Background: The aim of this study was to compare fetal thymus
volume in women who delivered at
Although twin birth rates have changed in recent years by maternal age and ethnicity, they are increasing and account for more than 3% of all live births [1, 2]. The risk of preterm birth for twins is 12 times higher than for single births. Twin pregnancies are high-risk pregnancies due to increased perinatal morbidity and mortality due to preterm delivery [3].
The two most researched methods for predicting preterm labor for multiple
pregnancies are cervical length and fetal fibronectin [4]. A meta-analysis showed
that in asymptomatic women with twins, a cervical length
Chorioamnionitis is associated with a significant reduction in thymic size at birth in very-low-birth-weight preterm infants [7]. Fetal thymus measurement may allow an early diagnosis of chorioamnionitis in PPROM cases [8, 9]. A recent study speculated that small fetal thymus may be associated with early preterm birth, chorioamnionitis, neonatal sepsis, and morbidity [10]. The fetal thymus’s role in preterm delivery for twin pregnancies is not well known, especially for twin pregnancies. A small thymus size may cause preterm delivery of twins.
The thymus, which is one of the primary lymphoid organs in the fetus, may undergo involution in some inflammatory processes. Whether this condition is affected by preterm labor caused by inflammation, which has a role in the etiopathogenesis of preterm labor, can be investigated. Previous studies [8] showed that fetal thymus tissue may lead to involution, which may lead to shrinkage of the thymus in chorioamnionitis. However, an exhaustive literature review failed to reveal any study investigating the role of fetal thymus size on preterm delivery rates of twins.
In this study, we aimed to evaluate dichorionic diamniotic pregnancies, a special patient group for preterm labor, in order to determine the relationship between preterm labor and thymus volume.
In this prospective cohort study, 40 spontaneous dichorionic diamniotic twin pregnant women admitted to our clinic for second-trimester (18–24 gestational week) anomaly screening were evaluated over a 12-month period. The Ethics Committee approved the study in 2017 (number 211), and the Scientific Research Ethics Committee of the authors’ faculty approved the research on 22 January 2018.
The study group consisted of women with spontaneous dichorionic diamniotic twin pregnancies (n = 40), 18–35 years of age, with no complaints at 18–24 weeks of gestation with normal renal, liver, and thyroid functions.
The exclusion criteria for the research group were as follows: (i)
endocrinopathies, systemic disease, collagen disorder, hypercholesterolemia
(familial or chronic hypercholesterolemia), sickle cell anemia, or a history of
neoplasm; (ii) patients with a history of coronary artery disease, angina, or
myocardial infarction; any history of known vascular, infectious (including
current urinary tract infection), or inflammatory disease; or hypertension,
coronary arteritis, electrocardiographic changes, or maternal autoimmune disease;
(iii) the use of any drug within 3 months before pregnancy; (iv) singleton
pregnancy; (vi) alcohol drinkers; (vii) abnormal renal, hepatic, and thyroid
function test results; (viii) refuse to participate in the study; (ix) abnormal
fetal findings in any baby (fetal anomaly, nuchal translucency increase,
first-/second-trimester screening test disorder); (x) abnormal fetal estimated
fetal weight (
After the participants signed the consent form, they were accepted into the study. Age, gravida, parity, body mass index (BMI), systolic and diastolic blood pressures at admission, biparietal diameter (BPD), abdominal circumference (AC), femur length (FL), estimated fetal weight (EFW), and cervical length (mm) data were recorded. Fetal thymus volumes were recorded for each fetus. Cases were followed up during pregnancy and the week of the delivery, and APGAR scores at the 1st and 5th minutes, neonatal weight, cord blood pH, and base excess (BE) values during delivery were recorded.
Preterm Birth Diagnosis: Although there is no definite limit for twin pregnancy,
all deliveries before 36 weeks were accepted as preterm births because the mean
birth week is 35 weeks in twins [11, 12]. The data of women experiencing preterm
labor (delivered at
The thymus is located superior to the fetal thorax. The thymus, fetal sternum,
3-vessel images (superior vena cava, aorta, and pulmonary artery), and fetal
lungs are observable when observing the transverse section of fetal thorax (Fig. 1). In the anterior mediastinum, the thymus is observed as a hypoechogenic 2-lobe
structure, as compared to other tissues. Each fetus’s thymus dimensions were
measured using VOLUSON E-10 ultrasonography, which can perform VOCAL measurement
with the 2-dimensional and 3-dimensional VOCAL program in 3-vessel trachea (3VT)
sections [6, 13, 14, 15, 16] (Fig. 1). The 3-dimensional fetal thymus volume data were
assessed using spatio-temporal image correlation with a standard 10-second
acquisition time and an acquisition sweep angle of 30
Each measurement was repeated 3 times in each fetus, and the mean size was determined. The interobserver variability and reproducibility were 3.1% and 98%, respectively [8]. The fetuses whose thymus could not be adequately visualized were not included in the final analysis.
