteristic | Value | ||||
| All | Cervical cancer patients | Endometrial cancer patients | |||
| n = 11 | n = 11 | ||||
| Age at the time of vaginal recurrence years, median, (range) | 63 (33–78) | 59 (33–78) | 63 (33–74) | ||
| Initial FIGO stage | 0 | 2 (9) | 2 (18) | 0 (0) | |
| I | 16 (73) | 7 (64) | 9 (82) | ||
| II | 2 (9) | 2 (18) | 0 (0) | ||
| III | 2 (9) | 0 (0) | 2 (18) | ||
| Histology | Squamous cell carcinoma | 4 (18) | 4 (36) | 0 (0) | |
| AdSqcc or Adenocarcinoma | 5 (23) | 5 (45) | 0 (0) | ||
| Endometrioid adenocarcinoma (G1) | 9 (41) | 0 (0) | 9 (82) | ||
| Others | 4 (18) | 2 (18) | 2 (18) | ||
| Initial surgery | Radical hysterectomy | 5 (23) | 5 (45) | 0 (0) | |
| Modified radical hysterectomy | 5 (23) | 2 (18) | 3 (27) | ||
| Abdominal total hysterectomy | 12 (54) | 4 (36) | 8 (73) | ||
| Initial pelvic lymph node dissection | Yes | 13 (59) | 7 (64) | 6 (55) | |
| No | 9 (41) | 4 (36) | 5 (45) | ||
| Location of vaginal recurrence | Vaginal stump and/or wall | 13 (59) | 6 (55) | 7 (64) | |
| Extravaginal extension | 9 (41) | 5 (45) | 4 (36) | ||
| Size of vaginal recurrence mm, mean (range) | 17 (0–45) | 12 (0–36) | 20 (0–45) | ||
| Pelvic lymph node metastasis at the time of recurrence | Yes | 3 (14) | 2 (18) | 1 (9) | |
| No | 19 (86) | 9 (82) | 10 (91) | ||
| Abbreviations: FIGO, International Federation of Gynecologists and Obstetricians; AdSqcc, adenosquamous cell carcinoma. | |||||
The volume and dose-volume parameters for CTV and OARs are also shown in Table 2. The CTV D90 was significantly higher for the ISBT group than for the ICBT
group (p
| Clinical/treatment features | ICBT group | ISBT group | p value | |
| (n = 10) | (n = 12) | |||
| Age at the time of vaginal recurrence (years), median (range) | 61 (33–74) | 65 (33–78) | 0.31 | |
| Primary disease | Cervical cancer | 4 | 5 | 0.69 |
| Endometrioid cancer | 6 | 7 | ||
| Size of vaginal recurrence (cm), median (range) | 0.6 (0.0–1.7) | 2.7 (1.2–4.5) | ||
| Site of vaginal recurrence | Vaginal stump and/or wall | 10 | 3 | |
| Extravaginal extension | 0 | 9 | ||
| Treatment | Radiotherapy alone | 9 | 6 | 0.13 |
| Chemoradiotherapy | 1 | 6 | ||
| EBRT | Yes | 5 | 12 | 0.02 |
| No | 5 | 0 | ||
| Dose of WPI without midline block (Gy), median (range) | 15 (0–40) | 30 (30–50) | 0.01 | |