 Fig. 1.
Fig. 1.Appearance the of fetal thymus in 3 vessel sections on ultrasonography (aorta, aortic artery; pulm artery, pulmonary artery; svc, superior vena cava; thymus, fetal thymus).
All of the data were entered and coded in the SPSS 13.0 software package. A
Kolmogorov-Smirnov test was used to test the normality assumption. Our data
failed to pass this test, so Mann-Whitney U tests and Pearson correlation
analyses were used for the computer statistical analysis. The data of the cases
with preterm birth (
A total of 32 cases were evaluated for the final analysis. All of the patients
were delivered spontaneously. In total, 18 women delivered at
| Demographic and sonographic factors | The group with preterm birth (n = 18) | The group without preterm birth (n = 14) | P |
| Age (years) | 31.83 ± 5.82 | 31.21 ± 4.49 | |
| Gravida (no) | 2.00 ± 1.03 | 2.07 ± 1.27 | |
| Parity (no.) | 0.61 ± 0.70 | 0.71 ± 0.83 | |
| Body mass index (kg/m |
31.01 ± 5.88 | 29.98 ± 4.22 | |
| Pregnancy week at the time of measurement (week) | 20.59 ± 1.02 | 21.48 ± 1.79 | |
| Gestational week at birth (week) | 33.01 ± 2.69 | 36.66 ± 0.71 | |
| Cervical length at first admission (mm) | 34.22 ± 6.58 | 37.50 ± 4.40 | |
| 1. fetus EFW (g) | 308.78 ± 91.23 | 318.64 ± 113.49 | |
| 1. fetus thymus volume (cm |
0.312 ± 0.205 | 0.893 ± 0.440 | |
| 2. fetus EFW (g) | 308.22 ± 95.63 | 320.71 ± 103.45 | |
| 2. fetus thymus volume (cm |
0.318 ± 0.176 | 0.939 ± 0.510 | |
| Average fetal thymus volume (cm |
0.315 ± 0.177 | 0.916 ± 0.416 | |
| Cesarean section (%) | %100 | %100 | — |
| 1st fetus birth weight (gr.) | 1888.61 ± 611.93 | 2628.93 ± 333.41 | |
| 1. fetus fetal PH (no.) | 7.32 ± 0.06 | 7.33 ± 0.04 | |
| 1. fetus BE (mmol/L) | −2.24 ± 3.78 | −1.47 ± 1.72 | |
| 2. fetus birth weight (gr.) | 1792.78 ± 606.67 | 2465.71 ± 286.27 | |
| 2. fetus fetal PH (no.) | 7.31 ± 0.08 | 7.33 ± 0.03 | |
| 2. fetus BE (mmol/L) | −2.53 ± 3.23 | −1.20 ± 2.78 | |
| Data are given as mean | |||
For the study group, Figs. 2,3 show the ROC analysis of the relationship
between fetal thymus size and preterm labor, measured in the first and second
fetuses at 18–24 weeks of gestation, within the scope of preterm labor
development. When the fetal thymus volume measured in the first infant was used
as a marker for the development of preterm labor, the sensitivity and specificity
were 83.3% and 85.7%, respectively, for a thymus volume of 0.5245 cm
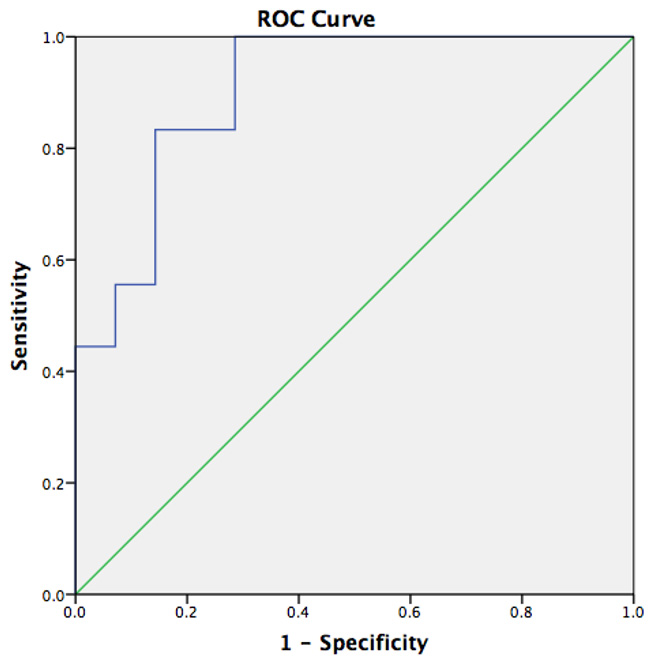 Fig. 2.
Fig. 2.ROC analysis showing the relationship between fetal thymus size and preterm labor in the first fetus for the study group.
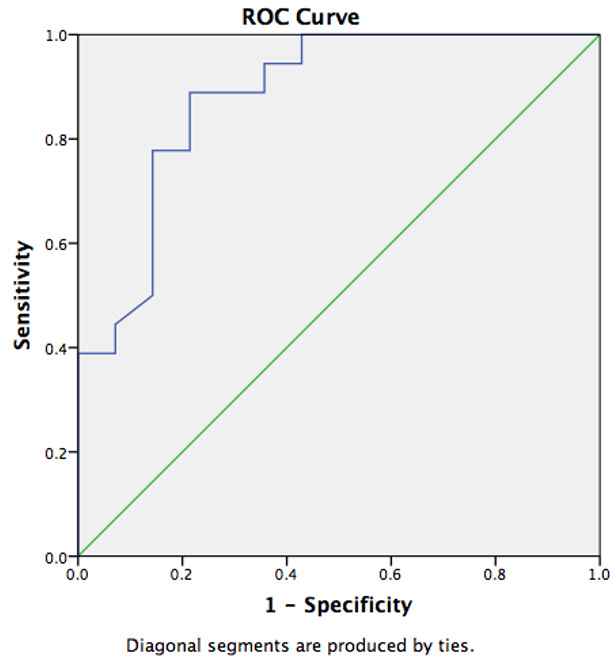 Fig. 3.
Fig. 3.ROC analysis showing the relationship between fetal thymus size and preterm labor in the second fetus for the study group.
Fetal thymus size (in terms both twins and average thymus size) was
significantly lower in women who developed preterm labor compared to those who
did not develop preterm labor. Fig. 4 shows ROC analysis of the the relationship
between mean fetal thymus size per twin and preterm labor. When the mean fetal
thymus volume measured per twin was used as a marker for the development of
preterm labor, the sensitivity and specificity were 88.9% and 92.9%,
respectively, for a thymus volume of 0.5310 cm
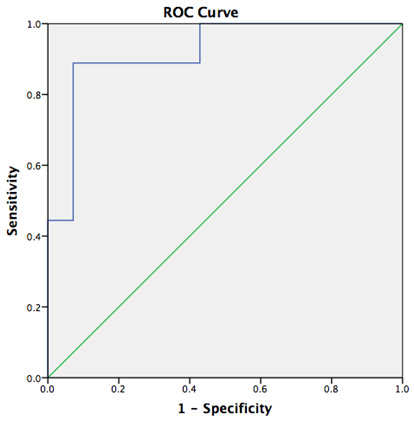 Fig. 4.
Fig. 4.ROC analysis showing the relationship between mean fetal thymus size per twin and preterm labor for the study group.
According to the binary logistic regression analysis performed to predict the development of preterm labor during pregnancy, low mean fetal thymus volume measured in both babies at 18–24 weeks can be used as a predictive factor (P = 0.008).
Given that twin pregnancies have a higher risk for preterm delivery, identifying these cases would enable the development of effective interventions and a better understanding of the disease mechanisms leading to spontaneous preterm labor so as to prevent the associated negative perinatal outcomes [17]. The current study may suggest the use of the fetal thymus for preventing complications associated with preterm delivery in twins.
The relationship between preterm birth and fetal thymus volume has been unknown [18]. One advantage of risk assessment with non-invasive markers such as fetal thymus volume may be the prevention of unnecessary and sometimes costly interventions for at-risk patients. In this study, fetal thymus volume was evaluated as a noninvasive marker that does not require additional cost. In dichorionic-diamniotic pregnancies, fetal thymus volume can be considered a marker for predicting preterm labor for each fetus in the second trimester.