| Volume for CTV (cc), median (IQR) | 6.6 (5.8–10.5) | 25.7 (16.9–53.4) | ||
| CTV D90 (Gy), median (IQR) | 60.9 (52.9–66.8) | 72.8 (71.9–75.7) | ||
| Bladder D2cc | 69.8 (50.3–81.9) | 70.2 (66.8–74.8) | 0.79 | |
| Intestine D2cc | 33.6 (6.8–39.3) | 36.1 (30.0–41.2) | 0.34 | |
| Sigmoid D2cc | 25.7 (1.7–33) | 42.8 (38.9–47.5) | ||
| Rectum D2cc | 36.4 (32.4–45.8) | 61.1 (53.8–66.0) | ||
| Abbreviations: IQR, interquartile range; CTV, clinical target volume; OAR, organs at risk; ICBT, intracavitary brachytherapy; ISBT, interstitial brachytherapy; EBRT, external beam radiotherapy; WPI, whole pelvic irradiation. | ||||
Complete response was reached in all patients. At the time of analysis, pelvic recurrence or distant metastasis were observed in 5 of 7 cervical cancer patients treated with ISBT. In contrast, there was no incidence of pelvic recurrence and distant metastasis in either endometrial cancer patients or cervical cancer patients treated with ICBT. Local recurrence was observed in one cervical cancer patient treated with ISBT at 6.9 months from radiotherapy, despite no residual tumor was observed on MRI and negative cytology was reported 2 months after radiotherapy. Although the sufficient dose (71.8 Gy) was administered to the CTV D90 in this patient with local recurrence, a part of the tumor edge adjacent to the pelvic wall enlarged. Inguinal lymph node metastasis and distant metastasis (i.e., lung, bone and peritoneal dissemination) were observed in 1 and 3 patients, respectively. The median follow-up duration was 58.7 months (range, 9.6–93.1 months). The 5-year OS, DFS and LC rates in the overall cohort were 84.8, 77.3 and 95.5%, respectively. Specifically, the 5-year OS rates (Fig. 2a) in cervical cancer patients and endometrial cancer patients were 70.1 and 100%, respectively (cervical cancer vs. endometrial cancer, p = 0.06), whereas the 5-year LC rates (Fig. 2b) were 90.9 and 100%, respectively (p = 0.32). There were no significant differences in OS and LC between the cervical cancer and endometrial cancer patients. Finally, the ICBT and ISBT groups reported 5-year LC rates of 100 and 90.9%, respectively (ICBT vs. ISBT, p = 0.36).
Grade 2 late rectal complications occurred in 2 patients (9%) who received
ISBT. No patients experienced grade
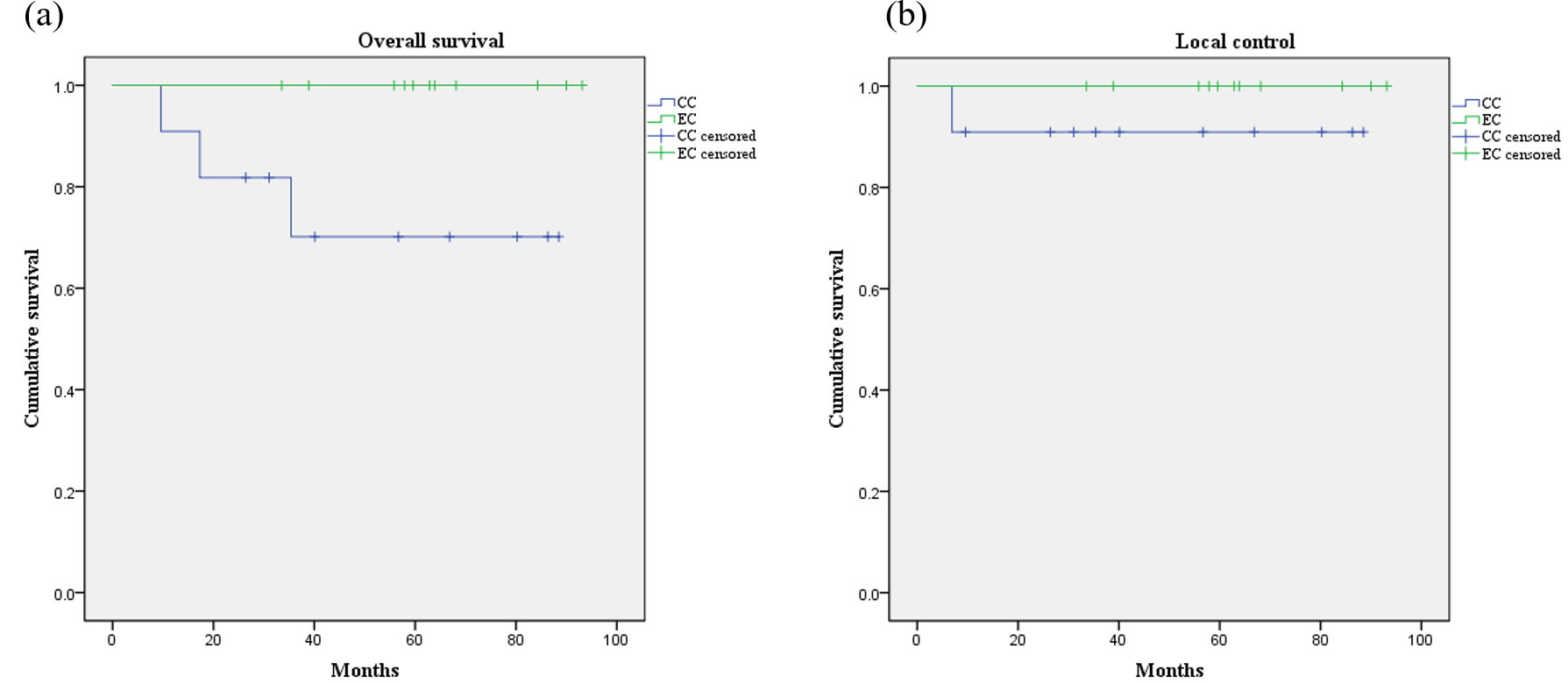 Fig. 2.