Many markers have been used to predict preterm birth. Yet, the effectiveness of
these markers in predicting preterm delivery is still controversial [17]. The
evaluations needed for predicting preterm delivery should be conducted prior to
24 weeks of gestation. Although preterm labor appears at the end of second
trimester and during the third trimester period, the first pathophysiological
changes may occur earlier. In particular, maternal and fetal diseases that affect
the fetus’s immunity can initiate preterm delivery [19]. Therefore, in the
current study, the fetal thymus volume was measured to evaluate the fetuses’
immune adaptation and predict preterm delivery before viability in the physiology
of preterm labor. The results showed that a small fetal thymus size may be a
marker for preterm delivery before other reported feto-maternal findings
(cervical opening, engagement, cervical remodeling, uterine contraction) appear
in preterm delivery. When the thymus volume is evaluated as a marker in
predicting preterm delivery, the cutoff value of 0.5280 cm
The fetal thymus produces thymosin alpha-1, which can be detected in maternal serum during pregnancy [19]. The thymus is the most important organ of the immune system and is responsible for immunity during the neonatal and infant periods. The idea that preterm delivery may be due to the weak immunity of the fetus was first introduced in 2007 in a chorioamnionitis study, in which the authors reported that thymus size decreased in patients who had preterm birth with chorioamnionitis [8]. As a result, they reported that the measurement of the fetal thymus might predict an early diagnosis of chorioamnionitis in PPROM cases [8]. Intrauterine infection and PPROM lead to fetal thymic involution [20]. An animal study reported that intrauterine inflammation on fetal thymus tissue decreased the number of CD8 T cells and altered the development and/or activation of CD4 T cells. Thus, the effects of chorioamnionitis in the thymus were also elucidated [21].
The fetal thymus responds sensitively to many systemic factors such as fetal chorioamnionitis. Fetal corticosteroids being released against systemic factors is the main factor of reduction in the thymus’s size. These processes increase the lymphocyte release from the fetal thymus cortex. According to pathology and necropsy studies, in case of fetal infection, the fetal thymus’s corticomedullary ratio changes, and the thymus shrinks [22]. Fetal thymus shrinkage, which has been well defined during fetal infections, may also possible in fetal latent inflammation. This inflammation may be associated with preterm birth in women with twin pregnancies. Furthermore, this situation may be predicted by measuring the fetal thymus’s volume in the second trimester of pregnancy.
A small fetal thymus may initiate or be the result of a fetal systemic inflammatory process that initiates preterm labor. The fetal thymus’s volume may not be large enough in response to the fetus’s systemic inflammatory condition, so the low number of required protective cells (T lymphocytes) may be unable to prevent preterm delivery. Thus, future molecular and biochemical studies are needed to evaluate the relationship among fetal systemic inflammatory response, fetal thymus, and preterm labor.
In a study on sheep, Yang et al. [23] reported that COX-2 and AKR1B1 dimer expression was upregulated in the maternal thymus in early pregnancy, and they concluded that the thymus was involved in immunomodulation during early pregnancy. The current results also suggest that a small fetal thymus volume affects maternal-fetal preterm delivery.
Compared to other commercially available preterm-prediction factors, fetal thymus measurement is cheap, easy to use. This is the first study to report on the role of fetal thymus measurement for predicting pre-term delivery of twins.
The strengths of the study were that a single physician made the measurements and that the method is easily applicable, with no additional costs, visits (it may be measured during routine screening), and additional time loss for the patient, as well as the prospective design of the study. In addition, the investigation of twin pregnancies—a specific group in which preterm labor is frequently seen—as well as the evaluation of two fetuses according to single-fetus pregnancies and the evaluation of the fetuses’ average thymus volume also make the research strong.
The limitations of the study included the margin of error of the VOCAL program, which is integrated with ultrasonography. Larger case series studies are needed to support this hypothesis due to the limited number of cases used in our study. In this prospective study, the thymuses were measured in the second trimester, but delivery was often months later. Missed intrauterine infections also may have caused small thymus volume. No placental histology was taken to confirm the presence of this condition. Further histopathologic studies are needed to exclude missed diagnoses of intrauterine infection.
In dichorionic-diamniotic twin pregnancies, fetal thymus volume measurement was
found to be a marker predicting the development of spontaneous preterm labor.
Small fetal thymus volume was a significant parameter in predicting preterm
labor. When the fetal thymus volume, measured 3-dimensionally by ultrasonography
in conjunction with the VOCAL program, was used as a marker for predicting
preterm delivery, the cut-off value was 0.5310 cm
All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. HS and ESGG conceived,designed and performed the experiment; SG. analyzed the data.
Clinical Trials Identifier Number: NCT04055701. All patients gave consent to participate the study.
We thank the numerous individuals who participated in this study.
This research received no external funding.
The authors declare no conflict of interest.