Fig. 2.Kaplan-Meier curve describing overall survival (a) and local control (b). Blue line: cervical cancer patients; green line: endometrial cancer patients. Abbreviations: CC, cervical cancer patients; EC, endometrial cancer patients.
Data on the outcomes of IGABT for uterine cancer patients with vaginal
recurrence are scarce. In this study, IGABT for vaginal recurrence could deliver
a sufficient CTV dose, with the OAR doses maintained within those recommended by
the American Brachytherapy Society (ABS) [11]. Therefore, an excellent 5-year LC
(95.5%) was obtained, while no patients experienced grade
In the dose-volume parameters of this study, CTV D90 in the ISBT group was
higher than that in the ICBT group. This could be considering that 5 out of 10
patients who received ICBT underwent ICBT alone. Moreover, the CTV D90 for
patients with thinner vaginal recurrence could have been underestimated due to
the contouring in the vaginal stump or wall. In the ICBT group, all 10 patients
presenting a small CTV obtained local control, in spite of lower dose of CTV D90
compared to those in ISBT group. This may be caused by underestimation of actual
delivered dose to CTV. Furthermore, the rectum and sigmoid D2ccs were higher in
the ISBT group compared to the ICBT group (both with p
Salvage 2D planning brachytherapy for endometrial cancer patients with vaginal
recurrence after radical surgery has reportedly led to 5-year LC rates of
65–100%, but also with high rates of grade 3 or 4 adverse effects (9–18%) [2, 3, 13, 14]. Over the last two decades, IGABT, which could evaluate the doses for
CTV and OARs, had been increasingly preferred over 2D planning brachytherapy
(Tan, 2011 [4]; WPI). Previous studies reported that IGABT for vaginal recurrence
could deliver higher doses to the CTV while being within the recommended doses
for OARs, hence leading to good LC (about 95%) and low rate of grade
Whylie et al. [14] reported that large vaginal recurrence predicted
poor LC and that 2D planning ICBT led to a 5-year LC of 65% in 49 endometrial
cancer patients whose median vaginal recurrence size was 2.0 cm. Furthermore, Ito
et al. [16] showed that for patients without palpable vaginal
recurrence, 2D planning ICBT achieved cumulative local failure of only 10%,
compared to the 49 and 63% for median- (
The limitations of this study include its retrospective design and the small sample size. Moreover, bias was present in the patients’ clinical background and the dose prescription. Thus, further multicenter studies that include a larger number of patients with uniform clinical background are needed to verify our findings. However, outcomes and dose-volume parameters evaluated in this study could encourage implementing IGABT for vaginal recurrence after being primarily managed by surgery.
CT-based IGABT based on the initial disease extent and tumor thickness enabled sufficient CTV dose administration, tolerable to organs at risk. CT-based brachytherapy has the potential to become essential to treat the vaginal recurrence of uterine cancer after being managed by surgery as it can achieve good LC without increasing the rate of late complications.
KNM, AO and HN designed the research study. RT performed the research. TO and HS provided help and advice on the ELISA experiments. RT analyzed. KNM wrote the manuscript. All authors contributed to editorial change in the manuscript. All authors read and approved the final manuscript.
This retrospective study was approved by the institutional review board of Tsukuba University (R01-277) and was conducted according to the tenets of the 1975 Declaration of Helsinki. The need for informed consent was waived owing to the retrospective nature of the study.
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
This research was funded by Japan Agency for Medical Research and Development (AMED), grant number 18xx0000000 h 0001.
The authors declare no conflict of interest.
